Ultra Short Course Chemotherapy for Early-Stage Non-Hodgkin’s Lymphoma in Children
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients
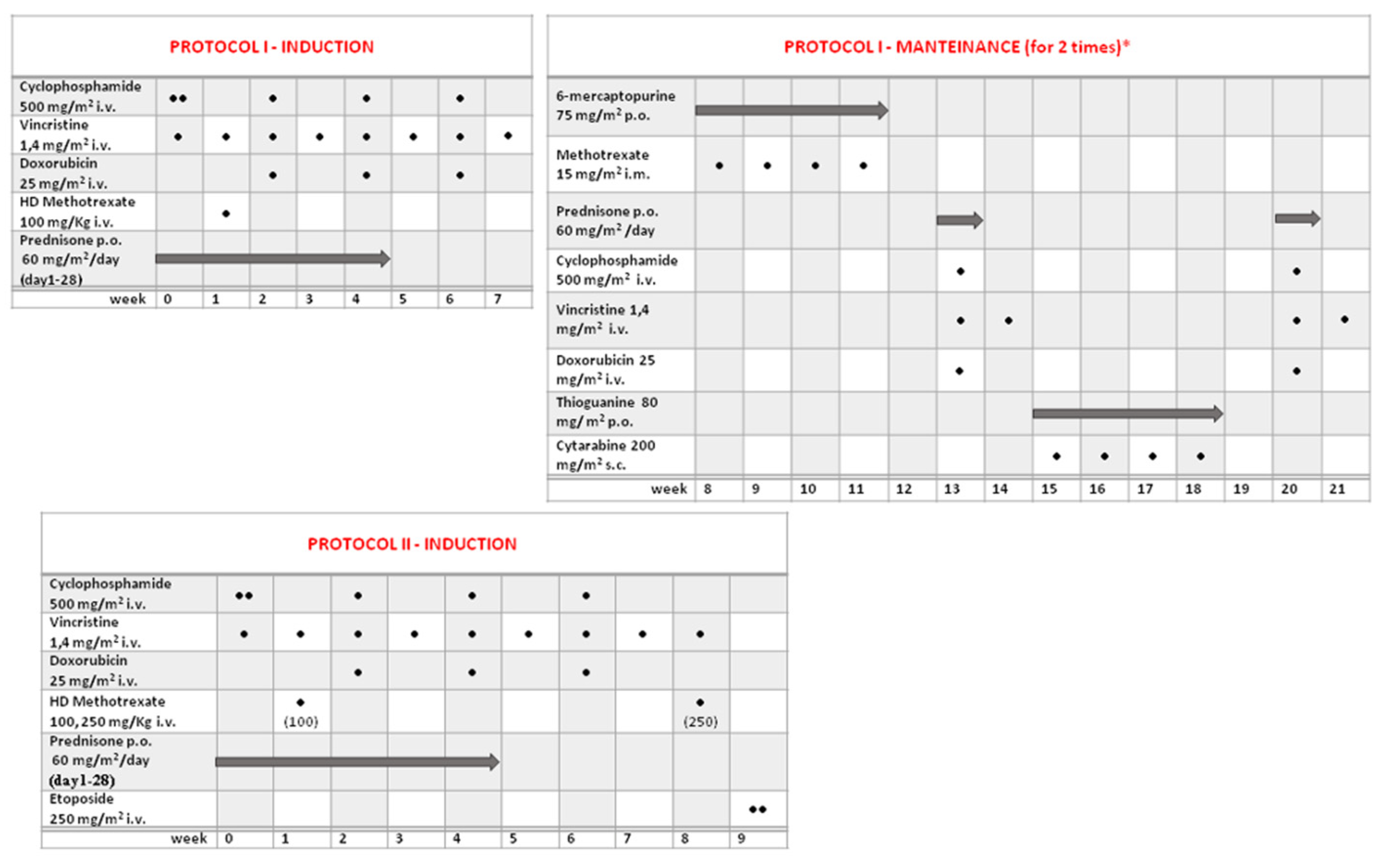
2.2. Treatment
2.3. Response Criteria and Follow-Up
2.4. Statistics
3. Results
3.1. Patients
3.2. Response to Treatment
3.3. Disease Relapse
3.4. Survival
3.5. Toxicities
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sandlund, J.T.; Downing, J.R.; Crist, W.M. Non-Hodgkin’s Lymphoma in Childhood. N. Engl. J. Med. 1996, 334, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.B.; Hustu, H.O.; Rivera, G.; Berard, C.W. End Results of Treating Children with Localized Non-Hodgkin’s Lymphomas with a Combined Modality Approach of Lessened Intensity. J. Clin. Oncol. 1983, 1, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Jenkin, R.D.T.; Anderson, J.R.; Chilcote, R.R. The Treatment of Localized Non-Hodgkin’s Lymphoma in Children: A Report from the Children’s Cancer Study Group. J. Clin. Oncol. 1984, 2, 88–97. [Google Scholar] [CrossRef]
- Anderson, J.R.; Jenkin, R.D.T.; Wilson, J.F.; Kjeldsberg, C.R.; Sposto, R.; Chilcote, R.R.; Coccia, P.F.; Exelby, P.R.; Siegel, S.; Meadows, A.T.; et al. Long-Term Follow-up of Patients Treated with COMP or LSA2L2 Therapy for Childhood Non-Hodgkin’s Lymphoma: A Report of CCG-551 from the Childrens Cancer Group. J. Clin. Oncol. 1993, 11, 1024–1032. [Google Scholar] [CrossRef]
- Link, M.P.; Donaldson, S.S.; Berard, C.W.; Shuster, J.J.; Murphy, S.B. Results of Treatment of Childhood Localized Non-Hodgkin’s Lymphoma with Combination Chemotherapy with or without Radiotherapy. N. Engl. J. Med. 1990, 322, 1169–1174. [Google Scholar] [CrossRef]
- Link, M.P.; Shuster, J.J.; Berard, C.W.; Murphy, S.B. Treatment of children and young adults with early-stage non-Hodgkin’s lymphoma. N. Engl. J. Med. 1997, 337, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Reiter, A.; Schrappe, M.; Parwaresch, R.; Henze, G.; Müller-Weihrich, S.; Sauter, S.; Sykora, K.W.; Ludwig, W.D.; Gadner, H.; Riehm, H. Non-Hodgkin’s Lymphomas of Childhood and Adolescence: Results of a Treatment Stratified for Biologic Subtypes and Stage-A Report of the Berlin-Frankfurt-Münster Group. J. Clin. Oncol. 1995, 13, 359–372. [Google Scholar] [CrossRef]
- Reiter, A.; Schrappe, M.; Tiemann, M.; Ludwig, W.D.; Yakisan, E.; Zimmermann, M.; Mann, G.; Chott, A.; Ebell, W.; Klingebiel, T.; et al. Improved Treatment Results in Childhood B-Cell Neoplasms with Tailored Intensification of Therapy: A Report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood 1999, 94, 3294–3306. [Google Scholar]
