Abstract
Recently, high entropy oxides (HEO) with special stabilization effects have been widely investigated as new anode materials for lithium-ion batteries. However, the lithium storage mechanism of HEO is still under debate. In this work, we applied a modified solution combustion synthesis method with a subsequent ball milling refinement process to prepare a six-component (FeNiCrMnMgAl)3O4 spinel high entropy oxide (6-SHEO). The novel 6-SHEO anode features outstanding electrochemical performance, enabling a stable capacity of 657 mAh g−1 at a current rate of 0.2 A g−1 after 200 cycles, and good high-rate capability with 350 mAh g−1 even at 4 A g−1. In addition, the lithium storage behavior of this 6-SHEO anode was explored in detail through in-situ XRD and ex-situ TEM approaches. Surprisingly, a reversible spinel to rock salt phase transition behavior and spinel phase residue phenomenon was firstly observed by this route. Furthermore, for better understanding of the phase change behavior in this 6-SHEO anode, a high-energy ball milling approach was applied to induce a similar spinel to rock salt phase transformation for the first time, which generates fresh insight into the mechanism of the phase change behavior in this 6-SHEO anode.
1. Introduction
As a key component of lithium-ion batteries, studies focused on anode materials have received much attention. At present, with low working potential and good cyclic stability, graphite has been successfully commercialized. However, low theoretical capacity (372 mAh g−1) cannot meet the ever-increasing demands for high specific energy devices. Thus, developing next-generation anode such as alloying anode (e.g., Si, P, Sb) and conversion anode (e.g., transition metal oxides) is essential.
Benefiting from the special high entropy effects, research on HEO anodes for lithium-ion batteries is in full swing [1,2]. Compared with single transition metal oxides, HEO possesses better dielectric properties and ionic conductivity [3,4,5,6]. Moreover, attributed to the active components in HEO with different lithium reaction potentials, the lithiation process of HEO is carried out in a stepwise way which can effectively alleviate electrode volume change and pulverization issues [7]. In addition, the high configuration entropy induced phase stabilization effect can promote the microscopic mechanical properties of electrodes, resulting in good structure stability while reacting with lithium [8]. All these advantages make it a promising next-generation anode material for lithium-ion batteries.
Since the concept of HEO anode was first proposed by Sarkar et al. in 2018, various HEO anode materials such as rock salt-type (RSHEO) [9], perovskite-type (PHEO) [10] and spinel-type (SHEO) [11] have been developed. Nevertheless, due to lack of accurate characterization, the lithium storage mechanism of the HEO anode has not been studied carefully [12].
X-ray diffraction (XRD) analysis for electrodes under different states of charge (SOC) is an effective method to investigate the phase transition and lithium storage process. Ex-situ XRD testing is convenient and fast. In recent years, this approach has been mostly used for the phase transition characterization of various new SHEO anodes. For example, Wang et al. performed an ex-situ XRD on (FeCoNiCrMn)3O4 SHEO and observed that the characteristic peaks of spinel gradually disappeared during the first discharge process with lithiation [11]. Different from the above work, Nguyen et al. observed a rock salt phase formation in (FeCoNiCrMn)3O4 SHEO during the first discharge process [13]. However, in their ex-situ XRD work, the observation of the crystalline Li2O phase is contrary to the shared understanding because the Li2O generated during conversion reaction in transition metal oxide (TMO) should be amorphous and prone to react with CO2 in the air to form Li2CO3, which is not observable by XRD without any protection [14]. Since the initial information cannot be fully reflected, these unprotective ex-situ XRD testing results still need to be verified.
Phase change information of electrodes under the operating state is more valuable. Operando XRD (in-situ XRD) techniques enable real-time monitoring during the charging/discharging process, and thus can reveal the internal structure evolution and phase changes of electrode materials [15]. However, due to weakened X-ray diffraction signal received from in-situ detecting device and uniformed amorphous structure in cycled HEO, the in-situ XRD studies on HEO are still far from satisfactory. To the best of our knowledge, only RSHEO (MgCoNiCuZn)O [9] and SHEO (NiCoMnFeTi)3O4 [16] have been carried out so far. In those works, characteristic peaks of rock salt or spinel gradually diminish to disappear during the lithiation process, and no other useful information could be supported. Therefore, finely designed in-situ XRD characterization for new SHEO anodes is of great value.
Herein, by modified solution combustion synthesis method with subsequent ball milling refinement process, a kind of six-component spinel high entropy oxide (FeNiCrMnMgAl)3O4 with higher configurational entropy was prepared and applied as anode for lithium-ion batteries. It should be noted that the multi-element approach for a single-phase is beneficial for tuning the physical and electrochemical properties of HEO. In the case of our 6-SHEO, transition metal elements (Fe, Ni, Cr, and Mn) were selected here as active components for lithium storage, while Mg and Al elements were selected as inactive components for buffering volume change and increasing whole entropy of our HEO. Compared with common high entropy oxide anodes as shown in Table 1, our (FeNiCrMnMgAl)3O4 anode exhibited outstanding electrochemical performance. To finely probe the lithium storage process of the 6-SHEO, an in-situ XRD characterization was carried out. Surprisingly, the phase transformation behavior of the 6-SHEO anode can be clearly characterized under this in-situ device, and for the first time, the reversible spinel to rock salt transition and parent spinel residue behaviors were observed in this 6-SHEO anode, which is highly instructive for investigating the lithium storage behaviors of SHEO anode.

