Human Mastadenovirus Infections in Children: A Review of the Current Status in the Arab World in the Middle East and North Africa
Abstract
1. Introduction
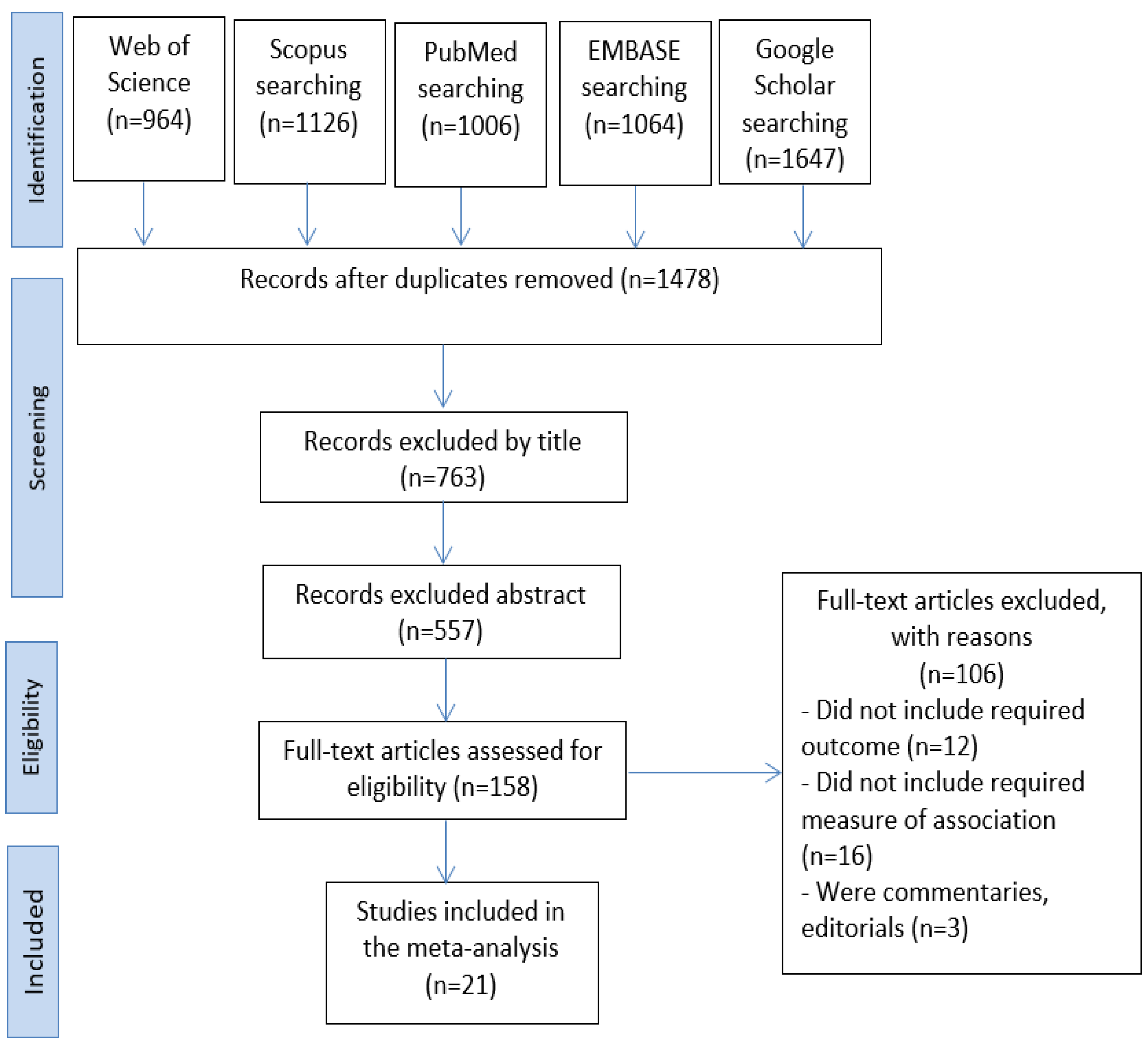
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samantaray, M.U.S.; Santra, M.P. Human Adenovirus Serotypes Efficiently Transducing HEK293 Cells: An In Vitro Propagation of HAdv. Int. J. Res. Appl. Sci. Biotechnol. 2021, 8, 17–21. [Google Scholar] [CrossRef]
- Rowe, W.P.; Huebner, R.J.; Gilmore, L.K.; Parrott, R.H.; Ward, T.G. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. 1953, 84, 570–573. [Google Scholar] [CrossRef]
- Moleirinho, M.G.; Feast, S.; Moreira, A.S.; Silva, R.J.; Alves, P.M.; Carrondo, M.J.; Huber, T.; Fee, C.; Peixoto, C. 3D-printed ordered bed structures for chromatographic purification of enveloped and non-enveloped viral particles. Sep. Purif. Technol. 2021, 254, 117681. [Google Scholar] [CrossRef]
- Takeuchi, A.; Hashimoto, K. Electron microscope study of experimental enteric adenovirus infection in mice. Infect. Immun. 1976, 13, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Krupovic, M.; Yutin, N. Evolution of double-stranded DNA viruses of eukaryotes: From bacteriophages to transposons to giant viruses. Ann. N. Y. Acad. Sci. 2015, 1341, 10–24. [Google Scholar] [CrossRef]
- Shaw, A.R.; Ziff, E.B. Transcripts from the adenovirus-2 major late promoter yield a single early family of 3′ coterminal mRNAs and five late families. Cell 1980, 22, 905–916. [Google Scholar] [CrossRef]
- Shek, L.P.-C.; Lee, B.-W. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr. Respir. Rev. 2003, 4, 105–111. [Google Scholar] [CrossRef]
- Abbas, K.Z.; Lombos, E.; Duvvuri, V.R.; Olsha, R.; Higgins, R.R.; Gubbay, J.B. Temporal changes in respiratory adenovirus serotypes circulating in the greater Toronto area, Ontario, during December 2008 to April 2010. Virol. J. 2013, 10, 15. [Google Scholar] [CrossRef]
- Chow, W.Z.; Chan, Y.F.; Oong, X.Y.; Ng, L.J.; Nor’E, S.S.; Ng, K.T.; Chan, K.G.; Hanafi, N.S.; Pang, Y.K.; Kamarulzaman, A. Genetic diversity, seasonality and transmission network of human metapneumovirus: Identification of a unique sub-lineage of the fusion and attachment genes. Sci. Rep. 2016, 6, 27730. [Google Scholar] [CrossRef]
- La Rosa, G.; Fratini, M.; Libera, S.D.; Iaconelli, M.; Muscillo, M. Viral infections acquired indoors through airborne, droplet or contact transmission. Ann. Dell’istituto Super. Di Sanita 2013, 49, 124–132. [Google Scholar]
- Arnold, A.; MacMahon, E. Adenovirus infections. Medicine 2017, 45, 777–780. [Google Scholar] [CrossRef]
- O’Brien, B.; Goodridge, L.; Ronholm, J.; Nasheri, N. Exploring the potential of foodborne transmission of respiratory viruses. Food Microbiol. 2021, 95, 103709. [Google Scholar] [CrossRef]
- Smith, J.G.; Wiethoff, C.M.; Stewart, P.L.; Nemerow, G.R. Adenovirus. In Cell Entry by Non-Enveloped Viruses; Springer: Berlin/Heidelberg, Germany, 2010; pp. 195–224. [Google Scholar]
- Murtagh, P.; Giubergia, V.; Viale, D.; Bauer, G.; Pena, H.G. Lower respiratory infections by adenovirus in children. Clinical features and risk factors for bronchiolitis obliterans and mortality. Pediatric Pulmonol. 2009, 44, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, R.C.; Uil, T.G. Adenovirus DNA replication. Cold Spring Harb. Perspect. Biol. 2013, 5, a013003. [Google Scholar] [CrossRef]