- Meadows, A.T.; Sposto, R.; Jenkin, R.D.T.; Kersey, J.H.; Chilcote, R.R.; Siegel, S.E.; Coccia, P.F.; Rosenstock, J.; Pringle, K.C.; Stolar, C.J.; et al. Similar Efficacy of 6 and 18 Months of Therapy with Four Drugs (COMP) for Localized Non-Hodgkin’s Lymphoma of Children: A Report from the Childrens Cancer Study Group. J. Clin. Oncol. 1989, 7, 92–99. [Google Scholar] [CrossRef]
- Patte, C.; Auperin, A.; Michon, J.; Behrendt, H.; Leverger, G.; Frappaz, D.; Lutz, P.; Coze, C.; Perel, Y.; Raphaël, M.; et al. The Société Française d’Oncologie Pédiatrique LMB89 Protocol: Highly Effective Multiagent Chemotherapy Tailored to the Tumor Burden and Initial Response in 561 Unselected Children with B-Cell Lymphomas and L3 Leukemia. Blood 2001, 97, 3370–3379. [Google Scholar] [CrossRef]
- Gerrard, M.; Cairo, M.S.; Weston, C.; Auperin, A.; Pinkerton, R.; Lambilliote, A.; Sposto, R.; McCarthy, K.; Lacombe, M.J.T.; Perkins, S.L.; et al. Excellent Survival Following Two Courses of COPAD Chemotherapy in Children and Adolescents with Resected Localized B-Cell Non-Hodgkin’s Lymphoma: Results of the FAB/LMB 96 International Study. Br. J. Haematol. 2008, 141, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Landmann, E.; Burkhardt, B.; Zimmermann, M.; Meyer, U.; Woessmann, W.; Klapper, W.; Wrobel, G.; Rosolen, A.; Pillon, M.; Escherich, G.; et al. Results and Conclusions of the European Intergroup EURO-LB02 Trial in Children and Adolescents with Lymphoblastic Lymphoma. Haematologica 2017, 102, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, M.; Lattuada, A.; Lombardi, F.; Rilke, F.; Gianni, C.; Fossati-Bellani, F. Childhood Non-Hodgkin’s Lymphoma: Prognostic Relevance of Clinical Stages and Histologic Subgroups. Am. J. Pediatr. Hematol. Oncol. 1983, 5, 161–171. [Google Scholar]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Lee Harris, N.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.B. Classification, Staging and End Results of Treatment of Childhood Non-Hodgkin’s Lymphomas: Dissimilarities from Lymphomas in Adults. Semin. Oncol. 1980, 7, 332–339. [Google Scholar] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Durrleman, S.; Simon, R. Flexible Regression Models with Cubic Splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef]
- Schemper, M.; Smith, T.L. A Note on Quantifying Follow-up in Studies of Failure Time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Flury, B.K.; Riedwyl, H. Standard Distance in Univariate and Multivariate Analysis. Am. Stat. 1986, 40, 249–25120. [Google Scholar]
