Approximate Models of Microbiological Processes in a Biofilm Formed on Fine Spherical Particles
Abstract
1. Introduction
2. Exact Mathematical Model
3. Approximate Mathematical Model Based on Laplace-Carson Transform
- Monod model:
- Haldane model:
- for
- for
- for
- Monod model:
- Haldane model:
4. Approximate Model Based on Pseudo-Steady State Approximation
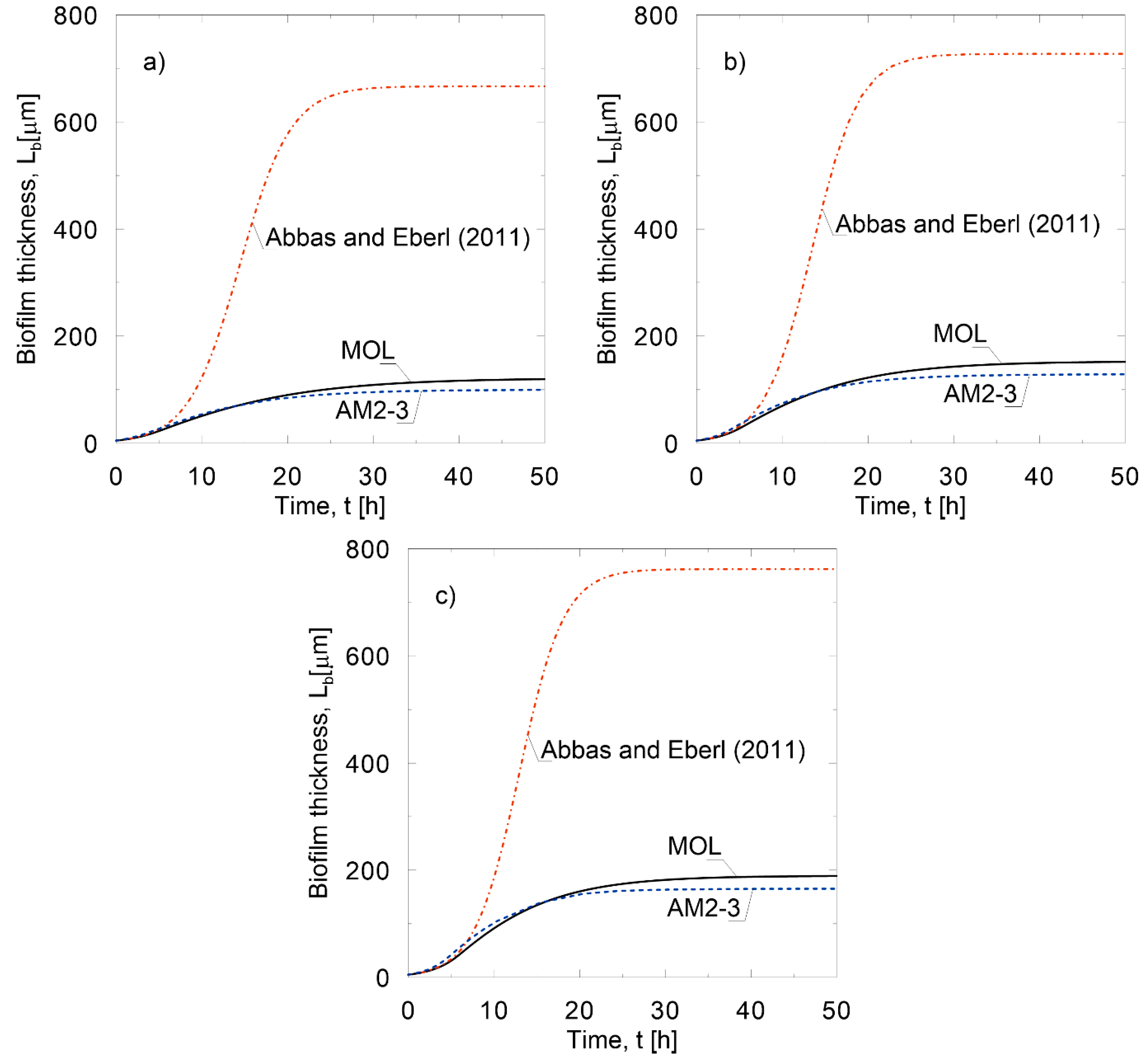
5. Accuracy and Efficiency Evaluation of the Approximate Model Based on the Laplace-Carson Transform
- The accuracy of the approximate models decreased with the increase in ;
- The nonlinear approximate model was more accurate than the linear one;
- The differences between the approximate models and the accurate model decreased with time.