- Lynch, J.P.; Fishbein, M.; Echavarria, M. Adenovirus. In Proceedings of the Seminars in Respiratory and Critical Care Medicine, Denver, Colorado, 13–18 May 2011; pp. 494–511. [Google Scholar]
- Gu, J.; Su, Q.-Q.; Zuo, T.-T.; Chen, Y.-B. Adenovirus diseases: A systematic review and meta-analysis of 228 case reports. Infection 2021, 49, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, G.L. The common cold. Prim. Care Clin. Off. Pract. 1996, 23, 657–675. [Google Scholar] [CrossRef]
- Shieh, W.-J. Human adenovirus infections in pediatric population-an update on clinic—Pathologic correlation. Biomed. J. 2021, 54, 38–49. [Google Scholar]
- Zhang, S.-Y.; Luo, Y.-P.; Huang, D.-D.; Fan, H.; Lu, Q.-B.; Wo, Y.; Chen, G.; Zhang, X.-A.; Li, Y.; Tong, Y.-G. Fatal pneumonia cases caused by human adenovirus 55 in immunocompetent adults. Infect. Dis. 2016, 48, 40–47. [Google Scholar] [CrossRef]
- Wang, H.-S. Updates in pediatrics. Biomed. J. 2022, 45, 9. [Google Scholar] [CrossRef]
- Ison, M.G.; Hayden, R.T. Adenovirus. In Diagnostic Microbiology of the Immunocompromised Host; Microbiology Spectrum, American Society for Microbiology Press: Washington, DC, USA, 2016; pp. 217–232. [Google Scholar]
- Matthes-Martin, S.; Boztug, H.; Lion, T. Diagnosis and treatment of adenovirus infection in immunocompromised patients. Expert Rev. Anti-Infect. Ther. 2013, 11, 1017–1028. [Google Scholar] [CrossRef]
- Bhatti, Z.; Dhamoon, A. Fatal adenovirus infection in an immunocompetent host. Am. J. Emerg. Med. 2017, 35, 1034.e1–1034.e2. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P., III; Kajon, A.E. Adenovirus: Epidemiology, global spread of novel serotypes, and advances in treatment and prevention. In Proceedings of the Seminars in Respiratory and Critical Care Medicine, San Francisco, CA, USA, 13–18 May 2016; pp. 586–602. [Google Scholar]
- Maschmeyer, G.; Ljungman, P. Infections in hematopoietic stem cell transplant recipients. In Principles and Practice of cancer Infectious Diseases; Springer: Berlin/Heidelberg, Germany, 2011; pp. 17–25. [Google Scholar]
- Rabaan, A.A.; Bakhrebah, M.A.; Nassar, M.S.; Natto, Z.S.; Al Mutair, A.; Alhumaid, S.; Aljeldah, M.; Garout, M.; Alfouzan, W.A.; Alshahrani, F.S. Suspected Adenovirus Causing an Emerging HEPATITIS among Children below 10 Years: A Review. Pathogens 2022, 11, 712. [Google Scholar] [CrossRef]
- Lion, T. Adenovirus persistence, reactivation, and clinical management. FEBS Lett. 2019, 593, 3571–3582. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, W.; Yang, B.; Qian, R.; Wu, F.; He, X.; Zhu, Q.; Liu, J.; Ni, Y.; Wang, J. Epidemiological and virological characteristics of respiratory tract infections in children during COVID-19 outbreak. BMC Pediatrics 2021, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-C.; Guo, Y.-H.; Qiu, F.-Z.; Wang, L.; Yang, S.; Feng, Z.-S.; Li, G.-X. Molecular and clinical characterization of human adenovirus associated with acute respiratory tract infection in hospitalized children. J. Clin. Virol. 2020, 123, 104254. [Google Scholar] [CrossRef]
- Hess, M. Detection and differentiation of avian adenoviruses: A review. Avian Pathol. 2000, 29, 195–206. [Google Scholar] [CrossRef]