- Spreafico, F.; Massimino, M.; Luksch, R.; Casanova, M.; Cefalo, G.S.; Collini, P.; Ferrari, A.; Polastri, D.; Terenziani, M.; Gasparini, M.; et al. Intensive, Very Short-Term Chemotherapy for Advanced Burkitt’s Lymphoma in Children. J. Clin. Oncol. 2002, 20, 2783–2788. [Google Scholar] [CrossRef]
- Robison, L.L. General Principles of the epidemiology of childhood cancer. In Principles and Practice of Pediatric Oncology, 2nd ed.; Lippincott, J.B., Pizzo, P.A., Poplack, D.G., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1993; pp. 3–10. [Google Scholar]
- Patte, C.; Auperin, A.; Gerrard, M.; Michon, J.; Pinkerton, R.; Sposto, R.; Weston, C.; Raphael, M.; Perkins, S.L.; McCarthy, K.; et al. Results of the Randomized International FAB/LMB96 Trial for Intermediate Risk B-Cell Non-Hodgkin Lymphoma in Children and Adolescents: It Is Possible to Reduce Treatment for the Early Responding Patients. Blood 2007, 109, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Woessmann, W.; Seidemann, K.; Mann, G.; Zimmermann, M.; Burkhardt, B.; Oschlies, I.; Ludwig, W.D.; Klingebiel, T.; Graf, N.; Gruhn, B.; et al. The Impact of the Methotrexate Administration Schedule and Dose in the Treatment of Children and Adolescents with B-Cell Neoplasms: A Report of the BFM Group Study NHL-BFM95. Blood 2005, 105, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Pillon, M.; Di Tullio, M.T.; Garaventa, A.; Cesaro, S.; Putti, M.C.; Favre, C.; Lippi, A.; Surico, G.; Di Cataldo, A.; D’Amore, E.; et al. Long-Term Results of the First Italian Association of Pediatric Hematology and Oncology Protocol for the Treatment of Pediatric B-Cell Non-Hodgkin Lymphoma (AIEOP LNH92). Cancer 2004, 101, 385–394. [Google Scholar] [CrossRef]
- Reiter, A.; Schrappe, M.; Ludwig, W.D.; Tlemann, M.; Parwaresch, R.; Zimmermann, M.; Schirg, E.; Henze, G.; Schellong, G.; Gadner, H.; et al. Intensive ALL-Type Therapy without Local Radiotherapy Provides a 90% Event-Free Survival for Children with T-Cell Lymphoblastic Lymphoma: A BFM Group Report. Blood 2000, 95, 416–421. [Google Scholar] [PubMed]
- Armenian, S.H.; Hudson, M.M.; Mulder, R.L.; Chen, M.H.; Constine, L.S.; Dwyer, M.; Nathan, P.C.; Tissing, W.J.E.; Shankar, S.; Sieswerda, E.; et al. Recommendations for Cardiomyopathy Surveillance for Survivors of Childhood Cancer: A Report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015, 16, e123–e136. [Google Scholar] [CrossRef]