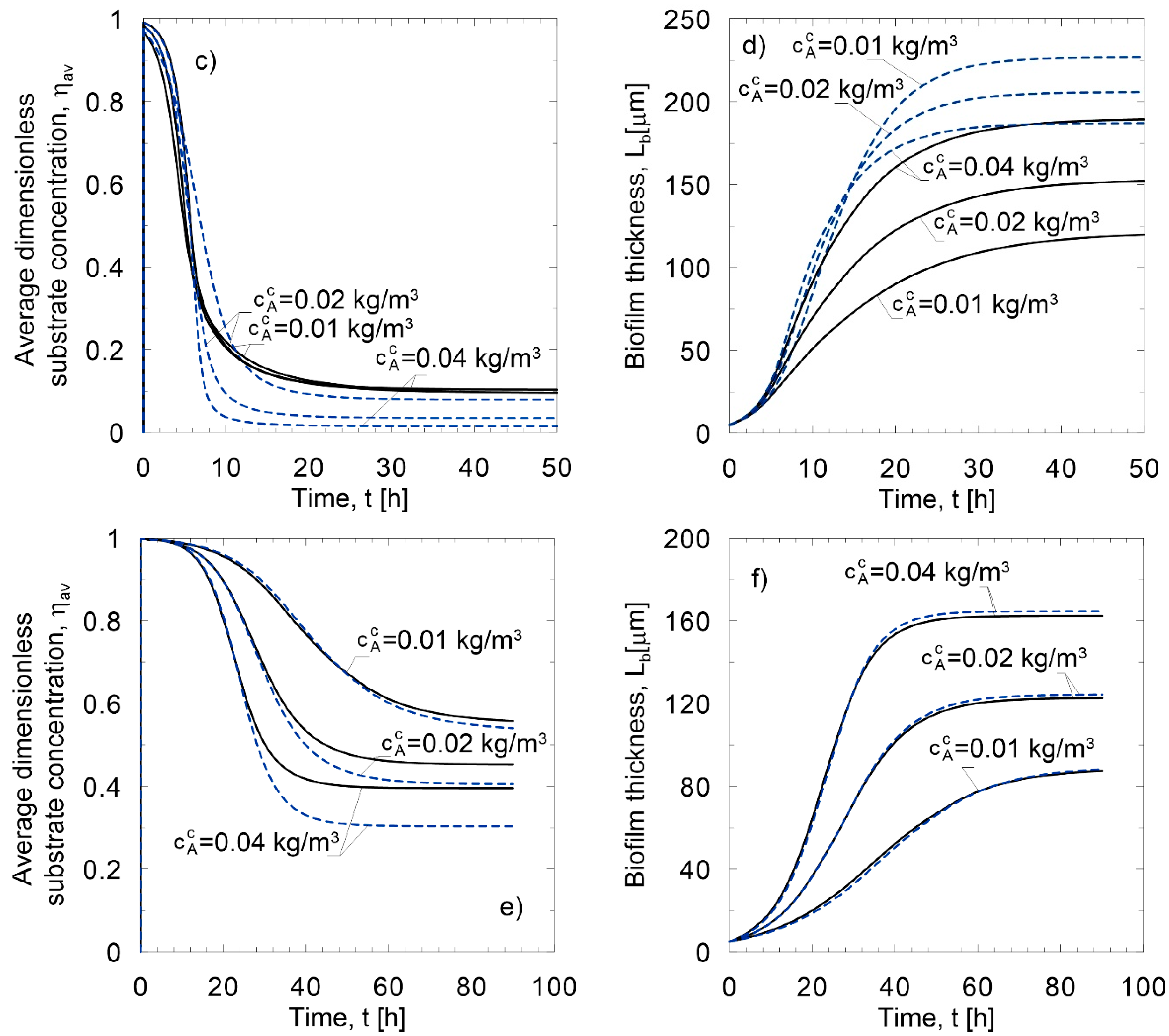
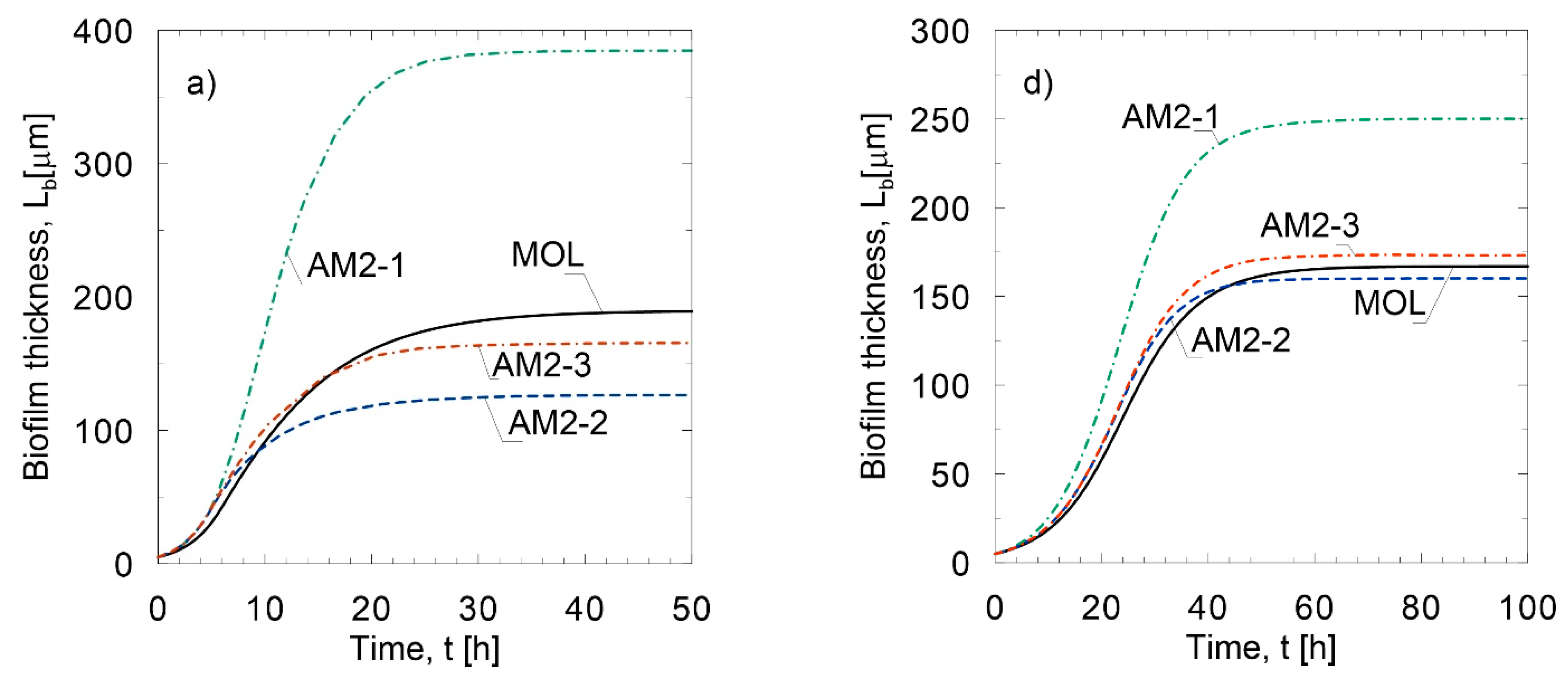
6. Application of Approximate Models to Conditions of the Dynamical Growth of Biofilm
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C | Laplace–Carson transform of limiting substrate concentration in the biofilm |
| mass concentration of limiting substrate in the biofilm (kg∙m−3) | |
| mass concentration of limiting substrate in the liquid phase (kg∙m−3) | |
| DeA | effective diffusion coefficient in biofilm (m2∙h−1) |
| k | maximum specific growth rate (h−1) |
| KA | saturation constant in kinetic equation (kg∙m−3) |
| kdet | detachment rate coefficient (m−1∙h−1) |
| dimensionless saturation constant in kinetic equation | |
| Kin | inhibition constant (kg∙m−3) |
| dimensionless inhibition constant in kinetic equation | |
| Lb | thickness of the biofilm (m) |
| derivative of thickness of the biofilm with respect to time | |
| p | complex variable |
| substrate uptake rate (kg A/m3∙h) | |
| rA,av | average substrate uptake rate (kg A/m3∙h) |
| rB | biomass growth rate (kg B/m3∙h) |
| rB,av | average biomass growth rate (kg B/m3∙h) |
| rb | bioparticle radius (rb = r0 + Lb) |
| r0 | inert particle radius |
| SR | stiffness ratio |
| t | time (h) |
| wBA | growth yield coefficient (kg B∙kg A−1) |
| x | space coordinate in the biofilm, m |
| z | dimensionless coordinate in the biofilm |
| δ | relative error |
| η | dimensionless substrate concentration |
| λ | degree of nonlinearity of the model |
| μ | parameter defined by Equation (33) |
| ρb | biomass concentration in the biofilm (kg∙m−3) |
| τ | dimensionless time |
| ΦA | Thiele modulus |
| modified Thiele modulus | |