Table 1.
Electrochemical performances of recently reported high entropy oxide anodes.
2. Materials and Methods
2.1. Preparation of the 6-SHEO Powders
(FeNiCrMnMgAl)3O4 powders in this work were prepared by a modified solution combustion synthesis (SCS) method [17] with subsequent refining treatment as shown in Figure 1. First, 0.01 mol metal nitrates Fe(NO3)2·5H2O (99%, Aladdin Industrial Inc., Shanghai, China), Mg(NO3)2 (99%, Sinopharm Chemical Reagent co., Ltd., Shanghai, China), Ni(NO3)2·6H2O (98%, Aladdin), Al(NO3)3·9H2O (99%, Aladdin), 50 wt% Mn(NO3)2 solution (AR, Aladdin), Cr(NO3)3·9H2O (99%, Aladdin), and 0.013 mol glycine (99%, Macklin Biochem. co., Ltd., Shanghai, China) were dissolved in 50 mL water and then dried into a brown gel. Afterward, the gel was placed in a muffle furnace at 780 °C for 30 min to prepare (FeNiCrMnMgAl)3O4 precursors. To refine the powders and narrow particle size distribution, the as-prepared 6-SHEO precursors were refined by planetary ball-milling with isopropyl alcohol for 10 h at a low-speed milling rate of 180 rpm. Specifically, the ball-material ratio is 30-1, and the size of stainless-steel balls is 8 mm. After that, the milled slurry was dried in a vacuum drying oven at 70 °C for 6 h.
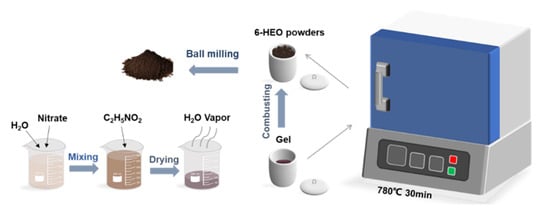
Figure 1.
Illustration for the preparation processes of the 6-SHEO powders.
2.2. Structure and Morphology Characterizations
The phase of samples was characterized by an X-ray diffractometer (XRD, Empyrean Alpha 1, PANalytical B.V., Almelo, The Netherlands) with Cu Kα radiation operating at 45 kV and 40 mA. The microstructure was investigated by field-emission scanning electron microscope (SEM, Carl Zeiss Supra 40 field, Zeiss Ltd., Oberkochen, Germany), and the element distributions of the 6-SHEO were observed by energy-dispersive X-ray spectroscopy (EDS, JEOL Ltd., Tokyo, Japan) attached with the SEM. The high-resolution transmission electron microscope (HRTEM, EOL JEM-2100, JEOL Ltd., Tokyo, Japan) observation was carried out at 200 kV. The analysis of particle size distribution was performed by a laser particle size analyzer (Bettesize 2600, Bettersize Instruments Ltd., Dandong, China).
For in-situ XRD testing, we designed an in-situ cell with beryllium (Be) disk as the current collector, glass fiber membrane as the separator, and lithium sheet as the counter electrode. Cycling of the in-situ cell was achieved by cell testing system (LAND-CT2001A, Wuhan LAND Electronic Co., Ltd., Wuhan, China) and the X-ray diffractometer (XRD, Empyrean Alpha 1, PANalytical B.V., Almelo, The Netherlands) was applied for collecting XRD information from cycling the in-situ cell. Specifically, under the working state of the in-situ cell, the 2θ scan was performed every 15 min with a step size of 0.013° and a duration of 40 s per step.
2.3. Electrochemistry Property Measurements
Coin cells (CR2016) were applied for electrochemical measurement here. To prepare the working electrode, active material, acetylene black (Super C-45, Kejing Materials Technology Co., Hefeng, China), and sodium carboxymethyl cellulose (CMC, Kejing) were mixed uniformly with a certain amount of water into electrode slurry at a weight ratio of 7:2:1. Afterward, the slurry was coated on the copper foil and dried into working electrode sheets (active material loading ~1.05 mg/cm2). Laboratory-made cells were assembled in an Ar-filled glove box (MIKROUNA super 750, Shanghai, China) with H2O and O2 contents less than 0.01 ppm. Lithium metal flakes were used as the counter electrode. LiPF6 solution contained with 1 mol L−1 ethylene carbonate (EC) and 1 mol L−1 diethyl carbonate (DEC) was used as the electrolyte (Tianci Materials Technology Co., Guangzhou, China). All the cells were tested galvanostatically under various current densities between 0.01 and 3 V (vs. Li/Li+) at room temperature through a cell testing system (LAND-CT2001A, Wuhan LAND Electronic Co., Ltd., Wuhan, China).
