Simultaneous Removal of Organic Matter and Nutrients from High Strength Organic Wastewater Using Sequencing Batch Reactor (SBR)
Abstract
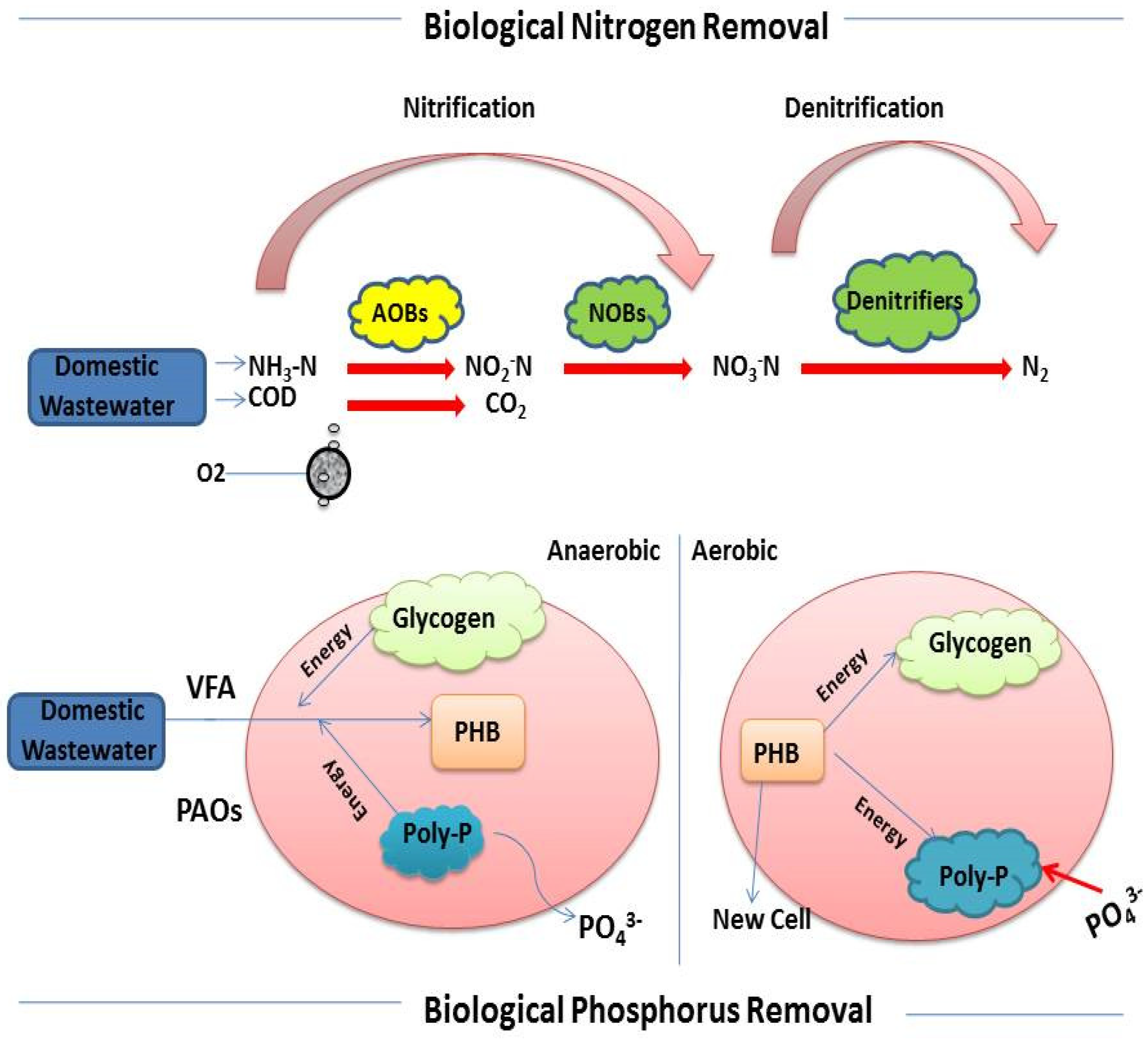
:1. Introduction
2. Materials and Methods
2.1. Reactor Setup and Operation
2.2. Seed Sludge and Wastewater Composition
2.3. Analytical Methods
2.4. Calculations
3. Results and Discussion
3.1. Effect of Cycle Time and C:N:P Ratios on Reactor Performance
3.1.1. COD Removal in SBR
3.1.2. N Removal
3.1.3. P Removal
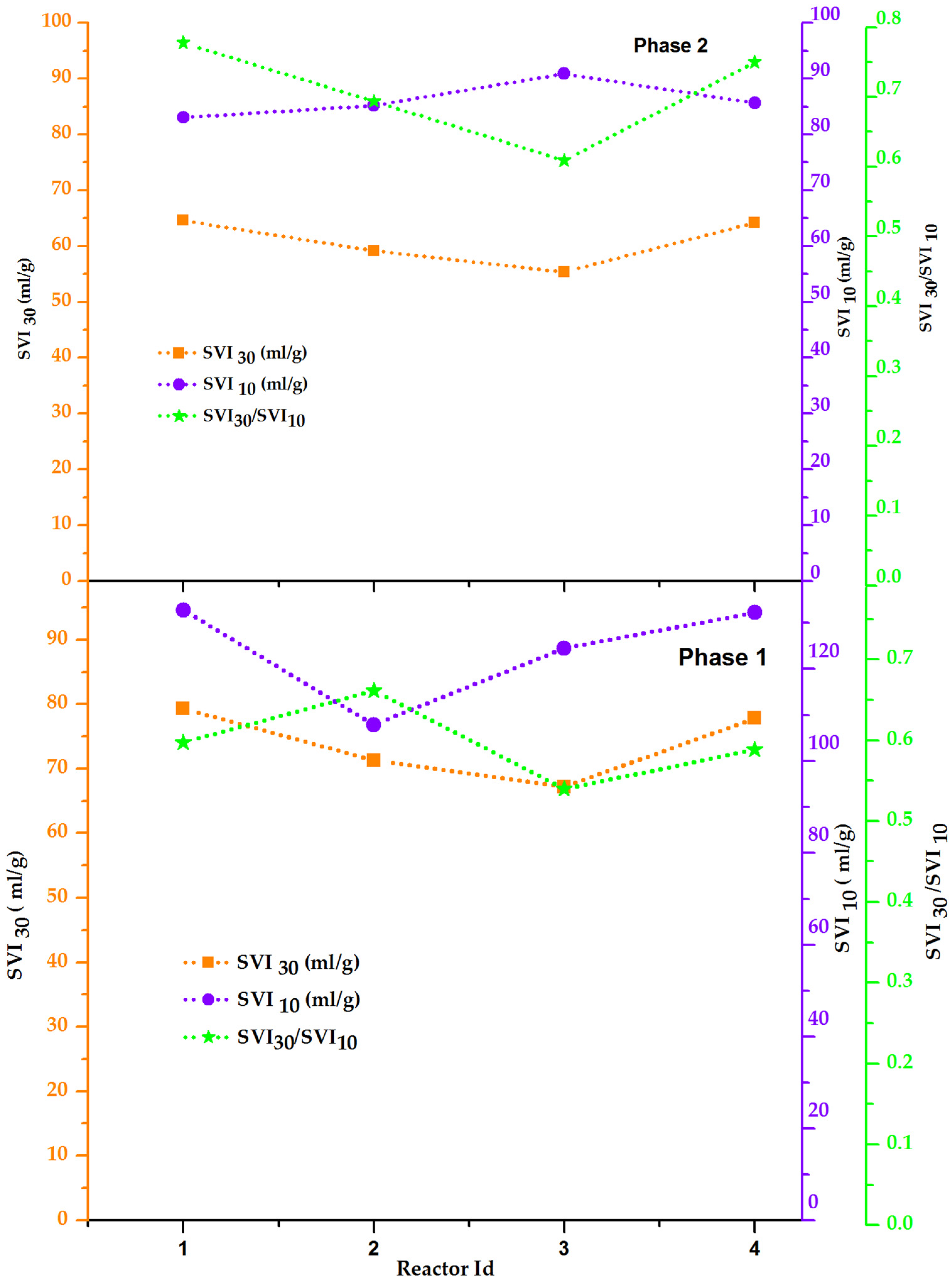
3.1.4. Sludge Volume Index
3.2. Statistical Analysis of Tested Variables
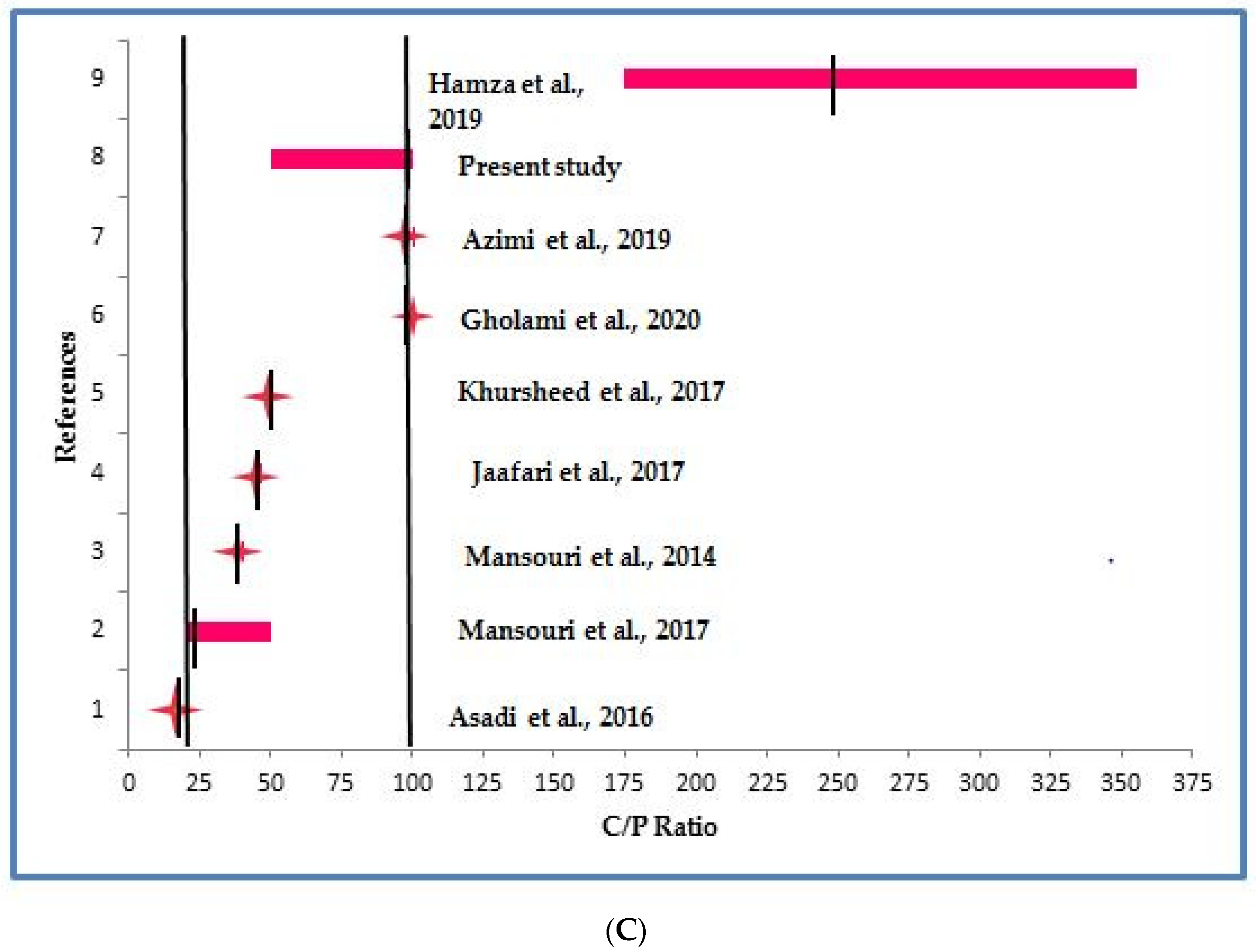
3.3. Comparison of Present Study with Literature
4. Conclusions
- The C:N:P ratio 100:5:1 (C/N = 20; C/P = 100) proposed for conventional activated sludge process was found to be sufficient for biomass growth and nutrient removal from high strength synthetic wastewater used in the present study;
- Excellent effluent quality with COD conc. < 50 mg/L and PO43− P conc. ~1 mg/L was attained at cycle time of 9 h in reactor R2. Almost complete NH3-N removal was also observed in the same. In addition, when the cycle time was reduced to 3 h, the removal efficiencies were quite encouraging (COD = 90%; NH3-N = 98.5%; PO43−P = 84.5%);
- Statistical analysis indicates that cycle time, carbon to nitrogen, and carbon to phosphorus all have significant individual main effects on NH3-N and PO43−-P removal at p < 0.05. COD removal, however, was not significantly affected by the C/N ratio. On NH3-N removal, there were also significant interaction effects between cycle time and C/N, cycle time and C/P, and C/N and C/P. Furthermore, the interaction effects of cycle time and C/N, as well as cycle time and C/P, were found to be insignificant for PO43−P removal;
- The coefficient of determination (R2) for COD, NH3-N and PO43− P removal was 0.841, 0.978, and 0.994, which suggested that there was very little variation in data that could not be explicated by the fitted model;
- SVI30 and SVI10 ratio were found to decrease with an increase in cycle time from 3 to 9 h;
- Further, the ratios of SVI30 and SVI10 were less than 1, which concluded that granulation was not complete in all the reactors.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, M.; Wang, Y.; Pan, Y.; Zhang, K.; Lyu, L.; Wang, M.; Zhu, T. Nitrogen removal from wastewater via simultaneous nitrification and denitrification using a biological folded non-aerated filter. Bioresour. Technol. 2019, 289, 121696. [Google Scholar] [CrossRef] [PubMed]