| Superscripts | |
| b | biofilm phase |
| c | liquid (continuous) phase |
| * | steady-state value |
| s | biofilm surface |
| Subscripts | |
| app | value obtained using the approximate model |
| av | average value |
| ex | exact value |
| s | biofilm surface |
| 0 | initial value |
Appendix A. Derivation of Equation (12)
Appendix B. Solution of the Exact Model Using the Method of Lines
References
- Abbasbandy, S. The application of homotopy analysis method to nonlinear equations arising in heat transfer. Phys. Lett. A 2006, 360, 109–113. [Google Scholar] [CrossRef]
- Stryjewski, W.S.; Tabiś, B.; Boroń, D. Dynamic behaviour of stirred tank bioreactors based on structured and unstructured kinetic models. A comparative study. Chem. Eng. Res. Des. 2015, 104, 541–550. [Google Scholar] [CrossRef]
- Skoneczny, S.; Tabiś, B. Dynamic properties of a continuous stirred tank biofilm bioreactor for aerobic processes. AIChE J. 2017, 63, 1818–1829. [Google Scholar] [CrossRef]
- Skoneczny, S.; Cioch, M. Modeling of continuous-flow bioreactors with a biofilm with the use of orthogonal collocation on finite elements. Chem. Eng. Commun. 2018, 205, 929–946. [Google Scholar] [CrossRef]
- Mera, N.S.; Elliott, L.; Ingham, D.B.; Lesnic, D. The boundary element solution of the Cauchy steady heat conduction problem in an anisotropic medium. Int. J. Numer. Methods Eng. 2000, 49, 481–499. [Google Scholar] [CrossRef]
- Liu, F.-B. A hybrid method for the inverse heat transfer of estimating fluid thermal conductivity and heat capacity. Int. J. Therm. Sci. 2011, 50, 718–724. [Google Scholar] [CrossRef]
