Effects of Alkaline-Reduced Water on Gastrointestinal Diseases
Abstract
1. Introduction
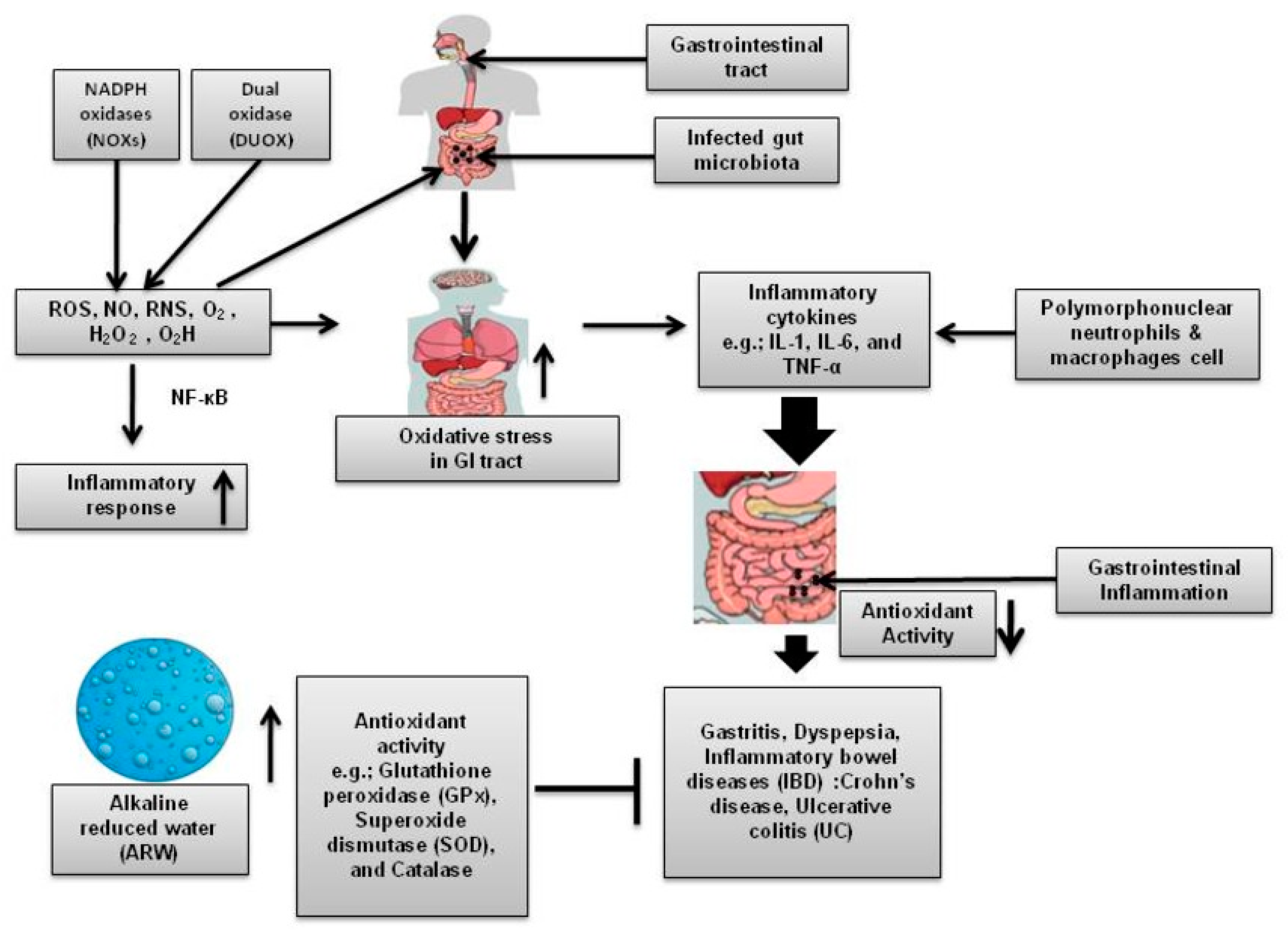
2. Oxidative Stress as a Contributing Factor of GI Diseases
3. ARW and Its Mechanism of Action
4. Development and Characteristics of ARW
5. ARW and Its Role in the Alleviation of Different GI Diseases
5.1. Gastritis
5.2. Dyspepsia
5.3. Inflammatory Bowel Disease
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIW | Alkaline ionized water |
| ARW | Alkaline reduced water |
| CD | Crohn’s disease |
| DUOX | Dual oxidase |
| EW | Electrolyzed water |
| GI | Gastrointestinal |
| GPx | Glutathione peroxidase |
| H2 | Hydrogen-rich molecules |
| H. pylori | Helicobacter pylori |
| HRW | Hydrogen-rich water |
| H2O2 | Hydrogen peroxide |
| HX | Hypoxanthine |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| IL | Interleukin |
| KFDA | Korea food and drug administration |
| MAPK | Mitogen-activated protein kinase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-kB | Nuclear factor-kappa B |
| NO | Nitric oxide |
| NOXs | NADPH oxidases |
| O2− | Superoxide |
| O2H | Hydroperoxyl radical |
| OH | Hydroxyl radical |
| ORP | Oxidation potential reduction |
| OS | Oxidative stress |
| PMNs | Polymorphonuclear neutrophils |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| SCFAs | Short-chain fatty acid |
| SOD | Superoxide dismutase |
| STAT3 | Signal transducer and activator of transcription 3 |
| TDS | Total dissolved solids |
| UC | Ulcerative colitis |
| XO | Xanthine oxidase |
References
- Hellier, M.D.; Williams, J.G. The burden of gastrointestinal disease: Implications for the provision of care in the uk. Gut 2007, 56, 165–166. [Google Scholar] [CrossRef]
- Oshima, T.; Miwa, H. Epidemiology of functional gastrointestinal disorders in japan and in the world. J. Neurogastroenterol. Motil. 2015, 21, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features and rome iv. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef]
- Gao, X.; Liu, J.; Li, L.; Liu, W.; Sun, M. A brief review of nutraceutical ingredients in gastrointestinal disorders: Evidence and suggestions. Int. J. Mol. Sci. 2020, 21, 1822. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, G.; Shah, A.; Morrison, M. Pathophysiology of functional gastrointestinal disorders: A holistic overview. Dig. Dis. 2017, 35 (Suppl. S1), 5–13. [Google Scholar] [CrossRef] [PubMed]
- Philpott, H.L.; Nandurkar, S.; Lubel, J.; Gibson, P.R. Drug-induced gastrointestinal disorders. Postgrad. Med. J. 2014, 90, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, P.; Bhatnagar, P.; Wickramasinghe, K.K.; Allender, S.; Foster, C.; Rayner, M. The economic burden of ill health due to diet, physical inactivity, smoking, alcohol and obesity in the uk: An update to 2006-07 nhs costs. J. Public Health 2011, 33, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Xavier, R.J. Gastrointestinal diseases. In Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Ryan, E.T., Hill, D.R., Solomon, T., Aronson, N.E., Endy, T.P., Eds.; Elsevier: London, UK, 2020; pp. 16–26. [Google Scholar]
- Wendland, B.E.; Aghdassi, E.; Tam, C.; Carrrier, J.; Steinhart, A.H.; Wolman, S.L.; Baron, D.; Allard, J.P. Lipid peroxidation and plasma antioxidant micronutrients in crohn disease. Am. J. Clin. Nutr. 2001, 74, 259–264. [Google Scholar] [CrossRef]