- Hojjati-Najafabadi, A.; Mansoorianfar, M.; Liang, T.; Shahin, K.; Wen, Y.; Bahrami, A.; Vasseghian, Y. Magnetic-MXene-based nanocomposites for water and wastewater treatment: A review. J. Water Process Eng. 2022, 47, 102696. [Google Scholar] [CrossRef]
- Kartal, B.; Kuenen, J.V.; Van Loosdrecht, M.C.M. Sewage treatment with anammox. Science 2010, 328, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Zuthi, M.F.R.; Guo, W.S.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I. Enhanced biological phosphorus removal and its modeling for the activated sludge and membrane bioreactor processes. Bioresour. Technol. 2013, 139, 363–374. [Google Scholar] [CrossRef]
- Bărbulescu, A.; Barbeş, L. Statistical methods for assessing water quality after treatment on a sequencing batch reactor. Sci. Total Environ. 2021, 752, 141991. [Google Scholar] [CrossRef]
- Wang, J.; Rong, H.; Cao, Y.; Zhang, C. Factors affecting simultaneous nitrification and denitrification (SND) in a moving bed sequencing batch reactor (MBSBR) system as revealed by microbial community structures. Bioprocess Biosyst. Eng. 2020, 43, 1833–1846. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Li, S.; He, P.; Wang, D. Optimization of Nitrogen Removal in Solid Carbon Source SND for Treatment of Low-Carbon Municipal Wastewater with RSM Method. Water 2018, 10, 827. [Google Scholar] [CrossRef]
- Jiang, J.Q. The role of coagulation in water treatment. Curr. Opin. Chem. Eng. 2015, 8, 36–44. [Google Scholar] [CrossRef]
- Wang, X.J.; Xia, S.Q.; Chen, L.; Zhao, J.F.; Renault, N.J.; Chovelon, J.M. Nutrients removal from municipal wastewater by chemical precipitation in a moving bed biofilm reactor. Process Biochem. 2006, 41, 824–828. [Google Scholar] [CrossRef]
- Huang, X.; Guida, S.; Jefferson, B.; Soares, A. Economic evaluation of ion-exchange processes for nutrient removal and recovery from municipal wastewater. NPJ Clean Water 2020, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Bolton, L.; Joseph, S.; Greenway, M.; Donne, S.; Munroe, P.; Marjo, C.E. Phosphorus adsorption onto an enriched biochar substrate in constructed wetlands treating wastewater. Ecol. Eng. 2019, 142, 100005. [Google Scholar] [CrossRef]
- Kumari, R.; Ankit, H.; Basu, S. Reclamation of water from dairy wastewater using membrane bioreactor (MBR)–Membrane filtration processes. Mater. Today Proc. 2021, 47, 1452–1456. [Google Scholar] [CrossRef]