- Kim, D.H. Approximations for unsteady-state diffusion and reaction in porous catalyst and their application to packed-bed reactor. AIChE J. 2008, 54, 2423–2431. [Google Scholar] [CrossRef]
- Wanner, O.; Eberl, H.J.; Morgenroth, E.; Noguera, D.R.; Picioreanu, C.; Rittmann, B.E.; van Loosdrecht, M.C.M. Mathematical Modeling of Biofilms; WA Scientific and Technical Report No. 18; IWA Task Group on Biofilm Modeling: London, UK, 2006. [Google Scholar]
- Nicolella, C.; van Loosdrecht, M.C.M.; Heijnen, S.J. Particle-based biofilm reactor technology. Trends Biotechnol. 2000, 18, 312–320. [Google Scholar] [CrossRef]
- Szukiewicz, M.K. An approximate model for diffusion and reaction in a porous pellet. Chem. Eng. Sci. 2002, 57, 1451–1457. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Liu, S.-B.; Keith, S. Approximate solution for the nonlinear model of diffusion and reaction in porous catalysts by the decomposition method. Chem. Eng. J. 2004, 102, 1–10. [Google Scholar] [CrossRef]
- Abbasbandy, S. Approximate solution for the nonlinear model of diffusion and reaction in porous catalysts by means of the homotopy analysis method. Chem. Eng. J. 2008, 136, 144–150. [Google Scholar] [CrossRef]
- Skoneczny, S.; Cioch-Skoneczny, M. Mathematical modelling and approximate solutions for microbiological processes in biofilm through homotopy-based methods. Chem. Eng. Res. Des. 2018, 139, 309–320. [Google Scholar] [CrossRef]
- Russo, M.E.; Maffettone, P.L.; Marzocchella, A.; Salatino, P. Bifurcational and dynamical analysis of a continuous biofilm reactor. J. Biotechnol. 2008, 135, 295–303. [Google Scholar] [CrossRef]