- Rahman, M.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Trinh, T.T.; Akter, R.; Jeong, Y.J.; Goh, S.H.; Kim, C.S.; Lee, K.J. Therapeutic Potential of Natural Products in Treating Neurodegenerative Disorders and Their Future Prospects and Challenges. Molecules 2021, 26, 5327. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, E.H.; Hahm, K.B. Oxidative stress in inflammation-based gastrointestinal tract diseases: Challenges and opportunities. J. Gastroenterol. Hepatol. 2012, 27, 1004–1010. [Google Scholar] [CrossRef]
- McQuaid, K.R. Drugs used in the treatment of gastrointestinal diseases. In Basic and Clinical Pharmacology; Katzung, B.G., Ed.; McGraw Hill: New York, NY, USA, 2007; pp. 1098–1099. [Google Scholar]
- Moayyedi, P.; Mearin, F.; Azpiroz, F.; Andresen, V.; Barbara, G.; Corsetti, M.; Emmanuel, A.; Hungin, A.P.S.; Layer, P.; Stanghellini, V.; et al. Irritable bowel syndrome diagnosis and management: A simplified algorithm for clinical practice. United Eur. Gastroenterol. J. 2017, 5, 773–788. [Google Scholar] [CrossRef]
- Grossi, F. Influence of mineral waters on functional dyspepsia. Clin. Ter. 1989, 129, 261–270. [Google Scholar] [PubMed]
- Ignacio, R.M.C.; Joo, K.B.; Lee, K.J. Clinical effect and mechanism of alkaline reduced water. J. Food Drug Anal. 2012, 20, 394–397. [Google Scholar]
- Shirahata, S.; Hamasaki, T.; Teruya, K. Advanced research on the health benefit of reduced water. Trends Food Sci. Technol. 2012, 23, 124–131. [Google Scholar] [CrossRef]
- Shirahata, S.; Li, Y.; Hamasaki, T.; Gadek, Z.; Teruya, K.; Kabayama, S.; Otsubo, K.; Morisawa, S.; Ishii, Y.; Katakura, Y. Redox regulation by reduced waters as active hydrogen donors and intracellular ros scavengers for prevention of type 2 diabetes. In Cell Technology for Cell Products; Smith, R., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 99–101. [Google Scholar]
- Tashiro, H.; Kitahora, T.; Fujiyama, Y.; Banba, T. Clinical evaluation of alkali-ionized water for chronic diarrhea e placebocontrolled double-blind study. Dig. Absorpt. 2000, 23, 52–56. [Google Scholar]
- Brown, K.; Molcan, E.; Rajendiran, E.; Nusrat, A.; Baker, J.; Ruscheinsky, S.; Gibson, D. Free radicals and gastrointestinal disorders. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin, Germany, 2014; pp. 1691–1727. [Google Scholar]
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Reactive oxygen species, oxidative damage and cell death. In Immunity and Inflammation in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 45–55. [Google Scholar]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol. 2021, 41, 101882. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, Q.; Luo, M.; Xiong, L. Gut Microbiota-Derived Metabolites in Irritable Bowel Syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 729346. [Google Scholar] [CrossRef]
- Van der Post, S.; Birchenough, G.M.; Held, J.M. NOX1-dependent redox signaling potentiates colonic stem cell proliferation to adapt to the intestinal microbiota by linking EGFR and TLR activation. Cell Rep. 2021, 35, 108949. [Google Scholar] [CrossRef]
- Aviello, G.; Knaus, U.G. Ros in gastrointestinal inflammation: Rescue or sabotage? Br. J. Pharmacol. 2017, 174, 1704–1718. [Google Scholar] [CrossRef]
