Non-Cystic Fibrosis Bronchiectasis in Pediatric Age: A Case Series in a Metropolitan Area of Northern Italy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
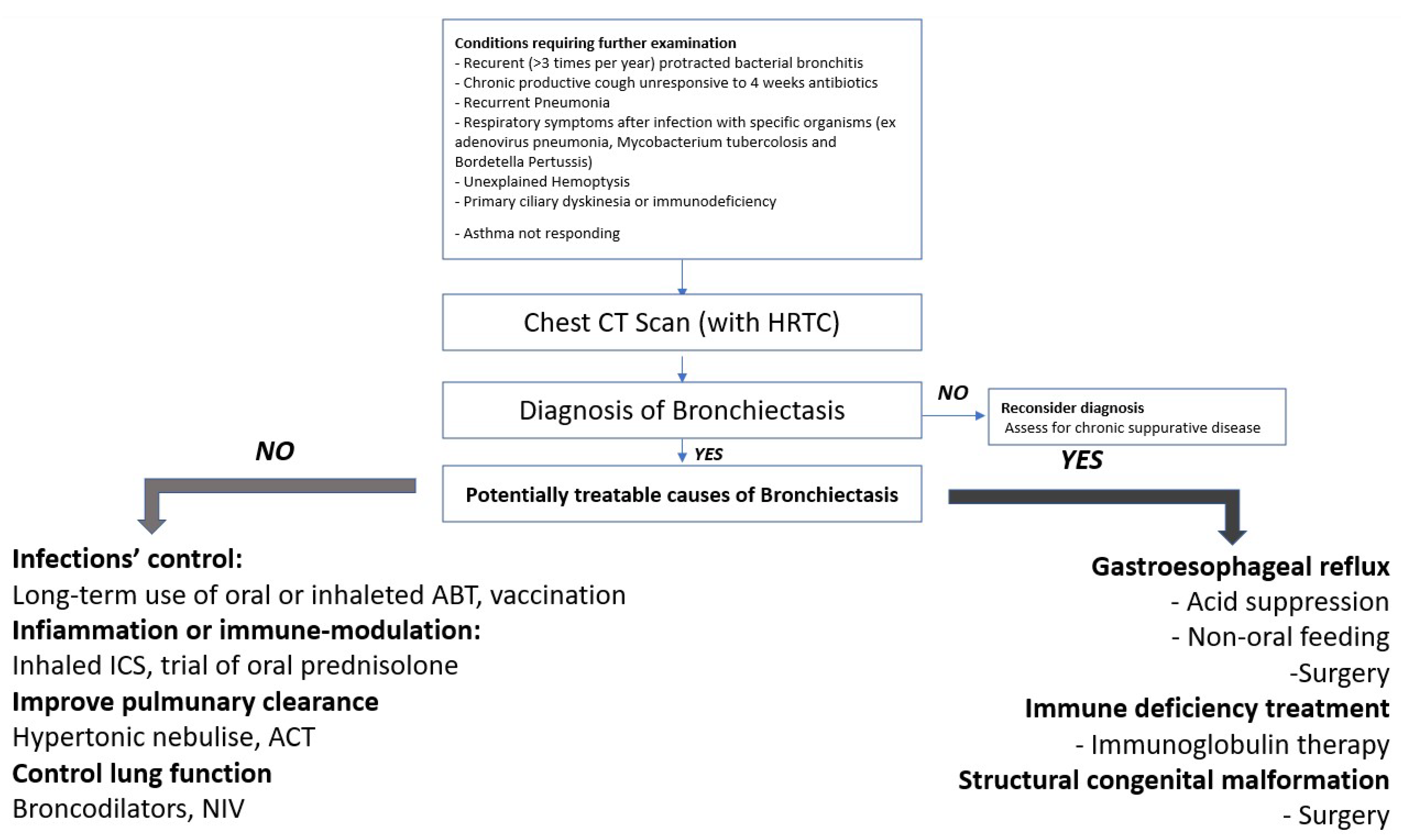
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brower, K.S.; Del Vecchio, M.T.; Aronoff, S.C. The etiologies of non-CF bronchiectasis in childhood: A systematic review of 989 subjects. BMC Pediatr. 2014, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- McCallum, G.B.; Binks, M.J. The epidemiology of chronic suppurative lung disease and bronchiectasis in children and adolescents. Front. Pediatr. 2017, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.; Karadag, B. Differences and similarities in non-cystic fibrosis bronchiectasis between developing and affluent countries. Paediatr. Respir. Rev. 2011, 12, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, N.; Porcaro, F.; Petreschi, F.; Cammerata, M.; Allegorico, A.; Negro, V.; Cutrera, R. Noncystic fibrosis bronchiectasis in children and adolescents: Follow-up over a decade. Pediatr. Pulmonol. 2021, 56, 3026–3034. [Google Scholar] [CrossRef]
- Santamaria, F.; Montella, S.; Pifferi, M.; Ragazzo, V.; De Stefano, S.; De Paulis, N.; Maglione, M.; Boner, A.L. A descriptive study of non-cystic fibrosis bronchiectasis in a pediatric population from central and southern Italy. Respiration 2009, 77, 160–165. [Google Scholar] [CrossRef]
- Goyal, V.; Grimwood, K.; Marchant, J.; Masters, I.B.; Chang, A.B. Pediatric bronchiectasis: No longer an orphan disease. Pediatr. Pulmonol. 2016, 51, 450–469. [Google Scholar] [CrossRef]
- Dosanjh, A. Airway Malformations and Bronchiectasis: A Pediatric Study. Ear Nose Throat J. 2020, 99, 44–46. [Google Scholar] [CrossRef]
- Cagdas, D.; Kızılkan, M.P.; Tagiyev, A.; Emiralioglu, N.; Kales, A.; Yalcun, E.; Dogru, D.; Ozcelik, U.; Kiper, N.; Tezcan, I. Primary Immunodeficiency Disorders in children with Non-Cystic Fibrosis Bronchiectasis. Eur. Ann. Allergy Clin. Immunol. 2020, 52, 271–276. [Google Scholar] [CrossRef]
- Lewiston, N.J. Bronchiectasis in childhood. Pediatr. Clin. N. Am. 1984, 31, 865–878. [Google Scholar] [CrossRef]
- McGuinness, G.; Naidich, D.P.; Leitman, B.S.; McCauley, D.I. Bronchiectasis: CT evaluation. AJR Am. J. Roentgenol. 1993, 160, 253–259. [Google Scholar] [CrossRef]