3. Results and Discussion
3.1. Structures and Morphologies of the 6-SHEO Powders
Figure 2a shows the XRD pattern of the refined (FeNiCrMnMgAl)3O4 powders, illustrating a perfect Fd-3m spinel characteristic (PDF: 00-028-0491). The HRTEM image and the corresponding Fast Fourier Transform (FFT) pattern of the sample are shown in Figure 2b. The HRTEM image exhibits well-defined lattice boundaries with d = 0.249, 0.287, and 0.465 nm, respectively, which should correspond to the (311), (220), and (111) planes of the spinel structure. Diffraction rings in the FFT pattern should correspond to the (440), (004), (022), and (111) planes of the FCC lattice. These results highly correspond to the XRD pattern.
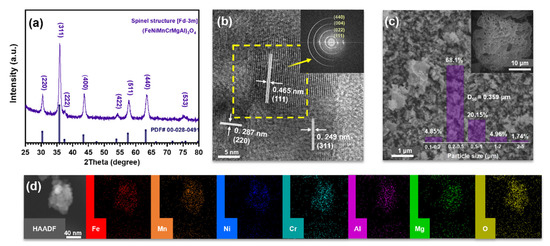
Figure 2.
Structure characterization of the 6-SHEO. (a) XRD pattern, (b) HRTEM image and corresponded FFT pattern of the selected area, (c) SEM images and particle size distribution, (d) STEM-EDS.
As displayed in SEM images in Figure 2c, the precursors of CSC made (FeNiCrMnMgAl)3O4 present a porous morphology, and the grounded product was fine and uniform, with an average size of about 0.36 μm. Furthermore, STEM-EDS elemental mapping within a specific region was investigated and recorded in Figure 2d, revealing the homogeneous element distribution of Fe, Ni, Cr, Mn, Mg, Al, and O in this high entropy oxide.
Figure 3a shows the survey XPS spectrum of the 6-SHEO, indicating the coexistence of element Fe, Ni, Cr, Al, Mn, Mg, Al, and O in our sample. To further demonstrate the chemical valance states of elements in (FeNiCrMnMgAl)3O4, the X-ray photoelectron spectroscopy (XPS) spectra of Fe 2p, Mg 1s, Cr 2p, Al 2p, Mn 2p, Ni 2p, and O 1s of the 6-SHEO are shown in Figure 3b–h respectively. The Fe 2p spectrum shows two spin-orbit peaks of Fe 2p1/2 and Fe 2p3/2 [18]. Binding energy of 724.2 eV and 713.1 eV are associated with Fe3+, and binding energy of 709.8 eV and 722.8 eV are related to Fe2+ [19]. For the XPS spectrum of Mg 1s, the peak centered at 1303.2 eV is indexed to Mg2+ [7]. Two main peaks located at 586.2 eV (Cr 2p1/2) and 576.2 eV (Cr 2p2/3) correspond to the Cr3+, and the peak centered at 591.1eV (Cr 2p2/3) is related with Cr6+ [20]. As for Al 2p spectra, the existence of Al3+ species is proved by a strong peak centered at 74 eV [21]. For the spectra of Mn 2p, two main peaks at 652.73 eV (Mn 2p3/2) and 641.18 eV (Mn 2p3/2) are indexed to Mn3+, the peak at 644.72 eV (Mn 2p3/2) is related to Mn3+ [11]. For the spectra of Ni 2p, the peaks centered at 874.1 eV (Ni 2p1/2) and 856.1 eV (Ni 2p2/3) should correspond to the Ni3+, the peaks centered at 872.3 eV (Ni 2p1/2) and 854.5 eV (Ni 2p2/3) are related with Ni4+ [22]. Figure 3h presents the O 1s spectra, and it was deconvoluted into two peaks at 529.5 eV and 531.1 eV, which correspond to lattice oxygen OL and oxygen vacancies OV [23,24]. For further quantitative analysis, based on the ratio of peak area, the proportions of OV to Ototal are approximately 46.5%.
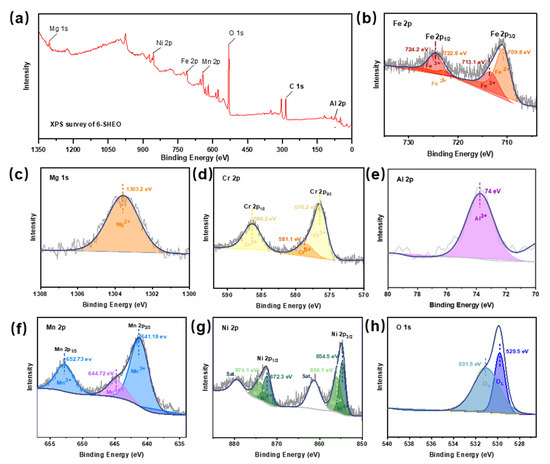
Figure 3.
High-resolution XPS spectra of the 6-SHEO. (a) Survey spectrum, (b) Fe 2p, (c) Mg 1s, (d) Cr 2p, (e) Al 2p, (f) Mn 2p, (g) Ni 2p, and (h) O 1s.