- Cao, S.T.; Wang, C.C.; Wu, H.; Zhang, Q.H.; Jiao, L.F.; Hu, C.H. Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets. J. Anim. Sci. 2018, 96, 1073–1083. [Google Scholar] [CrossRef]
- Crakes, K.R.; Rocha, C.S.; Grishina, I.; Hirao, L.A.; Napoli, E.; Gaulke, C.A.; Fenton, A.; Datta, S.; Arredondo, J.; Marco, M.L.; et al. Ppar alpha-targeted mitochondrial bioenergetics mediate repair of intestinal barriers at the host-microbe intersection during siv infection. Proc. Natl. Acad. Sci. USA 2019, 116, 24819–24829. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Perez, S.; Talens-Visconti, R.; Rius-Perez, S.; Finamor, I.; Sastre, J. Redox signaling in the gastrointestinal tract. Free Radic. Biol. Med. 2017, 104, 75–103. [Google Scholar] [CrossRef]
- Buffinton, G.D.; Doe, W.F. Altered ascorbic acid status in the mucosa from inflammatory bowel disease patients. Free Radic. Res. 1995, 22, 131–143. [Google Scholar] [CrossRef]
- Geerling, B.J.; von Houwelingen, A.C.; Badart-Smook, A.; Stockbrugger, R.W.; Brummer, R.J.M. The relation between antioxidant status and alterations in fatty acid profile in patients with crohn disease and controls. Scand. J. Gastroenterol. 1999, 34, 1108–1116. [Google Scholar] [CrossRef]
- Filippi, J.; Al-Jaouni, R.; Wiroth, J.B.; Hebuterne, X.; Schneider, S.M. Nutritional deficiencies in patients with crohn’s disease in remission. Inflamm. Bowel Dis. 2006, 12, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kruidenier, L.; Kuiper, I.; van Duijn, W.; Marklund, S.L.; van Hogezand, R.A.; Lamers, C.B.; Verspaget, H.W. Differential mucosal expression of three superoxide dismutase isoforms in inflammatory bowel disease. J. Pathol. 2003, 201, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Yoshikawa, T.; Ando, T.; Kishi, A.; Ueda, S.; Oyamada, H.; Kondo, M. Changes in superoxide dismutase activity in the gastric mucosa of peptic ulcer patients. J. Clin. Gastroenterol. 1992, 14 (Suppl. S1), S131–S134. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, M.; Iwane, A.H.; Shimokawa, T.; Cooke, R.; Yanagida, T. Brownian search-and-catch mechanism for myosin-vi steps. Nat. Chem. Biol. 2009, 5, 403–405. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, J.R.; Ryang, Y.S.; Kim, D.H.; Deung, Y.K.; Park, S.K.; Lee, K.J. A clinical trial of orally administered alkaline reduced water. Biomed. Sci. Lett. 2007, 13, 83–89. [Google Scholar]
- Kashiwagi, T.; Yan, H.; Hamasaki, T.; Kinjo, T.; Nakamichi, N.; Teruya, K.; Kabayama, S.; Shirahata, S. Electrochemically reduced water protects neural cells from oxidative damage. Oxid. Med. Cell. Longev. 2014, 2014, 869121. [Google Scholar] [CrossRef]
- Shirahata, S.; Kabayama, S.; Nakano, M.; Miura, T.; Kusumoto, K.; Gotoh, M.; Hayashi, H.; Otsubo, K.; Morisawa, S.; Katakura, Y. Electrolyzed-reduced water scavenges active oxygen species and protects DNA from oxidative damage. Biochem. Biophys. Res. Commun. 1997, 234, 269–274. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kim, Y.K.; Ryoo, K.K.; Lee, Y.B.; Park, E.J. Electrolyzed-reduced water protects against oxidative damage to DNA, rna, and protein. Appl. Biochem. Biotechnol. 2006, 135, 133–144. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.K. Anti-diabetic effects of electrolyzed reduced water in streptozotocin-induced and genetic diabetic mice. Life Sci. 2006, 79, 2288–2292. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Ryu, S.H.; Kim, H.W.; Yang, E.J.; Lim, S.J.; Ryang, Y.S.; Chung, C.H.; Park, S.K.; Lee, K.J. Anti-diabetic effect of alkaline-reduced water on oletf rats. Biosci. Biotechnol. Biochem. 2006, 70, 31–37. [Google Scholar] [CrossRef]