- Ko, G.; Cromeans, T.L.; Sobsey, M.D. Detection of infectious adenovirus in cell culture by mRNA reverse transcription-PCR. Appl. Environ. Microbiol. 2003, 69, 7377–7384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rohayem, J.; Berger, S.; Juretzek, T.; Herchenröder, O.; Mogel, M.; Poppe, M.; Henker, J.; Rethwilm, A. A simple and rapid single-step multiplex RT-PCR to detect Norovirus, Astrovirus and Adenovirus in clinical stool samples. J. Virol. Methods 2004, 118, 49–59. [Google Scholar] [CrossRef]
- Hiwarkar, P.; Kosulin, K.; Cesaro, S.; Mikulska, M.; Styczynski, J.; Wynn, R.; Lion, T. Management of adenovirus infection in patients after haematopoietic stem cell transplantation: State-of-the-art and real-life current approach: A position statement on behalf of the Infectious Diseases Working Party of the European Society of Blood and Marrow Transplantation. Rev. Med. Virol. 2018, 28, e1980. [Google Scholar]
- Jaff, D.O.; Aziz, T.A.; Smith, N.R. The incidence of rotavirus and adenovirus infections among children with diarrhea in Sulaimani Province, Iraq. J. Biosci. Med. 2015, 4, 124–131. [Google Scholar] [CrossRef]
- Harb, A.; Abraham, S.; Rusdi, B.; Laird, T.; O’Dea, M.; Habib, I. Molecular detection and epidemiological features of selected bacterial, viral, and parasitic enteropathogens in stool specimens from children with acute diarrhea in Thi-Qar Governorate, Iraq. Int. J. Environ. Res. Public Health 2019, 16, 1573. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.M.; Dove, W.; Abd-Eldayem, S.A.; Abu-Zeid, A.F.; Shamoon, H.E.; Hart, C.A. Molecular epidemiology and disease severity of respiratory syncytial virus in relation to other potential pathogens in children hospitalized with acute respiratory infection in Jordan. J. Med. Virol. 2008, 80, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Meqdam, M.M.; Nasrallah, G.; Al-Shurman, A. Detection of adenovirus infection in children in Jordan. Ann. Trop. Paediatr. 2001, 21, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, H.A.; Madi, N.M.; Al-Nakib, W. Analysis of viral diversity in stool samples from infants and children with acute gastroenteritis in Kuwait using Metagenomics approach. Virol. J. 2020, 17, 1–12. [Google Scholar] [CrossRef]
- Chehadeh, W.; Al-Adwani, A.; John, S.E.; Al-Dhufairi, S.; Al-Dousari, H.; Alkhaledi, M.; Al-Nakib, W. Adenovirus types associated with severe respiratory diseases: A retrospective 4-year study in Kuwait. J. Med. Virol. 2018, 90, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Al-Moyed, K.A.; Al-Jamrah, K.M.; Al-Robasi, A.B.A.; Nabhan, A.S.B.; Al-Haddad, A.M. Prevalence of enteric adenovirus among infants and young children suffering from acute gastroenteritis in Sana’a city, Yemen. Andal. J. Appl. Sci. 2015, 4, 79–81. [Google Scholar]
- Al Amad, M.A.; Al Mahaqri, A.A.; Al Serouri, A.A.; Khader, Y.S. Severe acute respiratory infections with influenza and noninfluenza respiratory viruses: Yemen, 2011–2016. INQUIRY J. Health Care Organ. Provis. Financ. 2019, 56, 1–7. [Google Scholar] [CrossRef]
- Aysha Waheed, A.; Muneera Abdulla, A.N.; Ghada Al, B.A. Adenovirus isolated from an outbreak of acute hemorrhagic conjunctivitis. Bahrain Med. Bull. 2016, 38, 224–226. [Google Scholar]