In general, the XPS result confirms that the mixed-valance state of Fe (+2 and +3), Ni (+3 and +4), Cr (+3 and +6), Mn (+3 and +4), Mg (+2), and Al (+3) in this (FeNiCrMnMgAl)3O4 anode. It should be noted that the presence of Mn4+ and Cr6+ may originate from the oxidation caused by the solution combustion process at high temperatures under the air atmosphere. Since such high-valence state components are not observed by XRD and TEM, we consider that they should only exist on the surface of the 6-SHEO. In addition, it has been proven that a certain amount of oxygen vacancies (OV) exists in this 6-SHEO. Considering that high concentration of OV can dramatically enhance the electron transport kinetics [25], it is reasonable that the (FeNiCrMnMgAl)3O4 anode enables such outstanding electrochemical performance as we mention below.
3.2. Electrochemical Performance of the 6-SHEO Anode
This S-HEO anode was assembled into half-cells for electrochemical analysis.
To estimate the capacity, galvanostatic cycling studies were carried out under a current density of 0.2 A g−1 within 0.01–3 V (vs. Li+/Li). As shown in Figure 4a, the discharge/charge specific capacities of the first cycle are 865.6 mAh g−1 and 550.4 mAh g−1, respectively, and the initial coulombic efficiency (ICE) is about 63.6%. It is generally believed that the low ICE of the conversion reaction anodes is mainly related to the formation of the solid electrolyte interface (SEI) film and the insufficient reversible phase transition [26]. Notably, the capacity of 6-SHEO increased in the range of 2–3 V after 60 cycles. It is generally believed that the increase in capacity of conversion anode in the higher voltage range is related to the decomposition of the electrolyte in cycling cells. Specifically, remained nano transition metal from the conversion reaction will catalyze the decomposition of the electrolyte, resulting in a rise to capacity.
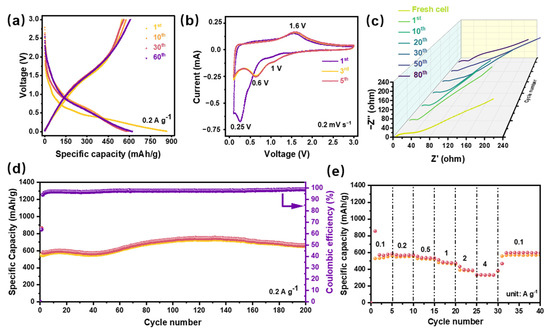
Figure 4.
Electrochemistry characterization of the 6-SHEO. (a) Voltage vs. capacity profile testing at 0.2 A g−1, (b) cyclic voltammetry at a scanning rate of 0.2 mV s−1, (c) comparison of electrochemical impedance spectroscopy (EIS) of different cycles at fully charged state, (d) capacity retention at 0.2 A g−1, (e) rate-performance measured at various current densities.
To explore the redox and structural transformation of the electrode, galvanostatic cyclic voltammetry (CV) was carried out at a scan rate of 0.2 mV s−1. As shown in Figure 4b, during the cathodic process, a broad cathode peak appeared at about 0.25 V in the first cycle, which is attributed to the stepwise reduction of transition metal oxide components (TM3+/TM0, TM4+/TM0) [27] and formation of the SEI film [28]. In subsequent cycles, as shown on the 3rd and 5th cycles, the two high-intensity cathode peaks were highly overlapped and shifted to about 0.6 V, which means the activation process and the reduction of electrode polarization have been completed [28]. In addition, we can also observe that the weak cathodic peak around 1.1 V is still retained. Coincidentally, the RSHEO (MgCoNiCuZn)O anode in Sarkar’s work has a strong cathodic peak at around 1.1 V [29], showing that the electrochemical behaviors of SHEO and RSHEO have a certain relationship. Correspondingly, the anodic scanning curves of these cycles are highly consistent, and the peak observed at about 1.6 V should represent the oxidation process of the transition metal components [30].
The cyclic performance is shown in Figure 4d, exhibiting excellent cycle stability at a current density of 0.2 A g−1. After 200 cycles, the reversible discharge specific capacity can be maintained at 670 mAh g−1, with a capacity retention rate of 99.8%. The excellent cycle performance might be attributed to the special structural stabilization effect of the high-entropy oxide. Notably, capacity increases during 50 to 120 cycles in our 6-SHEO anode; it is generally believed that the increase in capacity during long cycling is typical of conversion type materials which can be attributed to the activation processes. In addition, some researchers suggested that the capacity fluctuations of HEO anodes might be related to structural changes of the HEO [9].
Attractive rate capability under different current densities was shown in Figure 4e. Even at a high current density of 4 A g−1, it still maintains a reversible discharge capacity of 350 mAh g−1. The rate performance is closely related to the electronic conductivity of the electrode material and the ion transmission paths [31]. It is generally believed that the abundant oxygen vacancies in the HEO promote the charge transport kinetics, which leads to this excellent rate performance [32].