- Huang, K.C.; Yang, C.C.; Lee, K.T.; Chien, C.T. Reduced hemodialysis-induced oxidative stress in end-stage renal disease patients by electrolyzed reduced water. Kidney Int. 2003, 64, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Chycki, J.; Kurylas, A.; Maszczyk, A.; Golas, A.; Zajac, A. Alkaline water improves exercise-induced metabolic acidosis and enhances anaerobic exercise performance in combat sport athletes. PLoS ONE 2018, 13, e0205708. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Stojanovic, M.D. Hydrogen-rich water affected blood alkalinity in physically active men. Res. Sports Med. 2014, 22, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Koufman, J.A.; Johnston, N. Potential benefits of ph 8.8 alkaline drinking water as an adjunct in the treatment of reflux disease. Ann. Otol. Rhinol. Laryngol. 2012, 121, 431–434. [Google Scholar] [CrossRef]
- Shin, D.W.; Yoon, H.; Kim, H.S.; Choi, Y.J.; Shin, C.M.; Park, Y.S.; Kim, N.; Lee, D.H. Effects of alkaline-reduced drinking water on irritable bowel syndrome with diarrhea: A randomized double-blind, placebo-controlled pilot study. Evid. Based Complement. Alternat. Med. 2018, 2018, 9147914. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.R.; de Souza, C.R.T.; Modesto, A.A.C.; Moreira, F.C.; Teixeira, E.B.; Sarraf, J.S.; Allen, T.S.R.; Araujo, T.M.T.; Khayat, A.S. Effects of alkaline water intake on gastritis and mirna expression (mir-7, mir-155, mir-135b and mir-29c). Am. J. Transl. Res. 2020, 12, 4043–4050. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.E.; Khan, I.; Oh, D.H. Electrolyzed water as a novel sanitizer in the food industry: Current trends and future perspectives. Compr. Rev. Food Sci. Food Saf. 2016, 15, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Chambron, J. Physico-chemical, biological and therapeutic characteristics of electrolyzed reduced alkaline water (eraw). Water 2013, 5, 2094–2115. [Google Scholar] [CrossRef]
- Hanaoka, K.; Sun, D.; Lawrence, R.; Kamitani, Y.; Fernandes, G. The mechanism of the enhanced antioxidant effects against superoxide anion radicals of reduced water produced by electrolysis. Biophys. Chem. 2004, 107, 71–82. [Google Scholar] [CrossRef]
- Vadthya, P.; Thummalapalli, N.; Sundergopal, S. Ultrafiltration membrane assisted cost effective ionizer for production of therapeutic alkaline ionized water. J. Water Process Eng. 2019, 32, 100951. [Google Scholar] [CrossRef]
- Ostojic, S.M. Hydrogen-rich water as a modulator of gut microbiota? J. Funct. Foods 2021, 78, 104360. [Google Scholar] [CrossRef]
- Ohta, S. Recent progress toward hydrogen medicine: Potential of molecular hydrogen for preventive and therapeutic applications. Curr. Pharm. Des. 2011, 17, 2241–2252. [Google Scholar] [CrossRef]
- Ichihara, M.; Sobue, S.; Ito, M.; Ito, M.; Hirayama, M.; Ohno, K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen—Comprehensive review of 321 original articles. Med. Gas Res. 2015, 5, 12. [Google Scholar] [CrossRef]