- Dokianakis, S.N.; Kornaros, M.; Lyberatos, G. Effect of wall growth on the kinetic modeling of nitrite oxidation in a CSTR. Biotechnol. Bioeng. 2006, 93, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Ajbar, A. Classification of stability behavior of bioreactors with wall attachment and substrate-inhibited kinetics. Biotechnol. Bioeng. 2000, 72, 166–176. [Google Scholar] [CrossRef]
- Kornaros, M.; Dokianakis, S.N.; Lyberatos, G. Sensitivity analysis of a biofilm model describing mixed growth of nitrite oxidisers in a CSTR. Water Sci. Technol. 2006, 53, 313–320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mašić, A.; Eberl, H. Persistence in a Single Species CSTR Model with Suspended Flocs and Wall Attached Biofilms. Bull. Math. Biol. 2012, 74, 1001–1026. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Kojouharov, H.V.; Le, D.; Smith, H. The Freter model: A simple model of biofilm formation. J. Math. Biol. 2003, 47, 137–152. [Google Scholar] [CrossRef]
- De Beer, D.; Stoodley, P.; Roe, F.; Lewandowski, Z. Effects of biofilm structures on oxygen distribution and mass transport. Biotechnol. Bioeng. 1994, 43, 1131–1138. [Google Scholar] [CrossRef]
- Hibiya, K.; Nagai, J.; Tsuneda, S.; Hirata, A. Simple prediction of oxygen penetration depth in biofilms for wastewater treatment. Biochem. Eng. J. 2004, 19, 61–68. [Google Scholar] [CrossRef]
- Skoneczny, S. Nonlinear Steady-State Characteristics of Continuous Flow Bioreactors with Immobilized Biofilm. Ph.D. Thesis, Politechnika Krakowska, Kraków, Poland, 2013. [Google Scholar]
- Rittmann, B.E.; McCarty, P.L. Model of steady-state-biofilm kinetics. Biotechnol. Bioeng. 1980, 22, 2343–2357. [Google Scholar] [CrossRef]