To explore the reaction kinetics of this new 6-SHEO, electrochemical impedance characterization was carried out. As shown in the Nyquist diagram in Figure 4c, all spectra contain two parts, the semicircle in the high and medium frequency regions represent the SEI film resistance (RSEI) and the charge transfer impedance (RCT) together, and the sloping line in the low-frequency region of the second part depicts the Li+ transport in the bulk electrode. It can be noticed that similar to other SHEO anodes, the diameter of the first semicircle gradually decreases as the cycle proceeds [10,11]. It is generally believed that this may be related to the enhancement of the reaction kinetics and the stabilization of the SEI film during the electrode activation process. However, in the later stage of cycling, significant increase in the impedance of the fully charged cell after 80 cycles was observed. We suggest that this may be related to the damage of the 6-SHEO structure or phase change in long-cycle 6-SHEO.
As we have seen, such 6-SHEO exhibit outstanding electrochemical properties. We believe that the following factors contribute to the excellent performance of this 6-SHEO. Firstly, the higher configurational entropy from six components promotes a higher entropy effect (sluggish diffusion effect) [8], which suppresses the severe distortion of the 6-SHEO lattice during the lithiation process, and thus ensures good cyclic stability. Secondly, the ingenious selection of Mg and Al elements as inactive components can improve the buffer effect, which also facilitates cyclic stability. Lastly, the preparation route of this work (solution combustion and subsequent wet ball-milling grinding process) effectively controls and narrows the particle size distribution of this 6-SHEO material, leading to preferable charge transfer kinetics.
3.3. Lithium Storage Behaviors of the 6-SHEO Anode
For investigating the lithium storage mechanism of this 6-SHEO anode, ex-situ XRD was first carried out and the results, shown in Figure S1, reveal the evidence of spinel phase maintenance in the 6-SHEO anode during the first discharging and charging process. For better investigating the phase transformation behavior of this anode, in-situ XRD was performed during the first cycle and the second cycle of the discharge process.
As shown in Figure 5a, the spinel parent phase is maintained to some extent during the cycling process. In addition, shifts of (222), (400), and (440) peaks are visible during the first discharge process. It should be noted that this kind of offset is a typical in-situ XRD data feature of phase transition phenomena [33], and the position of the distortion endpoint should correspond to the rock salt phase (PDF: 00-002-1207). A detailed in-situ XRD (stacking diagram version) is shown in Figure S2.
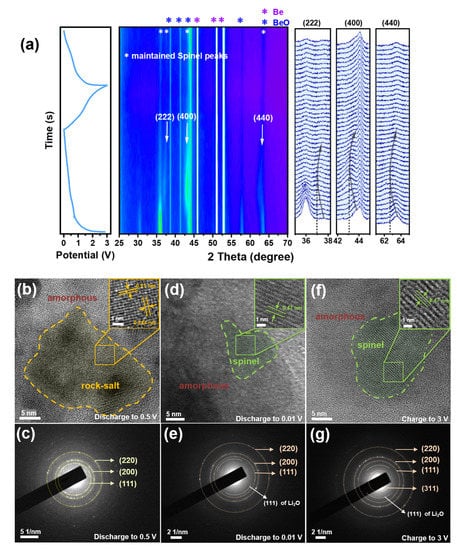
Figure 5.
(a) In-situ XRD of the 6-SHEO anode recorded along the initial lithiation-delithiation and second delithiation process, ex-situ TEM and corresponded SEAD of the 6-SHEO while (b,c) first discharged to 0.5 V, (d,e) first discharged to 0.01 V, and (f,g) first charged to 3 V.
Ex-situ TEM was carried out to better investigate the phase change of this 6-SHEO anode in the initial lithiation-delithiation process. As shown in Figure 5b, when discharged to 0.5 V, the 6-SHEO exhibits a typical rock salt phase with d = 0.21 and d = 0.24 nm related to the (200) and (111) planes, respectively [13]. Correspondingly, as shown in Figure 5c, the three diffraction rings for radius =6.65, 4.71, and 4.00 1/nm refer to the (220), (200), and (111) planes of salt-rock phase, respectively [9]. As shown in Figure 5d, the spinel phase can be observed while the 6-SHEO anode is further discharged to 0.01 V, and the plane with d = 0.47 nm corresponds to the (111) plane of the spinel phase [7]. Note that the reflection ring with radius = 3.69 1/nm should correspond to the (111) plane with d = 2.71 Å for Li2O [16]. Similarly, the HRTEM image (Figure 5f) of the 6-SHEO when charged to 3 V shows spinel features, too. In contrast, as shown in Figure 5e,g, the SEAD image of the further charged sample shows extra diffraction rings for the (311) plane, and diffraction rings are much clearer.
Therefore, we firmly believe that this 6-SHEO anode undergoes a reversible phase transition of spinel to rock salt then back to spinel phase during the first lithiation process, and the structure of the 6-SHEO will be repaired to better crystalline spinel after being further charged. In addition to that, detecting Li2O also indicates that the 6-SHEO is a kind of conversion anode, which can indirectly support the lithium storage mechanism we proposed below.Thus, what is the mechanism of this phase transition? Here we propose an explanation.
First of all, the transformed rock salt phase is not rock salt MgO. In the early years, researchers already introduced MgO, CaO, etc. into transition metal oxide systems to form bi/trimetallic oxides [34,35], and believe that these inactive components will in situ form a buffer matrix and inhibit the growth of active oxide crystal grains, namely as “spectator effect’’. In this work, since MgO is inactive, it will not gradually disappear during the further discharge (0.5–1 V) process.