- Xiao, H.W.; Li, Y.; Luo, D.; Dong, J.L.; Zhou, L.X.; Zhao, S.Y.; Zheng, Q.S.; Wang, H.C.; Cui, M.; Fan, S.J. Hydrogen-water ameliorates radiation-induced gastrointestinal toxicity via myd88’s effects on the gut microbiota. Exp. Mol. Med. 2018, 50, e433. [Google Scholar] [CrossRef]
- Higashimura, Y.; Baba, Y.; Inoue, R.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Hirai, Y.; Ushiroda, C.; Tanaka, Y.; Naito, Y. Effects of molecular hydrogen-dissolved alkaline electrolyzed water on intestinal environment in mice. Med. Gas Res. 2018, 8, 6–11. [Google Scholar] [CrossRef]
- Ikeda, M.; Shimizu, K.; Ogura, H.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Shimazu, T. Hydrogen-rich saline regulates intestinal barrier dysfunction, dysbiosis, and bacterial translocation in a murine model of sepsis. Shock 2018, 50, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Ji, X.; Zhang, Q.; Yao, W. Intestinal microbiota ecological response to oral administrations of hydrogen-rich water and lactulose in female piglets fed a fusarium toxin-contaminated diet. Toxins 2018, 10, 246. [Google Scholar] [CrossRef]
- Bordoni, L.; Gabbianelli, R.; Fedeli, D.; Fiorini, D.; Bergheim, I.; Jin, C.J.; Marinelli, L.; Di Stefano, A.; Nasuti, C. Positive effect of an electrolyzed reduced water on gut permeability, fecal microbiota and liver in an animal model of parkinson’s disease. PLoS ONE 2019, 14, e0223238. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.B.; Zhang, S.S.; Lu, Y.M.; Gong, W.J.; Jiang, X.P.; Wang, J.J.; Qiao, T.L.; Zhang, H.H.; Zhao, M.Q.; Wang, D.P.; et al. Effects of the long-term consumption of hydrogen-rich water on the antioxidant activity and the gut flora in female juvenile soccer players from suzhou, china. Med. Gas Res. 2018, 8, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Kim, C.S.; Shon, W.J.; Lee, Y.K.; Choi, E.Y.; Shin, D.M. Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: A randomized, double-blind, controlled trial. Sci. Rep. 2020, 10, 12130. [Google Scholar]
- Lian, N.Q.; Shen, M.X.; Zhang, K.; Pan, J.C.; Jiang, Y.; Yu, Y.; Yu, Y.H. Drinking hydrogen-rich water alleviates chemotherapy-induced neuropathic pain through the regulation of gut microbiota. J. Pain Res. 2021, 14, 681–691. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kiuchi, M.; Higashimura, Y.; Naito, Y.; Koyama, K. The effects of ingestion of hydrogen-dissolved alkaline electrolyzed water on stool consistency and gut microbiota: A double-blind randomized trial. Med. Gas. Res. 2021, 11, 138–144. [Google Scholar]
- Vorobjeva, N.V. Selective stimulation of the growth of anaerobic microflora in the human intestinal tract by electrolyzed reducing water. Med. Hypotheses 2005, 64, 543–546. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of functional food components on gut health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936. [Google Scholar] [CrossRef]
- Marcial, G.; Rodríguez, C.; Medici, M.; de Valdez, G.F. New approaches in gastritis treatment. In Gastritis and Gastric Cancer-New Insights in Gastroprotection, Diagnosis and Treatments; Tonino, P., Ed.; Intech: Rijeka, Croatia, 2011; pp. 153–176. [Google Scholar]
- Padmavathi, V.; Nagaraju, B.; Shampalatha, P.; Nirmala, M.; Fareeda, B.; Susan, T. Knowledge and factors influencing on gastritis among distant mode learners of various universities at selected study centers around bangalore city with a view of providing a pamphlet. Sch. J. Appl. Med. Sci. 2013, 1, 101–110. [Google Scholar]