- Zaraket, R.; Salami, A.; Bahmad, M.; El Roz, A.; Khalaf, B.; Ghssein, G.; Bahmad, H.F. Prevalence, risk factors, and clinical characteristics of rotavirus and adenovirus among Lebanese hospitalized children with acute gastroenteritis. Heliyon 2020, 6, e04248. [Google Scholar] [CrossRef]
- Alsuwaidi, A.R.; Al Dhaheri, K.; Al Hamad, S.; George, J.; Ibrahim, J.; Ghatasheh, G.; Issa, M.; Al-Hammadi, S.; Narchi, H. Etiology of diarrhea by multiplex polymerase chain reaction among young children in the United Arab Emirates: A case-control study. BMC Infect. Dis. 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Zaki, M.E.S.; El Kheir, N.A. Molecular study of astrovirus, adenovirus and norovirus in community acquired diarrhea in children: One Egyptian center study. Asian Pac. J. Trop. Biomed. 2017, 7, 987–990. [Google Scholar] [CrossRef]
- Elmahdy, E.M.; Ahmed, N.I.; Shaheen, M.N.; Mohamed, E.-C.B.; Loutfy, S.A. Molecular detection of human adenovirus in urban wastewater in Egypt and among children suffering from acute gastroenteritis. J. Water Health 2019, 17, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Allayeh, A.K.; El Baz, R.M.; Saeed, N.M.; Osman, M.E.S. Detection and genotyping of viral gastroenteritis in hospitalized children below five years old in Cairo, Egypt. Arch. Pediatric Infect. Dis. 2018, 6, e60288. [Google Scholar]
- Jroundi, I.; Mahraoui, C.; Benmessaoud, R.; Moraleda, C.; Tligui, H.; Seffar, M.; Kettani, S.C.; Benjelloun, B.S.; Chaacho, S.; Maaroufi, A. The epidemiology and aetiology of infections in children admitted with clinical severe pneumonia to a university hospital in Rabat, Morocco. J. Trop. Pediatrics 2014, 60, 270–278. [Google Scholar] [CrossRef]
- Bimouhen, A.; Regragui, Z.; El Falaki, F.; Ihazmade, H.; Benkerroum, S.; Cherkaoui, I.; Rguig, A.; Ezzine, H.; Benamar, T.; Triki, S.; et al. Viral aetiology of influenza-like illnesses and severe acute respiratory illnesses in Morocco, September 2014 to December 2016. J. Glob. Health. 2022, 12, 04062. [Google Scholar] [CrossRef]
- Elhag, W.I.; Saeed, H.A.; Omer, E.F.E.; Ali, A.S. Prevalence of rotavirus and adenovirus associated with diarrhea among displaced communities in Khartoum, Sudan. BMC Infect. Dis. 2013, 13, 1–6. [Google Scholar] [CrossRef]
- Adam, M.A.; Wang, J.; Enan, K.-A.; Shen, H.; Wang, H.; El Hussein, A.R.; Musa, A.B.; Khidir, I.M.; Ma, X. Molecular survey of viral and bacterial causes of childhood diarrhea in Khartoum state, Sudan. Front. Microbiol. 2018, 9, 112. [Google Scholar] [CrossRef]
- Derrar, F.; Izri, K.; Kaddache, C.; Boukari, R.; Hannoun, D. Virologic study of acute lower respiratory tract infections in children admitted to the paediatric department of Blida University Hospital, Algeria. New Microbes New Infect. 2019, 30, 100536. [Google Scholar] [CrossRef]
- Rahouma, A.; Klena, J.D.; Krema, Z.; Abobker, A.A.; Treesh, K.; Franka, E.; Abusnena, O.; Shaheen, H.I.; El Mohammady, H.; Abudher, A. Enteric pathogens associated with childhood diarrhea in Tripoli-Libya. Am. J. Trop. Med. Hyg. 2011, 84, 886. [Google Scholar] [CrossRef]
- Brini, I.; Guerrero, A.; Ezzine, I.K.; Orth-Höller, D.; Hetzer, B.; Würzner, R.; Hazgui, O.; Handous, I.; Nouri-Merchaoui, S.; Bouguila, J. Human adenoviruses associated with respiratory illness in neonates, infants, and children in the Sousse area of Tunisia. J. Med. Virol. 2020, 92, 3081–3092. [Google Scholar] [CrossRef]