Secondly, it should be noticed that a similar phase transition behavior happened when the 6-SHEO is refined by high-energy shaker ball-milling. As shown in Figure 6a, when the ball-milling time increases, peaks get broader as particle size decreases and the spinel HEO gradually transforms into the rock salt phase, which is similar to the phased change in 6-SHEO due to lithiation as shown in Figure S3. Moreover, the HRTEM and corresponding SEAD images shown in Figure 6b–e also confirm the transformation phenomenon of spinel to rock salt when the 6-SHEO are ball milled for 4 h. It is generally believed that during high-energy ball milling, the powder is subjected to huge impacts and stresses, resulting in states of crystalline irregularity, non-crystallization, and the formation of unstable phases with increased enthalpy [36]. When the ball-milling energy reaches the kinetic and thermodynamic conditions required for the phase transition, phase transitions will happen [37].
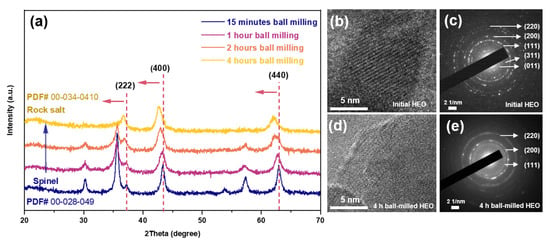
Figure 6.
(a) XRD patterns of 6-SHEO with different ball milling treatments. HRTEM and corresponded SEAD of (b,c) initial 6-SHEO, and (d,e) 4 h ball-milling treated 6-SHEO.
Here, we believe that this spinel to rock salt phase transition is a kind of diffusion-free continuous phase transition, and the ball-milling induced phase transition should originate from the stresses generated during the ball milling process. As for the phase transition in the 6-SHEO anode, it might result from the stresses generated by the reaction with lithium.
As illustrated in Figure 7, a lithium storage mechanism of the 6-SHEO was proposed.
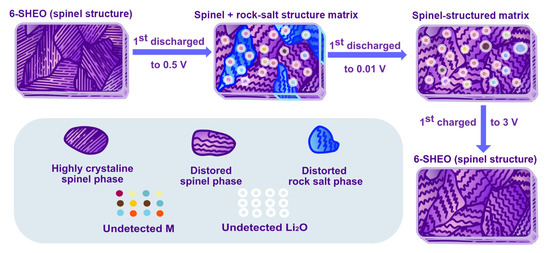
Figure 7.
Illustration for lithium storage mechanism of the 6-SHEO anode.
According to the result of our in-situ XRD measurement, during the discharging process, part of the 6-SHEO will undergo a reversible spinel to rock salt phase change process, and the rest will remain as the parent spinel phase. During the whole charging process, 6-SHEO will still remain as a spinel structure. It is obvious that the lithium storage process is based on these structure matrixes, and it is accepted that the high-entropy stabilization of the lattice contributed to such phenomena [9].
Considering many similar works dedicated to exploring the mechanism of HEO [9,16], it is generally believed that nano-Li2O and nano active M nuclei will form in HEO via a conversion reaction. Therefore, we believed that this 6-SHEO undergoes a similar conversion reaction, and after being fully charged, the participating ions can easily diffuse back to the 6-SHEO structure, leading to repaired parent spinel structure.
4. Conclusions
In summary, by modified solution combustion synthesis method with subsequent ball milling refinement process, a new 6-SHEO with enhanced electrochemical properties was prepared. Compared with conventional SHEO, this 6-SHEO demonstrates greatly improved capacity, with a reversible capacity of 657 mAh g−1 at a current rate of 0.2 A g−1 after 200 cycles. Furthermore, the lithium storage behavior of this 6-SHEO has been fully explored. As confirmed by in-situ XRD and ex-situ TEM, unlike conventional conversion anodes, the reversible spinel to rock salt phase transformation phenomenon and spinel residue behavior of the 6-SHEO anode were first demonstrated. Furthermore, a high-energy ball milling approach was first applied in this work for induing phase change in the 6-SHEO. Surprisingly, similar spinel to rock salt phase transformation behavior was observed in the ball-milled 6-SHEO, which shed light on exploring the mechanism of the phase change behavior in SHEO anodes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr10010049/s1, Figure S1: Evidence of spinel phase maintenance-Ex-situ XRD of (FeNiCrMnMgAl)3O4, Figure S2: In-situ XRD of 6-SHEO anode recorded along the initial lithiation-delithiation and second delithiation process (stacking diagram version), Figure S3: XRD information of solution combustion made (FeNiCrMnMgAl)3O4 precursor, planetary ball-milled refined 6-SHEO, 6-SHEO first lithiated to 0.5 V and shaker ball-milled 6-SHEO.