- Jannathul, F.; Noorzaid, M.; Norain, A.; Dini, S.; Nurul, H.; Nurulnasuha, N. A descriptive study on lifestyle factors influencing gastritis among university students of unikl rcmp in malaysia. Indian J. Nat. Sci. 2016, 6, 10753–10756. [Google Scholar]
- Khanzode, S.S.; Khanzode, S.D.; Dakhale, G.N. Serum and plasma concentration of oxidant and antioxidants in patients of helicobacter pylori gastritis and its correlation with gastric cancer. Cancer Lett. 2003, 195, 27–31. [Google Scholar] [CrossRef]
- Jia, Y.T.; Wei, W.; Ma, B.; Xu, Y.; Liu, W.J.; Wang, Y.; Lv, K.Y.; Tang, H.T.; Wei, D.; Xia, Z.F. Activation of p38 mapk by reactive oxygen species is essential in a rat model of stress-induced gastric mucosal injury. J. Immunol. 2007, 179, 7808–7819. [Google Scholar] [CrossRef]
- Kishino, M.; Nakamura, S.; Shiratori, K. Clinical and endoscopic features of undifferentiated gastric cancer in patients with severe atrophic gastritis. Intern. Med. 2016, 55, 857–862. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Richter, J.E. Dyspepsia: Organic causes and differential characteristics from functional dyspepsia. Scand. J. Gastroenterol. Suppl. 1991, 182, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J.; Goodsall, T.; Potter, M. Functional dyspepsia. Aust. Prescr. 2017, 40, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Futagami, S.; Wakabayashi, M.; Sakasegawa, N.; Agawa, S.; Higuchi, K.; Kodaka, Y.; Iwakiri, K. Management of functional dyspepsia: State of the art and emerging therapies. Ther. Adv. Chronic Dis. 2018, 9, 23–32. [Google Scholar] [CrossRef]
- Liebregts, T.; Adam, B.; Bredack, C.; Gururatsakul, M.; Pilkington, K.R.; Brierley, S.M.; Blackshaw, L.A.; Gerken, G.; Talley, N.J.; Holtmann, G. Small bowel homing t cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am. J. Gastroenterol. 2011, 106, 1089–1098. [Google Scholar] [CrossRef]
- Addula, M.; Wilson, V.E.D.; Reddymasu, S.; Agrawal, D.K. Immunopathological and molecular basis of functional dyspepsia and current therapeutic approaches. Expert Rev. Clin. Immunol. 2018, 14, 831–840. [Google Scholar] [CrossRef]
- Xue, J.; Shang, G.; Tanaka, Y.; Saihara, Y.; Hou, L.; Velasquez, N.; Liu, W.; Lu, Y. Dose-dependent inhibition of gastric injury by hydrogen in alkaline electrolyzed drinking water. BMC Complement. Altern. Med. 2014, 14, 81. [Google Scholar] [CrossRef]
- Bertoni, M.; Olivieri, F.; Manghetti, M.; Boccolini, E.; Bellomini, M.G.; Blandizzi, C.; Bonino, F.; Del Tacca, M. Effects of a bicarbonate-alkaline mineral water on gastric functions and functional dyspepsia: A preclinical and clinical study. Pharmacol. Res. 2002, 46, 525–531. [Google Scholar] [CrossRef]
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory bowel disease: Clinical aspects and treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef]
- Seril, D.N.; Liao, J.; Yang, G.Y.; Yang, C.S. Oxidative stress and ulcerative colitis-associated carcinogenesis: Studies in humans and animal models. Carcinogenesis 2003, 24, 353–362. [Google Scholar] [CrossRef]
- Barrett, J.C.; Hansoul, S.; Nicolae, D.L.; Cho, J.H.; Duerr, R.H.; Rioux, J.D.; Brant, S.R.; Silverberg, M.S.; Taylor, K.D.; Barmada, M.M.; et al. Genome-wide association defines more than 30 distinct susceptibility loci for crohn’s disease. Nat. Genet. 2008, 40, 955–962. [Google Scholar] [CrossRef]