- Swain, K.; Abbassi, B.; Kinsley, C. Combined electrocoagulation and chemical coagulation in treating brewery wastewater. Water 2020, 12, 726. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Zeynali, V.; Masoudi, S.M.A.; Sargolzaei, J.; Dehkordi, F.S. The effect of key factors in aerobic bioreactor and optimization by different strategies. Environ. Technol. Innov. 2020, 18, 100724. [Google Scholar] [CrossRef]
- Akın, B.S.; Ugurlu, A. Monitoring and control of biological nutrient removal in a sequencing batch reactor. Process Biochem. 2005, 40, 2873–2878. [Google Scholar] [CrossRef]
- Water Environment Federation; Environmental and Water Resources Institute (U.S.). Biological Nutrient Removal (BNR) Operation in Wastewater Treatment Plants; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Singh, M.; Srivastava, R.K. Sequencing batch reactor technology for biological wastewater treatment: A review. Asia-Pac. J. Chem. Eng. 2011, 6, 3–13. [Google Scholar] [CrossRef]
- Mansouri, A.M.; Zinatizadeh, A.A.; Irandoust, M.; Akhbari, A. Statistical analysis and optimization of simultaneous biological nutrients removal process in an intermittently aerated SBR. Korean J. Chem. Eng. 2014, 31, 88–97. [Google Scholar] [CrossRef]
- Eddy, M.; Abu-Orf, M.; Bowden, G.; Burton, F.L.; Pfrang, W.; Stensel, H.D.; AECOM. Wastewater Engineering: Treatment and Resource Recovery; McGraw Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Han, F.; Ye, W.; Wei, D.; Xu, W.; Du, B.; Wei, Q. Simultaneous nitrification-denitrification and membrane fouling alleviation in a submerged biofilm membrane bioreactor with coupling of sponge and biodegradable PBS carrier. Bioresour. Technol. 2018, 270, 156–165. [Google Scholar] [CrossRef]
- Khursheed, A.; Gaur, R.Z.; Sharma, M.K.; Tyagi, V.K.; Khan, A.A.; Kazmi, A.A. Dependence of enhanced biological nitrogen removal on carbon to nitrogen and rbCOD to sbCOD ratios during sewage treatment in sequencing batch reactor. J. Clean. Prod. 2018, 171, 1244–1254. [Google Scholar] [CrossRef]
- Azimi, S.C.; Shirini, F.; Pendashteh, A. Evaluation of COD and turbidity removal from woodchips wastewater using biologically sequenced batch reactor. Process Saf. Environ. Prot. 2019, 128, 211–227. [Google Scholar] [CrossRef]