Author Contributions
Most of the experimental work, original manuscript preparation, and revision, Y.Z.; manuscript review and editing, X.W. and X.L.; supervision, data analysis, and funding acquisition, R.H. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding was received for this study.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (51831009, 52071144, 51621001, and 51822104).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tian, L.Y.; Zhang, Z.; Liu, S.; Li, G.R.; Gao, X.P. High-Entropy Spinel Oxide Nanofibers as Catalytic Sulfur Hosts Promise the High Gravimetric and Volumetric Capacities for Lithium–Sulfur Batteries. Energy Environ. Mater. 2021, 1–10. [Google Scholar] [CrossRef]
- Liu, H.; Syama, L.; Zhang, L.; Lee, C.; Liu, C.; Dai, Z.; Yan, Q. High-entropy alloys and compounds for electrocatalytic energy conversion applications. SusMat 2021, 1–24. [Google Scholar] [CrossRef]
- Fan, M.; Chang, X.; Meng, Q.; Wan, L.; Guo, Y. Progress in the sustainable recycling of spent lithium-ion batteries. SusMat 2021, 1, 241–254. [Google Scholar] [CrossRef]
- Pu, F.; Bai, Y.; Lv, J.; Zhao, X.; Wu, G.; Kong, C.; Lei, B.; Zhang, X.; Jin, H.; Yang, Z. Yolk–Shell Cu2O@CuO-decorated RGO for High-Performance Lithium-Ion Battery Anode. Energy Environ. Mater. 2020, 1–8. [Google Scholar] [CrossRef]
- Bérardan, D.; Franger, S.; Dragoe, D.; Meena, A.K.; Dragoe, N. Colossal dielectric constant in high entropy oxides. Phys. Status Solidi-Rapid Res. Lett. 2016, 10, 328–333. [Google Scholar] [CrossRef] [Green Version]
- Bérardan, D.; Franger, S.; Meena, A.K.; Dragoe, N. Room temperature lithium superionic conductivity in high entropy oxides. J. Mater. Chem. A 2016, 4, 9536–9541. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Qiu, N.; Wu, B.; Yang, Z.; Sun, S.; Wang, Y. A new spinel high-entropy oxide (Mg0.2Ti0.2Zn0.2Cu0.2Fe0.2)3O4 with fast reaction kinetics and excellent stability as an anode material for lithium ion batteries. RSC Adv. 2020, 10, 9736–9744. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Wang, Q.; Schiele, A.; Chellali, M.R.; Bhattacharya, S.S.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B. High-Entropy Oxides: Fundamental Aspects and Electrochemical Properties. Adv. Mater. 2019, 31, 1806236. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; de Biasi, L.; Kübel, C.; Brezesinski, T.; Bhattacharya, S.S.; Hahn, H.; et al. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 3400. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Wang, D.; Zhang, X.; Li, J.; Du, Q.; Liu, X.; Zhang, J.; Qi, X. A high-entropy perovskite titanate lithium-ion battery anode. J. Mater. Sci. 2020, 55, 6942–6951. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, S.; Duan, C.; Mao, J.; Dong, Y.; Dong, K.; Wang, Z.; Luo, S.; Liu, Y.; Qi, X. Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance. J. Alloys Compd. 2020, 844, 156158. [Google Scholar] [CrossRef]
- Ghigna, P.; Airoldi, L.; Fracchia, M.; Callegari, D.; Anselmi-Tamburini, U.; D’Angelo, P.; Pianta, N.; Ruffo, R.; Cibin, G.; de Souza, D.O.; et al. Lithiation Mechanism in High-Entropy Oxides as Anode Materials for Li-Ion Batteries: An Operando XAS Study. ACS Appl. Mater. Interfaces 2020, 12, 50344–50354. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.X.; Patra, J.; Chang, J.K.; Ting, J.M. High entropy spinel oxide nanoparticles for superior lithiation-delithiation performance. J. Mater. Chem. A 2020, 8, 18963–18973. [Google Scholar] [CrossRef]
- Hu, R.; Chen, D.; Waller, G.; Ouyang, Y.; Chen, Y.; Zhao, B.; Rainwater, B.; Yang, C.; Zhu, M.; Liu, M. Dramatically enhanced reversibility of Li2O in SnO2-based electrodes: The effect of nanostructure on high initial reversible capacity. Energy Environ. Sci. 2016, 9, 595–603. [Google Scholar] [CrossRef]
- Xia, M.; Liu, T.; Peng, N.; Zheng, R.; Cheng, X.; Zhu, H.; Yu, H.; Shui, M.; Shu, J. Lab-Scale In Situ X-ray Diffraction Technique for Different Battery Systems: Designs, Applications, and Perspectives. Small Methods 2019, 3, 1–23. [Google Scholar] [CrossRef]
- Chen, T.Y.; Wang, S.Y.; Kuo, C.H.; Huang, S.C.; Lin, M.H.; Li, C.H.; Chen, H.Y.T.; Wang, C.C.; Liao, Y.F.; Lin, C.C.; et al. In operando synchrotron X-ray studies of a novel spinel (Ni0.2Co0.2Mn0.2Fe0.2Ti0.2)3O4high-entropy oxide for energy storage applications. J. Mater. Chem. A 2020, 8, 21756–21770. [Google Scholar] [CrossRef]