- Svensson, L.; Wadell, G.; Uhnoo, I.; Johansson, M.; Von Bonsdorff, C.-H. Cross-reactivity between enteric adenoviruses and adenovirus type 4: Analysis of epitopes by solid-phase immune electron microscopy. J. Gen. Virol. 1983, 64, 2517–2520. [Google Scholar] [CrossRef] [PubMed]
- Grimwood, K.; Carzino, R.; Barnes, G.L.; Bishop, R.F. Patients with enteric adenovirus gastroenteritis admitted to an Australian pediatric teaching hospital from 1981 to 1992. J. Clin. Microbiol. 1995, 33, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Saderi, H.; Roustai, M.; Sabahi, F.; Sadeghizadeh, M.; Owlia, P.; De Jong, J. Incidence of enteric adenovirus gastroenteritis in Iranian children. J. Clin. Virol. 2002, 24, 1–5. [Google Scholar] [CrossRef]
- Subekti, D.; Lesmana, M.; Tjaniadi, P.; Safari, N.; Frazier, E.; Simanjuntak, C.; Komalarini, S.; Taslim, J.; Campbell, J.; Oyofo, B. Incidence of Norwalk-like viruses, rotavirus and adenovirus infection in patients with acute gastroenteritis in Jakarta, Indonesia. FEMS Immunol. Med. Microbiol. 2002, 33, 27–33. [Google Scholar] [CrossRef]
- Koh, H.; Baek, S.Y.; Shin, J.I.; Chung, K.S.; Jee, Y.M. Coinfection of viral agents in Korean children with acute watery diarrhea. J. Korean Med. Sci. 2008, 23, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Bányai, K.; Esona, M.; Liu, A.; Wang, Y.; Tu, X.; Jiang, B. Molecular detection of novel adenoviruses in fecal specimens of captive monkeys with diarrhea in China. Vet. Microbiol. 2010, 142, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Tekin, A. Mardin\′deki akut gastroenteritli çocuklarda Rotavirüs ve Enterik Adenovirüs sıklığı. J. Clin. Exp. Investig. 2010, 1, 41–45. [Google Scholar]
- Costa, L.C.P.d.N.; Siqueira, J.A.M.; Portal, T.M.; Sousa, E.C.; Linhares, A.d.C.; Gabbay, Y.B.; Resque, H.R. Detection and genotyping of human adenovirus and sapovirus in children with acute gastroenteritis in Belém, Pará, between 1990 and 1992: First detection of GI. 7 and GV. 2 sapoviruses in Brazil. Rev. Da Soc. Bras. De Med. Trop. 2017, 50, 621–628. [Google Scholar] [CrossRef]
- CDC. Adenovirus VIS: Vaccine Information Statements (VISs); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020. [Google Scholar]
- Kujawski, S.A.; Lu, X.; Schneider, E.; Blythe, D.; Boktor, S.; Farrehi, J.; Haupt, T.; McBride, D.; Stephens, E.; Sakthivel, S.K. Outbreaks of adenovirus-associated respiratory illness on 5 college campuses in the United States, 2018–2019. Clin. Infect. Dis. 2021, 72, 1992–1999. [Google Scholar] [CrossRef]
- Roy, S.; Sandhu, A.; Medina, A.; Clawson, D.S.; Wilson, J.M. Adenoviruses in fecal samples from asymptomatic rhesus macaques, United States. Emerg. Infect. Dis. 2012, 18, 1081. [Google Scholar] [CrossRef]
- Banatvala, J.E.; Griffiths, P.; Schoub, B.; Mortimer, P. Principles and Practice of Clinical Virology; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Singh-Naz, N.; Rodriguez, W.; Kidd, A.; Brandt, C. Monoclonal antibody enzyme-linked immunosorbent assay for specific identification and typing of subgroup F adenoviruses. J. Clin. Microbiol. 1988, 26, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Guerrant, R.L.; Hughes, J.M.; Lima, N.L.; Crane, J. Diarrhea in developed and developing countries: Magnitude, special settings, and etiologies. Rev. Infect. Dis. 1990, 12, S41–S50. [Google Scholar] [CrossRef] [PubMed]