- Asadi, A.; Zinatizadeh, A.A.; Van Loosdrecht, M. High rate simultaneous nutrients removal in a single air lift bioreactor with continuous feed and intermittent discharge regime: Process optimization and effect of feed characteristics. Chem. Eng. J. 2016, 301, 200–209. [Google Scholar] [CrossRef]
- Abdulgader, M.; Yu, J.; Zinatizadeh, A.A.; Williams, P.; Rahimi, Z. Process analysis and optimization of single stage flexible fibre biofilm reactor treating milk processing industrial wastewater using response surface methodology (RSM). Chem. Eng. Res. Des. 2019, 149, 169–181. [Google Scholar] [CrossRef]
- Shao, Y.; Shi, Y.; Mohammed, A.; Liu, Y. Wastewater ammonia removal using an integrated fixed-film activated sludge-sequencing batch biofilm reactor (IFAS-SBR): Comparison of suspended flocs and attached biofilm. Int. Biodeterior. Biodegrad. 2017, 116, 38–47. [Google Scholar] [CrossRef]
- de Sousa Rollemberg, S.L.; Barros, A.R.M.; de Lima, J.P.M.; Santos, A.F.; Firmino, P.I.M.; dos Santos, A.B. Influence of sequencing batch reactor configuration on aerobic granules growth: Engineering and microbiological aspects. J. Clean. Prod. 2019, 238, 117906. [Google Scholar] [CrossRef]
- Yadu, A.; Sahariah, B.P.; Anandkumar, J. Influence of COD/ammonia ratio on simultaneous removal of NH4+-N and COD in surface water using moving bed batch reactor. J. Water Process Eng. 2018, 22, 66–72. [Google Scholar] [CrossRef]
- Wang, H.; Song, Q.; Wang, J.; Zhang, H.; He, Q.; Zhang, W.; Li, H. Simultaneous nitrification, denitrification and phosphorus removal in an aerobic granular sludge sequencing batch reactor with high dissolved oxygen: Effects of carbon to nitrogen ratios. Sci. Total Environ. 2018, 642, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.A.; Zaghloul, M.S.; Iorhemen, O.T.; Sheng, Z.; Tay, J.H. Optimization of organics to nutrients (COD: N: P) ratio for aerobic granular sludge treating high-strength organic wastewater. Sci. Total Environ. 2019, 650, 3168–3179. [Google Scholar] [CrossRef]
- Li, D.; Zhang, S.; Li, S.; Zeng, H.; Zhang, J. Aerobic granular sludge operation and nutrients removal mechanism in a novel configuration reactor combined sequencing batch reactor and continuous-flow reactor. Bioresour. Technol. 2019, 292, 122024. [Google Scholar] [CrossRef]
- Abramov, V.O.; Abramova, A.V.; Cravotto, G.; Nikonov, R.V.; Fedulov, I.S.; Ivanov, V.K. Flow-mode water treatment under simultaneous hydrodynamic cavitation and plasma. Ultrason. Sonochemistry 2021, 70, 105323. [Google Scholar] [CrossRef]