- Mao, A.; Xiang, H.Z.; Zhang, Z.G.; Kuramoto, K.; Yu, H.; Ran, S. Solution combustion synthesis and magnetic property of rock-salt (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O high-entropy oxide nanocrystalline powder. J. Magn. Magn. Mater. 2019, 484, 245–252. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Gu, S.; Zhu, A. Graphene nanosheets loaded Fe3O4 nanoparticles as a promising anode material for lithium ion batteries. J. Alloy. Compd. 2020, 813, 152160. [Google Scholar] [CrossRef]
- Bao, B.; Liu, J.; Xu, H.; Liu, B.; Zhang, K.; Jin, Z. Insight into a high temperature selective oxidation of HP40 alloy under a H2-H2O environment. RSC Adv. 2017, 7, 8589–8597. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.Y.; Hsu, C.C.; Ho, H.P.; Wu, S.H. Sol-gel synthesis of aluminum doped lithium titanate anode material for lithium ion batteries. Electrochim. Acta 2013, 87, 126–132. [Google Scholar] [CrossRef]
- Ma, L.; Lyu, S.S.; Dai, Y.; Pei, X.Y.; Mo, D.C.; Fu, Y.X. Lithium storage properties of NiO/reduced graphene oxide composites derived from different oxidation degrees of graphite oxide. J. Alloys Compd. 2019, 810, 151954. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Zhao, B.; Chen, H.; Liu, X.; Zhao, R.; Wang, X.; Liu, J.; Chen, Y.; Liu, M. Improving the Activity for Oxygen Evolution Reaction by Tailoring Oxygen Defects in Double Perovskite Oxides. Adv. Funct. Mater. 2019, 29, 1–12. [Google Scholar] [CrossRef]
- Sarkar, A.; Khan, G.G. The formation and detection techniques of oxygen vacancies in titanium oxide-based nanostructures. Nanoscale 2019, 11, 3414–3444. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Chen, Z.; Gong, Y.; Zhang, R.; Liu, H.; Tang, P.; Chen, X.; Passerini, S.; Liu, J. The Role of Cation Vacancies in Electrode Materials for Enhanced Electrochemical Energy Storage: Synthesis, Advanced Characterization, and Fundamentals. Adv. Energy Mater. 2020, 10, 1903780. [Google Scholar] [CrossRef]
- Ji, G.; Ma, Y.; Lee, J.Y. Mitigating the initial capacity loss (ICL) problem in high-capacity lithium ion battery anode materials. J. Mater. Chem. 2011, 21, 9819–9824. [Google Scholar] [CrossRef]
- Cherian, C.T.; Zheng, M.; Reddy, M.V.; Chowdari, B.V.R.; Sow, C.H. Zn2SnO4 nanowires versus nanoplates: Electrochemical performance and morphological evolution during Li-cycling. ACS Appl. Mater. Interfaces 2013, 5, 6054–6060. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Qiu, N.; Chen, H.; Yang, Z.; Sun, S.; Wang, Y.; Cui, Y. A high entropy oxide (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2O) with superior lithium storage performance. J. Alloys Compd. 2019, 777, 767–774. [Google Scholar] [CrossRef]
- Cao, K.; Jin, T.; Yang, L.; Jiao, L. Recent progress in conversion reaction metal oxide anodes for Li-ion batteries. Mater. Chem. Front. 2017, 1, 2213–2242. [Google Scholar] [CrossRef]
- Jow, T.R.; Delp, S.A.; Allen, J.L.; Jones, J.P.; Smart, M.C. Factors Limiting Li+ Charge Transfer Kinetics in Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A361–A367. [Google Scholar] [CrossRef]
- Lökçü, E.; Toparli, Ç.; Anik, M. Electrochemical Performance of (MgCoNiZn)1-xLixO High-Entropy Oxides in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 23860–23866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Brady, A.B.; Pelliccione, C.J.; Bock, D.C.; Bruck, A.M.; Li, J.; Sarbada, V.; Hull, R.; Stach, E.A.; Takeuchi, K.J.; et al. Investigation of Structural Evolution of Li1.1V3O8 by In Situ X-ray Diffraction and Density Functional Theory Calculations. Chem. Mater. 2017, 29, 2364–2373. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, W.; Huo, N.; Yang, S. Synthesis of Vesicle-Like MgFe2O4/Graphene 3D Network Anode Material with Enhanced Lithium Storage Performance. ACS Sustain. Chem. Eng. 2017, 5, 563–570. [Google Scholar] [CrossRef]
- Huo, N.; Yin, Y.; Liu, W.; Zhang, J.; Ding, Y.; Wang, Q.; Shi, Z.; Yang, S. Facile synthesis of MgFe2O4/C composites as anode materials for lithium-ion batteries with excellent cycling and rate performance. New J. Chem. 2016, 40, 7068–7074. [Google Scholar] [CrossRef]
- Zhang, D.L. Processing of advanced materials using high-energy mechanical milling. Prog. Mater. Sci. 2004, 49, 537–560. [Google Scholar] [CrossRef]
- Luo, K.; Ni, S.; Song, M. Fabricating a bulk FCC Hf by a combination of high-energy ball milling and spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2018, 75, 107–110. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).