- Chang, A.B.; Fortescue, R.; Grimwood, K.; Alexopoulou, E.; Bell, L.; Boyd, J.; Bush, A.; Chalmers, J.D.; Hill, A.T.; Karadag, B.; et al. European Respiratory Society guidelines for the management of children and adolescents with bronchiectasis. Eur. Respir. J. 2021, 58, 2002990. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.B.; Bruce, K.D.; Martin, M.L.; Burr, L.D.; Serisier, D.J. The effect of long- term macrolide treatment on respiratory microbiota composition in non-cystic fibrosis bronchiectasis: An analysis from the randomised, double-blind, placebo-controlled BLESS trial. Lancet Respir. Med. 2014, 2, 988–996. [Google Scholar] [CrossRef]
- Lee, A.L.; Button, B.M.; Tannenbaum, E.L. Airway-Clearance Techniques in Children and Adolescents with Chronic Suppurative Lung Disease and Bronchiectasis. Front. Pediatr. 2017, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- El Boustany, P.; Gachelin, E.; Colomban, C.; Cernoia, J.; Sudour, P.; Carsin, A.; Dubus, J.-C. A review of non-cystic fibrosis bronchiectasis in children with a focus on the role of long-term treatment with macrolides. Pediatr. Pulmonol. 2019, 54, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M.; Di Palmo, E.; Bertelli, L.; Camela, F.; Ricci, G.; Pession, A. A pediatric disease to keep in mind: Diagnostic tools and management of bronchiectasis in pediatric age. Ital. J. Pediatr. 2017, 43, 117. [Google Scholar] [CrossRef]
- Bhalla, M.; Turcios, N.; Aponte, V.; Jenkins, M.; Leitman, B.S.; McCauley, D.I.; Naidich, D.P. Cystic fibrosis: Scoring system with thin-section CT. Radiology 1991, 179, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.A.; Metcalfe, R.; Milne, D.G.; Thompson, J.; Byrnes, C.A. Retrospective review of children presenting with non cystic fibrosis bronchiectasis: HRCT features and clinical relationships. Pediatr. Pulmonol. 2003, 36, 87–93. [Google Scholar] [CrossRef]
- Webb, W.R.; Muller, N.L.; Naidich, D.P. High-Resolution CT of the Lung; Lippincott: New York, NY, USA; Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 71–193. [Google Scholar]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. ATS/ERS Task Force. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Global Initiative for Asthma Global Strategy for Asthma Management and Prevention. Updated 2021. Available online: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf (accessed on 22 February 2021).