- Zaman, M.; Kim, M.; Nakhla, G. Simultaneous nitrification-denitrifying phosphorus removal (SNDPR) at low DO for treating carbon-limited municipal wastewater. Sci. Total Environ. 2021, 760, 143387. [Google Scholar] [CrossRef]
- Meng, Q.; Yang, F.; Liu, L.; Meng, F. Effects of COD/N ratio and DO concentration on simultaneous nitrifcation and denitrifcation in an airlift internal circulation membrane bioreactor. J. Environ. Sci. 2008, 20, 933–939. [Google Scholar] [CrossRef]
- Gholami, F.; Zinatizadeh, A.A.; Zinadini, S.; McKay, T.; Sibali, L. An innovative jet loop-airlift bioreactor for simultaneous removal of carbon and nitrogen from soft drink industrial wastewater: Process performance and kinetic evaluation. Environ. Technol. Innov. 2020, 19, 100772. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Shen, J.; Zhao, X.; Kang, J. Impact of carbon to nitrogen ratio on the performance of aerobic granular reactor and microbial population dynamics during aerobic sludge granulation. Bioresour. Technol. 2019, 271, 258–265. [Google Scholar] [CrossRef]
- Mansouri, A.M.; Zinatizadeh, A.A. A comparative study of an up-flow aerobic/anoxic sludge fixed film bioreactor and sequencing batch reactor with intermittent aeration in simultaneous nutrients (N, P) removal from synthetic wastewater. Water Sci. Technol. 2017, 76, 1044–1058. [Google Scholar] [CrossRef]
- Lackner, S.; Terada, A.; Horn, H.; Henze, M.; Smets, B.F. Nitritation performance in membrane-aerated biofilm reactors differs from conventional biofilm systems. Water Res. 2010, 44, 6073–6084. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.H.; Dharmawan, F.; Palmer, C.G. Roles of polyurethane foam in aerobic moving and fixed bed bioreactors. Bioresour. Technol. 2010, 101, 1435–1439. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Akhbari, A.; Zinatizadeh, A.A.L.; Mohammadi, P.; Irandoust, M.; Mansouri, Y. Process modeling and analysis of biological nutrients removal in an integrated RBC-AS system using response surface methodology. Chem. Eng. J. 2011, 168, 269–279. [Google Scholar] [CrossRef]
- Jaafari, J.; Mesdaghinia, A.; Nabizadeh, R.; Hoseini, M.; Mahvi, A.H. Influence of upflow velocity on performance and biofilm characteristics of Anaerobic Fluidized Bed Reactor (AFBR) in treating high-strength wastewater. J. Environ. Health Sci. Eng. 2014, 12, 139. [Google Scholar] [CrossRef]