- Zeng, A.-P.; Deckwer, W.-D. A kinetic model for substrate and energy consumption of microbial growth under substrate-sufficient conditions. Biotechnol. Prog. 1995, 11, 71–79. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Kumar, S. Biodegradation kinetics of phenol and catechol using Pseudomonas putida MTCC 1194. Biochem. Eng. J. 2005, 22, 151–159. [Google Scholar] [CrossRef]
- Skoneczny, S.; Stryjewski, W.; Bizon, K.; Tabiś, B. Three-phase fluidized bed bioreactor modelling and simulation. Biochem. Eng. J. 2017, 121, 118–130. [Google Scholar] [CrossRef]
- Kumar, P.; Qureshi, S. Laplace-Carson integral transform for exact solutions of non-integer order initial value problems with Caputo operator. J. Appl. Math. Comput. Mech. 2020, 19, 57–66. [Google Scholar] [CrossRef]
- Makarov, A.M. Application of the laplace-carson integral transform method to the theory of nonstationary flows of a viscoplastic medium. J. Eng. Phys. 1970, 19, 870–874. [Google Scholar] [CrossRef]
- Aggarwal, S.; Sharma, S.D.; Vyas, A. Laplace-Carson Transform for the Primitive of Convolution Type Volterra Integro-Differential Equation of First Kind. Int. J. Res. Innov. Appl. Sci. 2020, V, 2454–6194. [Google Scholar]
- Szukiewicz, M.K. New approximate model for diffusion and reaction in a porous catalyst. AIChE J. 2000, 46, 661–665. [Google Scholar] [CrossRef]
- Szukiewicz, M.; Petrus, R. Approximate Model for Diffusion and Reaction in a Porous Pellet and an Effectiveness Factor. Chem. Eng. Sci. 2004, 59, 479–483. [Google Scholar] [CrossRef]
- Picioreanu, C.; van Loosdrecht, M.C.M.; Heijnen, J.J. A new combined differential-discrete cellular automaton approach for biofilm modeling: Application for growth in gel beads. Biotechnol. Bioeng. 1998, 57, 718–731. [Google Scholar] [CrossRef]