- Lamps, L.W. Infective disorders of the gastrointestinal tract. Histopathology 2007, 50, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, A.; Baumgart, D. Viral gastroenteritis in adults. Recent Pat. Antiinfect. Drug Discov. 2011, 6, 54–63. [Google Scholar] [CrossRef]
- Thewainy, H.T.; Hasony, H. Enteric adenovirus associated with acute gastroenteritis among hospitalized and healthy childern under five-years of age in Basrah, Iraq. Med. J. Basrah Univ. 2019, 37, 37–44. [Google Scholar]
- Wu, B.; Qi, X.; Xu, K.; Ji, H.; Zhu, Y.; Tang, F.; Zhou, M. Genetic characteristics of the coxsackievirus A24 variant causing outbreaks of acute hemorrhagic conjunctivitis in Jiangsu, China, 2010. PLoS ONE 2014, 9, e86883. [Google Scholar] [CrossRef]
| Region | Country | Study | Study Design | Targeted Children | Sample Size | Sample Location | Disease | Prevalence Rate |
|---|---|---|---|---|---|---|---|---|
| Middle East | Iraq | Ali Harb et al., 2019 | A descriptive cross-sectional observational study | Children below five years with acute diarrhea | 320 children | Stool specimens | Gastroenteritis | 4.5% |
| Iraq | Dilshad Jaff et al., 2016 | A descriptive cross-sectional observational study | Children below five years of age with gastroenteritis | 100 children | Stool specimens | Gastroenteritis | 3.0% | |
| Jordan | Nasser Kaplan et al., 2008 | A descriptive prospective cross-sectional study | Children below five years with an acute respiratory infection | 326 children | Nasopharyngeal aspirates | Acute respiratory infection | 18.0% | |
| Jordan | Mamdoh Meqdam et al., 2001 | A descriptive cross-sectional observational study | Children below thirteen years old with respiratory tract infections | 350 children | Nasopharyngeal aspirates | Acute respiratory infection | 15.4% | |
| Kuwait | Hawraa Mohammad et al., 2020 | A 4-year descriptive retrospective study | Children below ten years with gastroenteritis | 84 children | Stool samples | Gastroenteritis | 23.2% | |
| Kuwait | Wassim Chehadeh et al., 2018 | A 4-year descriptive retrospective study | Children below four years with severe respiratory disease | 743 children | Nasopharyngeal aspirate and Nasopharyngeal swab | Severe respiratory infections | 3.6% | |
| Yemen | Khaled Al-Moyed et al., 2015 | A descriptive cross-sectional observational study | Children below five years with acute gastroenteritis | 326 children | Stool samples | Gastroenteritis | 11% | |
| Yemen | Mohammad Al Amad et al., 2019 | A descriptive retrospective study | Children below fifteen years of age with severe acute respiratory infections | 1413 children | Nasopharyngeal and oropharyngeal swabs | Acute respiratory infection | 7.0% | |
| Bahrain | Aysha Agab et al., 2016 | A case study | A seven-year-old male and a five-year-old female | Two Bahraini siblings | Conjunctival swabs | Acute hemorrhagic conjunctivitis | NM | |
| Lebanon | Rasha Zaraket et al., 2020 | A 12-months descriptive retrospective study | Children below twelve years with acute gastroenteritis | 308 children | Stool samples | Gastroenteritis | 25.3% | |