- Chen, C.; Guo, W.S.; Ngo, H.H.; Chang, S.W.; Nguyen, D.D.; Zhang, J.; Zhang, X.B. Effects of C/N ratio on the performance of a hybrid sponge-assisted aerobic moving bed-anaerobic granular membrane bioreactor for municipal wastewater treatment. Bioresour. Technol. 2018, 247, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Masoudi, S.M.A.; Hedayati Moghaddam, A.; Sargolzaei, J.; Darroudi, A.; Zeynali, V. Investigation and optimization of the SND–SBR system for organic matter and ammonium nitrogen removal using the central composite design. Environ. Prog. Sustain. Energy 2018, 37, 1638–1646. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, P.; Li, G.; Yin, J.; Li, J.; Zhao, X. Effect of COD/N ratio on nitrogen removal in a membrane-aerated biofilm reactor. Int. Biodeterior. Biodegrad. 2016, 113, 74–79. [Google Scholar] [CrossRef]
- Ding, S.; Bao, P.; Wang, B.; Zhang, Q.; Peng, Y. Long-term stable simultaneous partial nitrification, anammox and denitrification (SNAD) process treating real domestic sewage using suspended activated sludge. Chem. Eng. J. 2018, 339, 180–188. [Google Scholar] [CrossRef]
- Asadi, A.; Zinatizadeh, A.A.L.; Isa, M.H. Performance of intermittently aerated up-flow sludge bed reactor and sequencing batch reactor treating industrial estate wastewater: A comparative study. Bioresour. Technol. 2012, 123, 495–506. [Google Scholar] [CrossRef]
- Palm, J.; Jenkins, D. Relationship Between Organic Loading, Dissolved Oxygen Concentration and Sludge Settleability in the Completely Mixed Activated Sludge Process. J. WPCF 1980, 10, 2484. [Google Scholar]
- Sathian, S.; Rajasimman, M.; Radha, G.; Shanmugapriya, V.; Karthikeyan, C. Performance of SBR for the treatment of textile dye wastewater: Optimizationand kinetic studies. Alex. Eng. J. 2014, 53, 417–426. [Google Scholar] [CrossRef]
- Zinatizadeh, A.A.L.; Mansouri, Y.; Akhbari, A.; Pashaei, S. Biological treatmentof a synthetic dairy wastewater in a sequencing batch biofilm reactor: Statisticalmodeling using optimization using response surface methodology. Chem. Ind. Chem. Eng. Quart. 2011, 17, 485–495. [Google Scholar] [CrossRef]
- Ni, B.J.; Xie, W.M.; Liu, S.G.; Yu, H.Q.; Wang, Y.Z.; Wang, G.; Dai, X.L. Granulation of activated sludge in a pilot-scale sequencing batch reactor for the treatment of low-strength municipal wastewater. Water Res. 2009, 43, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Derlon, N.; Wagner, J.; da Costa, R.H.R.; Morgenroth, E. Formation of aerobic granules for the treatment of real and low-strength municipal