- Nikolaizik, W.H.; Warner, J.O. Aetiology of chronic suppurative lung disease. Arch. Dis. Child. 1994, 70, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Wurzel, D.F.; Marchant, J.M.; Yerkovich, S.T.; Upham, J.W.; Petsky, H.L.; Smith-Vaughan, H.; Masters, B.; Buntain, H.; Chang, A.B. Protracted bacterial bronchitis in children: Natural history and risk factors for bronchiectasis. Chest 2016, 150, 1101–1108. [Google Scholar] [CrossRef] [Green Version]
- Gallucci, M.; Pedretti, M.; Giannetti, A.; di Palmo, E.; Bertelli, L.; Pession, A.; Ricci, G. When the Cough Does Not Improve: A Review on Protracted Bacterial Bronchitis in Children. Front. Pediatr. 2020, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S.; Barbato, A.; Collins, S.A.; Goutaki, M.; Behan, L.; Caudri, D.; Dell, S.; Eber, E.; Escudier, E.; Hirst, R.A.; et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur. Respir. J. 2017, 49, 1601090. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, H.; Schmidts, M.; Werner, C.; Onoufriadis, A.; Loges, N.T.; Raidt, J.; Banki, N.F.; Shoemark, A.; Burgoyne, T.; Al Turki, S.; et al. UK10K Consortium. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am. J. Hum. Genet. 2012, 91, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Shoemark, A.; Pinto, A.L.; Patel, M.P.; Daudvohra, F.; Hogg, C.; Mitchison, H.M.; Burgoyne, T. PCD Detect: Enhancing ciliary features through image averaging and classification. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L1048–L1060. [Google Scholar] [CrossRef] [PubMed]
- Pennekamp, P.; Dworniczak, B.; Omran, H. SPEF2- and HYDIN-Mutant Cilia Lack the Central Pair-associated Protein SPEF2, Aiding Primary Ciliary Dyskinesia Diagnostics. Am. J. Respir. Cell Mol. Biol. 2020, 62, 382–396. [Google Scholar] [CrossRef]
- McDonnell, M.; O’Toole, D.; Ward, C.; Pearson, J.; Lordan, J.; De Soyza, A.; Loebinger, M.; Chalmers, J.; Laffey, J.; Rutherford, R. A qualitative synthesis of gastro-oesophageal reflux in bronchiectasis: Current understanding and future risk. Respir. Med. 2018, 141, 132–143. [Google Scholar] [CrossRef]
- Douros, K.; Sardeli, O.; Prountzos, S.; Galani, A.; Moriki, D.; Alexopoulou, E.; Priftis, K.N. Asthma-Like Features and Anti-Asthmatic Drug Prescription in Children with Non-CF Bronchiectasis. J. Clin. Med. 2020, 9, 4009. [Google Scholar] [CrossRef]
- Goyal, V.; Chang, A.B. Combination inhaled corticosteroids and long-acting beta2-agonists for children and adults with bronchiectasis. Cochrane Database Syst. Rev. 2014, 2014, CD010327. [Google Scholar] [CrossRef]
- Säynäjäkangas, O.; Keistinen, T.; Tuuponen, T.; Kivelä, S.L. The course of childhood bronchiectasis: A case report and considerations of hospital use. Int. J. Circumpolar Health 1998, 57, 276–279. [Google Scholar]
- Hnin, K.; Nguyen, C.; Carson, K.V.; Evans, D.J.; Greenstone, M.; Smith, B.J. Prolonged antibiotics for non-cystic fibrosis bronchiectasis in children and adults. Cochrane Database Syst. Rev. 2015, 2015, CD001392. [Google Scholar] [CrossRef]
- Pasteur, M.C.; Bilton, D.; Hill, A.T. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010, 65 (Suppl. 1), i1–i58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, C.; Chalmers, J.D.; Crossingham, I.; Relph, N.; Felix, L.M.; Evans, D.J.; Milan, S.J.; Spencer, S. Macrolide antibiotics for bronchiectasis. Cochrane Database Syst. Rev. 2018, 3, CD012406. [Google Scholar] [CrossRef] [PubMed]
- Somayaji, R.; Goss, C.H. Duration of antibiotic therapy in non-cystic fibrosis bronchiectasis. Curr. Pulmonol. Rep. 2019, 8, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Valery, P.C.; Morris, P.S.; A Byrnes, C.; Grimwood, K.; Torzillo, P.J.; A Bauert, P.; Masters, I.B.; Diaz, A.; McCallum, G.B.; Mobberley, C.; et al. Long-term azithromycin for Indigenous children with non-cystic-fibrosis bronchiectasis or chronic suppurative lung disease (Bronchiectasis Intervention Study): A multicentre, double-blind, randomised controlled trial. Lancet Respir. Med. 2013, 1, 610–620, Erratum in Lancet Respir. Med. 2015, 3, e29. [Google Scholar] [CrossRef]