| United Arab Emirates | Ahmed Alsuwaidi et al., 2021 | A descriptive case-control observational study | Children below five years with diarrhea | 203 children as a case | Stool samples | Acute diarrhea | 17.2% | |
| North Africa | Egypt | Abdou Kamal Allayeh et al., 2018 | A descriptive cross-sectional observational study | Children below five years with gastroenteritis | 119 children | Fecal diarrhea samples | Gastroenteritis | 6.7% |
| Egypt | Maysaa El Sayed Zaki et al., 2017 | A descriptive cross-sectional observational study | Children below five years with acute diarrhea | 100 children | Stool sample | Acute diarrhea | 20.0% | |
| Egypt | Elmahdy Elmahdy et al., 2019 | A descriptive cross-sectional observational study | Children below five years with gastroenteritis | 60 children | Stool samples | Gastroenteritis | 28.3% | |
| Morocco | Imane Jroundi et al., 2014 | A descriptive prospective cross-sectional study | Children below five years with respiratory symptomatology | 700 children | Nasopharyngeal aspirates | Acute respiratory infection | 17.0% | |
| Morocco | Marcil Sarrah et al., 2018 | A descriptive prospective cross-sectional study | Children below fourteen years with severe acute viral respiratory infections | 103 children | Nasopharyngeal aspirates | Acute respiratory infection | 16.5% | |
| Sudan | Wafa Elhag et al., 2013 | A descriptive cross-sectional observational study | Children below fourteen years old with acute diarrhea | 511 children | Stool samples | Acute diarrhea | 1.5% | |
| Sudan | Mosab Adam et al., 2018 | A descriptive cross-sectional observational study | Children below five years with acute diarrhea | 437 children | Stool samples | Acute diarrhea | 1.6% | |
| Algeria | Fawzi Derrar et al., 2019 | A descriptive prospective cross-sectional study | Children below two years with respiratory tract infections | 117 children | Nasal or nasopharyngeal aspiration | Acute respiratory infection | 7.5% | |
| Libya | Amal Rahouma et al., 2011 | A descriptive cross-sectional observational study | Children below five years with acute diarrhea | 239 children | Stool specimens | Gastroenteritis | 7.1% | |
| Tunisia | Ines Brini et al., 2020 | A descriptive cross-sectional observational study | Children below five years with acute respiratory infections | 583 children | Nasopharyngeal aspirate | Acute respiratory infection | 19.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qashqari, F.S.I. Human Mastadenovirus Infections in Children: A Review of the Current Status in the Arab World in the Middle East and North Africa. Children 2022, 9, 1356. https://doi.org/10.3390/children9091356
Qashqari FSI. Human Mastadenovirus Infections in Children: A Review of the Current Status in the Arab World in the Middle East and North Africa. Children. 2022; 9(9):1356. https://doi.org/10.3390/children9091356
Chicago/Turabian StyleQashqari, Fadi S. I. 2022. "Human Mastadenovirus Infections in Children: A Review of the Current Status in the Arab World in the Middle East and North Africa" Children 9, no. 9: 1356. https://doi.org/10.3390/children9091356
APA StyleQashqari, F. S. I. (2022). Human Mastadenovirus Infections in Children: A Review of the Current Status in the Arab World in the Middle East and North Africa. Children, 9(9), 1356. https://doi.org/10.3390/children9091356