wastewater using a sequencing batch reactor operated at constant volume. Water Res. 2016, 105, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Younesi, H.; Najafpour, G.; Zinatizadeh-Lorestani, A.A. Application of response surface methodology for simultaneous carbon and nitrogen (SND) removal from dairy wastewater in batch systems. Int. J. Environ. Stud. 2012, 69, 962–986. [Google Scholar] [CrossRef]
- Jaafari, J.; Seyedsalehi, M.; Safari, G.H.; Ebrahimi Arjestan, M.; Barzanouni, H.; Ghadimi, S.; Haratipour, P. Simultaneous biological organic matter and nutrient removal in an anaerobic/anoxic/oxic (A2O) moving bed biofilm reactor (MBBR) integrated system. Int. J. Environ. Sci. Technol. 2017, 14, 291–304. [Google Scholar] [CrossRef]
- Zinatizadeh, A.A.L.; Ghaytooli, E. Simultaneous nitrogen and carbon removal from wastewater at different operating conditions in a moving bed biofilm reactor (MBBR): Process modeling and optimization. J. Taiwan Inst. Chem. Eng. 2015, 53, 98–111. [Google Scholar] [CrossRef]
- Ray, S.; Mohanty, A.; Mohanty, S.S.; Mishra, S.; Chaudhury, G.R. Optimization of biological elimination of ammonia and chemical oxygen demand from wastewater using response surface methodology. CLEAN–Soil Air Water 2014, 42, 1744–1750. [Google Scholar] [CrossRef]






| Cycle Time (h) | Fill | Anaerobic (min) | Aerobic (min) | Settle (min) | Decant (min) |
|---|---|---|---|---|---|
| 3 h | Instantaneous | 30 | 135 | 10 | 5 |
| 9 h | Instantaneous | 60 | 455 | 20 | 5 |
| S. No. | Chemical Name | Conc. (g/L) |
|---|---|---|
| 1 | Glucose | 40 |
| 2 | NH4Cl | 7.638 |
| 3 | KH2PO4 | 4.39 |
| 4 | NaHCO3 | 13.76 |
| 5 | Trace Elements (1 mL/L each) | |
| FeCl3.6H2O | 2 | |
| MnSO4.H2O | 1 | |
| Boric Acid | 2 |
| Parameter | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| NH3-N (mg/L) | 50 | 50 | 100 | 100 |
| PO43−P (mg/L) | 20 | 10 | 10 | 20 |
| VER (%) | 50 | 50 | 50 | 50 |
| MLSS (mg/L) | 5420 | 5750 | 5060 | 5140 |
| (a) | |||||
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. |
| Corrected Model | 175.131 a | 7 | 25.019 | 36.284 | <0.001 * |
| Intercept | 491,512.731 | 1 | 491,512.731 | 712,829.238 | <0.001 * |
| CT | 123.611 | 1 | 123.611 | 179.271 | <0.001 * |
| CN | 3.703 | 1 | 3.703 | 5.370 | 0.025 * |
| CP | 11.703 | 1 | 11.703 | 16.972 | <0.001 * |
| CT * CN | 22.126 | 1 | 22.126 | 32.088 | <0.001 * |
| CT * CP | 10.286 | 1 | 10.286 | 14.917 | <0.001 * |
| CN * CP | 0.000 | 1 | 0.000 | 0.000 | 1.000 * |