- Pawlowsky, U.; Howell, J.A. Mixed culture biooxidation of phenol. I. Determination of kinetic parameters. Biotechnol. Bioeng. 1973, 15, 889–896. [Google Scholar] [CrossRef]
- Dormand, J.R.; Prince, P.J. A family of embedded Runge-Kutta formulae. J. Comput. Appl. Math. 1980, 6, 19–26. [Google Scholar] [CrossRef]
- Gear, C.W. Algorithm 407: DIFSUB for solution of ordinary differential equations [D2]. Commun. ACM 1971, 14, 185–190. [Google Scholar] [CrossRef]
- Lardon, L.A.; Merkey, B.V.; Martins, S.; Dötsch, A.; Picioreanu, C.; Kreft, J.-U.; Smets, B.F. iDynoMiCS: Next-generation individual-based modelling of biofilms. Environ. Microbiol. 2011, 13, 2416–2434. [Google Scholar] [CrossRef] [PubMed]
- Tabiś, B.; Grzywacz, R. Numerical and technological properties of bubble column bioreactors for aerobic processes. Comput. Chem. Eng. 2011, 35, 212–219. [Google Scholar] [CrossRef]
- Bakke, R.; Trulear, M.G.; Robinson, J.A.; Characklis, W.G. Activity of Pseudomonas aeruginosa in biofilms: Steady state. Biotechnol. Bioeng. 1984, 26, 1418–1424. [Google Scholar] [CrossRef]
- Abbas, F.; Eberl, H.J. Analytical substrate flux approximation for the Monod boundary value problem. Appl. Math. Comput. 2011, 218, 1484–1494. [Google Scholar] [CrossRef]
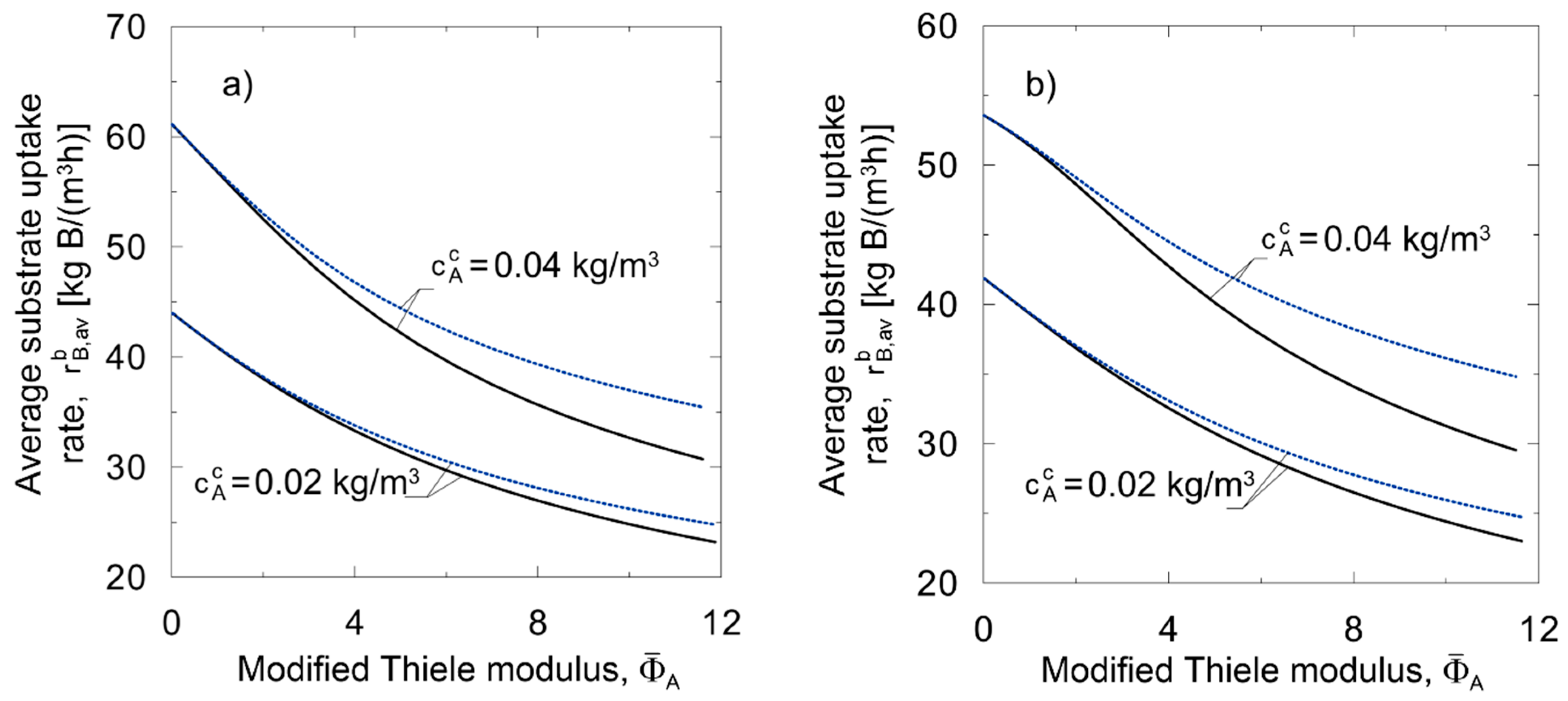

 Method of lines;
Method of lines;  Linear approximate model;
Linear approximate model;  Nonlinear approximate model; (a) oxidation of nitrite; (b) glucose utilization.
Nonlinear approximate model; (a) oxidation of nitrite; (b) glucose utilization.
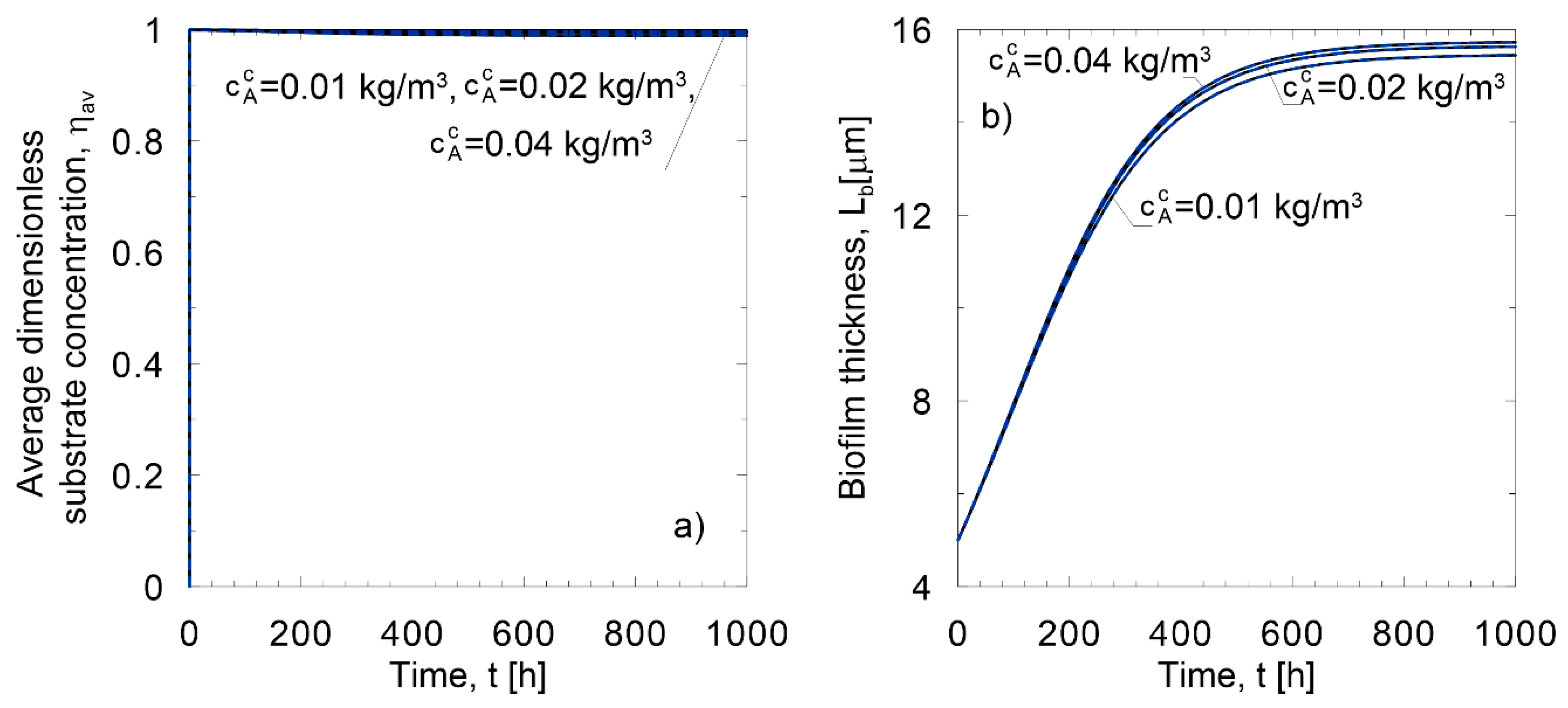
 Method of lines;
Method of lines;  Linear approximate model;