- Green, D.M.; Nolan, V.G.; Goodman, P.J.; Whitton, J.A.; Srivastava, D.K.; Leisenring, W.M.; Neglia, J.P.; Sklar, C.A.; Kaste, S.C.; Hudson, M.M.M.; et al. The Cyclophosphamide Equivalent Dose as an Approach for Quantifying Alkylating Agent Exposure: A Report from the Childhood Cancer Survivor Study. Pediatr. Blood Cancer 2014, 61, 53–67. [Google Scholar] [CrossRef] [Green Version]




| Protocol I | Protocol II | All | |
|---|---|---|---|
| No. of patients | 21 | 25 | 46 |
| Median age, years (range) | 10, 9 (2.2–18.5) | 9, 2 (4.3–19.8) | 10, 3 (2.2–19.8) |
| Male:Female | 12:9 | 13:12 | 25:21 |
| “Resected” tumor at diagnosis | 12 | 14 | 26 |
| Histological Classification | |||
| Burkitt L | 10 | 15 | 25 |
| Precursor B lymphoblastic L | 3 | 2 | 5 |
| MALT L | 3 | 1 | 4 |
| DLBCL | 3 | 5 | 8 |
| ALCL | 2 | 0 | 2 |
| Follicular lymphoma | 0 | 1 | 1 |
| Not-otherwised-specified high–grade B lymphoma | 0 | 1 | 1 |
| Relapses/failure to induction | 5 | 2 | 7 |
| Death related to L | 1 | 0 | 1 |
| Death for other causes | 2 | 1 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiavello, E.; Spreafico, F.; Barretta, F.; Meraviglia, G.; Biassoni, V.; Terenziani, M.; Boschetti, L.; Gattuso, G.; Chiaravalli, S.; Bergamaschi, L.; et al. Ultra Short Course Chemotherapy for Early-Stage Non-Hodgkin’s Lymphoma in Children. Children 2022, 9, 1279. https://doi.org/10.3390/children9091279
Schiavello E, Spreafico F, Barretta F, Meraviglia G, Biassoni V, Terenziani M, Boschetti L, Gattuso G, Chiaravalli S, Bergamaschi L, et al. Ultra Short Course Chemotherapy for Early-Stage Non-Hodgkin’s Lymphoma in Children. Children. 2022; 9(9):1279. https://doi.org/10.3390/children9091279
Chicago/Turabian StyleSchiavello, Elisabetta, Filippo Spreafico, Francesco Barretta, Giulia Meraviglia, Veronica Biassoni, Monica Terenziani, Luna Boschetti, Giovanna Gattuso, Stefano Chiaravalli, Luca Bergamaschi, and et al. 2022. "Ultra Short Course Chemotherapy for Early-Stage Non-Hodgkin’s Lymphoma in Children" Children 9, no. 9: 1279. https://doi.org/10.3390/children9091279
APA StyleSchiavello, E., Spreafico, F., Barretta, F., Meraviglia, G., Biassoni, V., Terenziani, M., Boschetti, L., Gattuso, G., Chiaravalli, S., Bergamaschi, L., Puma, N., Sironi, G., Nigro, O., Podda, M., Meazza, C., Casanova, M., Ferrari, A., Luksch, R., & Massimino, M. (2022). Ultra Short Course Chemotherapy for Early-Stage Non-Hodgkin’s Lymphoma in Children. Children, 9(9), 1279. https://doi.org/10.3390/children9091279