| CT * CN * CP | 3.703 | 1 | 3.703 | 5.370 | 0.025 * |
| Error | 33.097 | 48 | 0.690 | ||
| Total | 491,720.960 | 56 | |||
| Corrected Total | 208.229 | 55 | |||
| (b) | |||||
| Corrected Model | 10,443.882 a | 7 | 1491.983 | 309.480 | <0.001 * |
| Intercept | 432,266.631 | 1 | 432,266.631 | 89,664.544 | <0.001 * |
| CT | 1407.390 | 1 | 1407.390 | 291.933 | <0.001 * |
| CN | 7133.790 | 1 | 7133.790 | 1479.753 | <0.001 * |
| CP | 197.934 | 1 | 197.934 | 41.057 | <0.001 * |
| CT * CN | 1200.300 | 1 | 1200.300 | 248.977 | <0.001 * |
| CT * CP | 170.417 | 1 | 170.417 | 35.349 | <0.001 * |
| CN * CP | 188.410 | 1 | 188.410 | 39.082 | <0.001 * |
| CT * CN * CP | 145.641 | 1 | 145.641 | 30.210 | <0.001 * |
| Error | 231.405 | 48 | 4.821 | ||
| Total | 442,941.918 | 56 | |||
| Corrected Total | 10,675.287 | 55 | |||
| (c) | |||||
| Corrected Model | 16,974.379 a | 7 | 2424.911 | 1089.765 | <0.001 * |
| Intercept | 274,623.247 | 1 | 274,623.247 | 123,416.787 | <0.001 * |
| CT | 978.643 | 1 | 978.643 | 439.806 | <0.001 * |
| CN | 210.908 | 1 | 210.908 | 94.783 | <0.001 * |
| CP | 15,230.240 | 1 | 15,230.240 | 6844.531 | <0.001 * |
| CT * CN | 2.155 | 1 | 2.155 | 0.969 | 0.330 * |
| CT * CP | 22.360 | 1 | 22.360 | 10.049 | 0.003 * |
| CN * CP | 507.572 | 1 | 507.572 | 228.105 | <0.001 * |
| CT * CN * CP | 22.501 | 1 | 22.501 | 10.112 | 0.003 * |
| Error | 106.808 | 48 | 2.225 | ||
| Total | 291,704.435 | 56 | |||
| Corrected Total | 17,081.187 | 55 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Bhatti, M.S. Simultaneous Removal of Organic Matter and Nutrients from High Strength Organic Wastewater Using Sequencing Batch Reactor (SBR). Processes 2022, 10, 1903. https://doi.org/10.3390/pr10101903
Sharma A, Bhatti MS. Simultaneous Removal of Organic Matter and Nutrients from High Strength Organic Wastewater Using Sequencing Batch Reactor (SBR). Processes. 2022; 10(10):1903. https://doi.org/10.3390/pr10101903
Chicago/Turabian StyleSharma, Ambika, and Manpreet Singh Bhatti. 2022. "Simultaneous Removal of Organic Matter and Nutrients from High Strength Organic Wastewater Using Sequencing Batch Reactor (SBR)" Processes 10, no. 10: 1903. https://doi.org/10.3390/pr10101903
APA StyleSharma, A., & Bhatti, M. S. (2022). Simultaneous Removal of Organic Matter and Nutrients from High Strength Organic Wastewater Using Sequencing Batch Reactor (SBR). Processes, 10(10), 1903. https://doi.org/10.3390/pr10101903