- E Kobbernagel, H.; Buchvald, F.F.; Haarman, E.G.; Casaulta, C.; A Collins, S.; Hogg, C.; E Kuehni, C.; Lucas, J.S.; Moser, C.; Quittner, A.L.; et al. Efficacy and safety of azithromycin maintenance therapy in primary ciliary dyskinesia (BESTCILIA): A multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir. Med. 2020, 8, 493–505. [Google Scholar] [CrossRef]
- Lee, E.; Sol, I.S.; Kim, J.D.; Yang, H.J.; Min, T.K.; Jang, G.C.; Hwang, Y.H.; Cho, H.J.; Suh, D.I.; Kim, K.; et al. Long-term macrolide treatment for non-cystic fibrosis bronchiectasis in children: A meta-analysis. Sci. Rep. 2021, 11, 24287. [Google Scholar] [CrossRef]
- Bush, A. Azithromycin is the answer in paediatric respiratory medicine, but what was the question? Paediatr. Respir. Rev. 2020, 34, 67–74. [Google Scholar] [CrossRef]
- Wilson, L.M.; Morrison, L.; Robinson, K.A. Airway clearance techniques for cystic fibrosis: An overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 2019, 1, CD011231. [Google Scholar] [CrossRef]
- Poeta, M.; Maglione, M.; Borrelli, M.; Santamaria, F. Non-cystic fibrosis bronchiectasis in children and adolescents: Neglected and emerging issues. Pediatr. Neonatol. 2020, 61, 255–262. [Google Scholar] [CrossRef]
- Lee, A.L.; Burge, A.T.; Holland, A.E. Positive expiratory pressure therapy versus other airway clearance techniques for bronchiectasis. Cochrane Database Syst. Rev. 2017, 9, CD011699. [Google Scholar] [CrossRef]
| Associated Conditions | Number of Patients |
|---|---|
| Previous lower airways infections | 8 |
| Primary ciliary dyskinesia | 1 |
| Immune deficiency | 2 |
| Gastroesophageal reflux disease | 2 |
| Right-sided aortic arch | 1 |
| Syndromic unknown disease | 1 |
| Lung neuroblastoma | 1 |
| Asthma/atopy | 6 |
| Protracted Bacterial Bronchitis | 1 |
| Respiratory distress or intubation during perinatal period | 2 |
| Symptoms | Number of Patients |
|---|---|
| Recurrent wheezing in first year of life | 5 |
| Recurrent wheezing | 11 |
| Wet Cough | 15 |
| Dyspnea | 1 |
| Persistent purulent expectoration | 4 |
| Recurrent infections of lower respiratory tract | 15 |
| Sinusitis | 2 |
| Laryngotracheitis | 1 |
| Hemoptysis | 0 |
| Radiologic Findings | N patients | Associated Conditions (Number Subjects) | Localization |
|---|---|---|---|
| Cystic | 1 | GER | lower left lobe |
| Cylindric | 9 | PI (5) CVID/EVANS (1) NB (1) EA (1) CP (1) | 2 medium lobe, 2 lower left lobe, 1 right lower lobe bilateral Lower and apical left lobe lower left lobe medium lobe |
| Varicose | 1 | PCD (1) | medium lobe, lower right lobe, lower left lobe |
| Non-specific morphology | 4 | ID (1) PI (3) | medium lobe 1 lower left lobe, 1 bilateral upper lobe 1 medium lobe and lower left lobe |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallucci, M.; Candela, E.; Di Palmo, E.; Miniaci, A.; Pession, A. Non-Cystic Fibrosis Bronchiectasis in Pediatric Age: A Case Series in a Metropolitan Area of Northern Italy. Children 2022, 9, 1420. https://doi.org/10.3390/children9091420
Gallucci M, Candela E, Di Palmo E, Miniaci A, Pession A. Non-Cystic Fibrosis Bronchiectasis in Pediatric Age: A Case Series in a Metropolitan Area of Northern Italy. Children. 2022; 9(9):1420. https://doi.org/10.3390/children9091420
Chicago/Turabian StyleGallucci, Marcella, Egidio Candela, Emanuela Di Palmo, Angela Miniaci, and Andrea Pession. 2022. "Non-Cystic Fibrosis Bronchiectasis in Pediatric Age: A Case Series in a Metropolitan Area of Northern Italy" Children 9, no. 9: 1420. https://doi.org/10.3390/children9091420
APA StyleGallucci, M., Candela, E., Di Palmo, E., Miniaci, A., & Pession, A. (2022). Non-Cystic Fibrosis Bronchiectasis in Pediatric Age: A Case Series in a Metropolitan Area of Northern Italy. Children, 9(9), 1420. https://doi.org/10.3390/children9091420