- Schindler, C.; Levy, D.E.; Decker, T. Jak-stat signaling: From interferons to cytokines. J. Biol. Chem. 2007, 282, 20059–20063. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A. New il-12-family members: Il-23 and il-27, cytokines with divergent functions. Nat. Rev. Immunol. 2005, 5, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Atreya, I.; Atreya, R.; Neurath, M.F. Nf-kappab in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K. Role of stat3 in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 5110–5114. [Google Scholar] [CrossRef] [PubMed]
- D’Inca, R.; Cardin, R.; Benazzato, L.; Angriman, I.; Martines, D.; Sturniolo, G.C. Oxidative DNA damage in the mucosa of ulcerative colitis increases with disease duration and dysplasia. Inflamm. Bowel Dis. 2004, 10, 23–27. [Google Scholar] [CrossRef]
- Hussain, S.P.; Amstad, P.; Raja, K.; Ambs, S.; Nagashima, M.; Bennett, W.P.; Shields, P.G.; Ham, A.J.; Swenberg, J.A.; Marrogi, A.J.; et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: A cancer-prone chronic inflammatory disease. Cancer Res. 2000, 60, 3333–3337. [Google Scholar]
- Fujita, K.; Seike, T.; Yutsudo, N.; Ohno, M.; Yamada, H.; Yamaguchi, H.; Sakumi, K.; Yamakawa, Y.; Kido, M.A.; Takaki, A.; et al. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of parkinson’s disease. PLoS ONE 2009, 4, e7247. [Google Scholar] [CrossRef] [PubMed]
- Sobue, S.; Yamai, K.; Ito, M.; Ohno, K.; Ito, M.; Iwamoto, T.; Qiao, S.; Ohkuwa, T.; Ichihara, M. Simultaneous oral and inhalational intake of molecular hydrogen additively suppresses signaling pathways in rodents. Mol. Cell. Biochem. 2015, 403, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Molecular hydrogen: An inert gas turns clinically effective. Ann. Med. 2015, 47, 301–304. [Google Scholar] [CrossRef] [PubMed]

| Author and Year | Disease/Condition | Route of Administration | Dose and Duration | Outcomes | Reference |
|---|---|---|---|---|---|
| Tashiro et al., 2000 | Abdominal complaints | Oral | pH 9.5, 0.5 L/d, 30 d | Improvement in abdominal complaints | [18] |
| Shirahata et al., 2007 | Type 2 diabetes | Oral | 2 L/d, 6 d | Decreased ROS level and improvement in blood cholesterol, low-density lipoprotein, and serum creatinine | [17] |
| Yang et al., 2007 | Senile disease | Oral | pH 9.5, 1.5 L/d, 60 d | Improvement in blood parameters | [37] |
| Ostojic et al., 2014 | Exercise-induced acidosis | Oral | pH 9.3, 2 L/d, 14 d | Significantly increased fasting arterial blood pH | [45] |
| Chycki et al., 2018 | Exercise-induced metabolic acidosis | Oral | pH 9.13, 2.6–3.2 L/d, 21 d | Enhances hydration, improves acid-base balance and anaerobic exercise performance | [44] |
| Shin et al., 2018 | IBS with Diarrhea | Oral | pH 8.5–10, 2 L/d, 57 d | Improvement in abdominal pain, IBS quality-of-life score significantly increased | [47] |
| Chaves et al., 2020 | Gastritis | Oral | pH 8.5–10, 5 months | Clinical benefit, observed higher expression of miR-135b and miR-29c | [48] |
| Oxidation Reaction at Anode | Reduction Reaction at Cathode |
|---|---|
| 4H2O + 4e− → 4OH− + 4H+ + 4e− | 2H2O + 2e− → 2OH− + 2H+ + 2e− |
| 4OH− → O2↑ + 2H2O + 4e− | 2H+ + 2e− → H2↑ 2H+ + 2e− → 2H (Active hydrogen) |