Linear approximate model;  Nonlinear approximate model; (a) oxidation of nitrite; (b) glucose utilization.
Nonlinear approximate model; (a) oxidation of nitrite; (b) glucose utilization.


| Process | k [1/h] | KA [kg/m3] | wBA [kg B/kg A] |
|---|---|---|---|
| Nitrite oxidation | 7.917 × 10−3 | 2.4 × 10−4 | 0.23 |
| k [1/h] | KA [kg/m3] | Kin [kg/m3] | wBA [kg B/kg A] |
|---|---|---|---|
| 0.26 | 0.0254 | 0.173 | 0.616 |
| Model | Exact Model | Linear Approximate Model | Nonlinear Approximate Model |
|---|---|---|---|
| Integration algorithm | Dormand-Prince (4/5) (MOL) | Dormand-Prince (4/5) | |
| Normalized time of execution | 1 | 0.0468 | 0.0327 |
| Integration algorithm | Gear’s method (MOL) | Gear’s method | |
| Normalized time of execution | 0.0816 | 0.0561 | 0.0535 |
| k [1/h] | KA [kg/m3] | wBA [kg B/kg A] |
|---|---|---|
| 0.4 | 0.002 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoneczny, S.; Cioch-Skoneczny, M. Approximate Models of Microbiological Processes in a Biofilm Formed on Fine Spherical Particles. Processes 2022, 10, 48. https://doi.org/10.3390/pr10010048
Skoneczny S, Cioch-Skoneczny M. Approximate Models of Microbiological Processes in a Biofilm Formed on Fine Spherical Particles. Processes. 2022; 10(1):48. https://doi.org/10.3390/pr10010048
Chicago/Turabian StyleSkoneczny, Szymon, and Monika Cioch-Skoneczny. 2022. "Approximate Models of Microbiological Processes in a Biofilm Formed on Fine Spherical Particles" Processes 10, no. 1: 48. https://doi.org/10.3390/pr10010048
APA StyleSkoneczny, S., & Cioch-Skoneczny, M. (2022). Approximate Models of Microbiological Processes in a Biofilm Formed on Fine Spherical Particles. Processes, 10(1), 48. https://doi.org/10.3390/pr10010048