| 2H2O → O2↑+ 4H+ + 4e− (Overall reaction) | 2H2O + 2e− → H2↑ + 2OH− (Overall reaction) |
| Water | pH | ORP | TDS |
|---|---|---|---|
| Tap water | 7.31 | 551 | 122 |
| ARW | 9.52 | −99 | 127 |
| Author & Year | Model | Disease/Condition | Route of Administration | Dose and Duration | Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Xiao et al., 2018 | Mice | Radiation-induced intestinal toxicity | Oral | H2 0.8 mM, 5 d | Improvement in radiation-mediated gastrointestinal toxicity, tract functions, and the epithelial layer of intestinal integrity | [56] |
| Higashimura et al., 2018 | Mice | Intestinal flora | Oral | H2 0.32 mM, 4 wks | Significantly increased a marker of intestinal fermentation (weight of cecal contents), produced significant more SCFAs contents, and showed a distinct microbiota composition favorable to gut | [57] |
| Ikeda et al., 2018 | Mice | Sepsis model | Oral | HRW 15 mL/kg, 7 d | HRW prevent intestinal dysbiosis, hyperpermeability, and bacterial translocation | [58] |
| Zheng et al., 2018 | Piglet | Fusarium mycotoxins diet | Oral | H2 0.6 mM, 25 d | Decreased diarrhea rate, increased acetate, butyrate, total SCFAs, increased relative abundance of specific taxa | [59] |
| Bordoni et al., 2019 | Rat | Parkinson’s disease | Oral | ARW, H2 0.4–0.9 mM, 15 d | Increased barrier integrity, increased butyric acid, and butyrate-producing bacteria | [60] |
| Sha et al., 2019 | Human | Female football athletes | Oral | HRW1.5–2 L/d, 60 d | Increased blood hemoglobin, malondialdehyde, SOD, total antioxidant capacity, species diversity of gut and fecal microbiota | [61] |
| Shim et al., 2020 | Human | Healthy adults | Oral | HRW (H2 0.753 ± 0.012 mg/L) 1.5 L/d, 4 wks | Reduced cell death and inflammatory responses by modulating transcriptional networks of TLR-NFκB signaling | [62] |
| Lian et al., 2021 | Mice | Chemotherapy-induced neuropathic pain | Oral | H2 0.8–1 ppm, 20 d | Reduction in the microbial diversity and modifies the structure of gut microbiota and reverse excessive production of inflammatory cytokines and OS | [63] |
| Tanaka et al., 2021 | Human | Healthy volunteers gut microbiota and stool condition | Oral | ARW (pH 9.5, H2 0.3 mg/L) 500 mL/d, 14 d | Increase in specific gut bacterial species Bifidobacterium | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajgai, J.; Kim, C.-S.; Rahman, M.H.; Jeong, E.-S.; Jang, H.-Y.; Kim, K.-E.; Choi, J.; Cho, I.-Y.; Lee, K.-J.; Lee, M. Effects of Alkaline-Reduced Water on Gastrointestinal Diseases. Processes 2022, 10, 87. https://doi.org/10.3390/pr10010087
Bajgai J, Kim C-S, Rahman MH, Jeong E-S, Jang H-Y, Kim K-E, Choi J, Cho I-Y, Lee K-J, Lee M. Effects of Alkaline-Reduced Water on Gastrointestinal Diseases. Processes. 2022; 10(1):87. https://doi.org/10.3390/pr10010087
Chicago/Turabian StyleBajgai, Johny, Cheol-Su Kim, Md. Habibur Rahman, Eun-Sook Jeong, Hong-Young Jang, Ka-Eun Kim, JaeHo Choi, Il-Young Cho, Kyu-Jae Lee, and Mihyun Lee. 2022. "Effects of Alkaline-Reduced Water on Gastrointestinal Diseases" Processes 10, no. 1: 87. https://doi.org/10.3390/pr10010087
APA StyleBajgai, J., Kim, C.-S., Rahman, M. H., Jeong, E.-S., Jang, H.-Y., Kim, K.-E., Choi, J., Cho, I.-Y., Lee, K.-J., & Lee, M. (2022). Effects of Alkaline-Reduced Water on Gastrointestinal Diseases. Processes, 10(1), 87. https://doi.org/10.3390/pr10010087







