Double-Inlet Left Ventricle
Abstract
:1. Introduction
2. Pathologic Anatomy
3. Pathophysiology
4. Clinical Features
5. Chest X-ray
6. Electrocardiogram
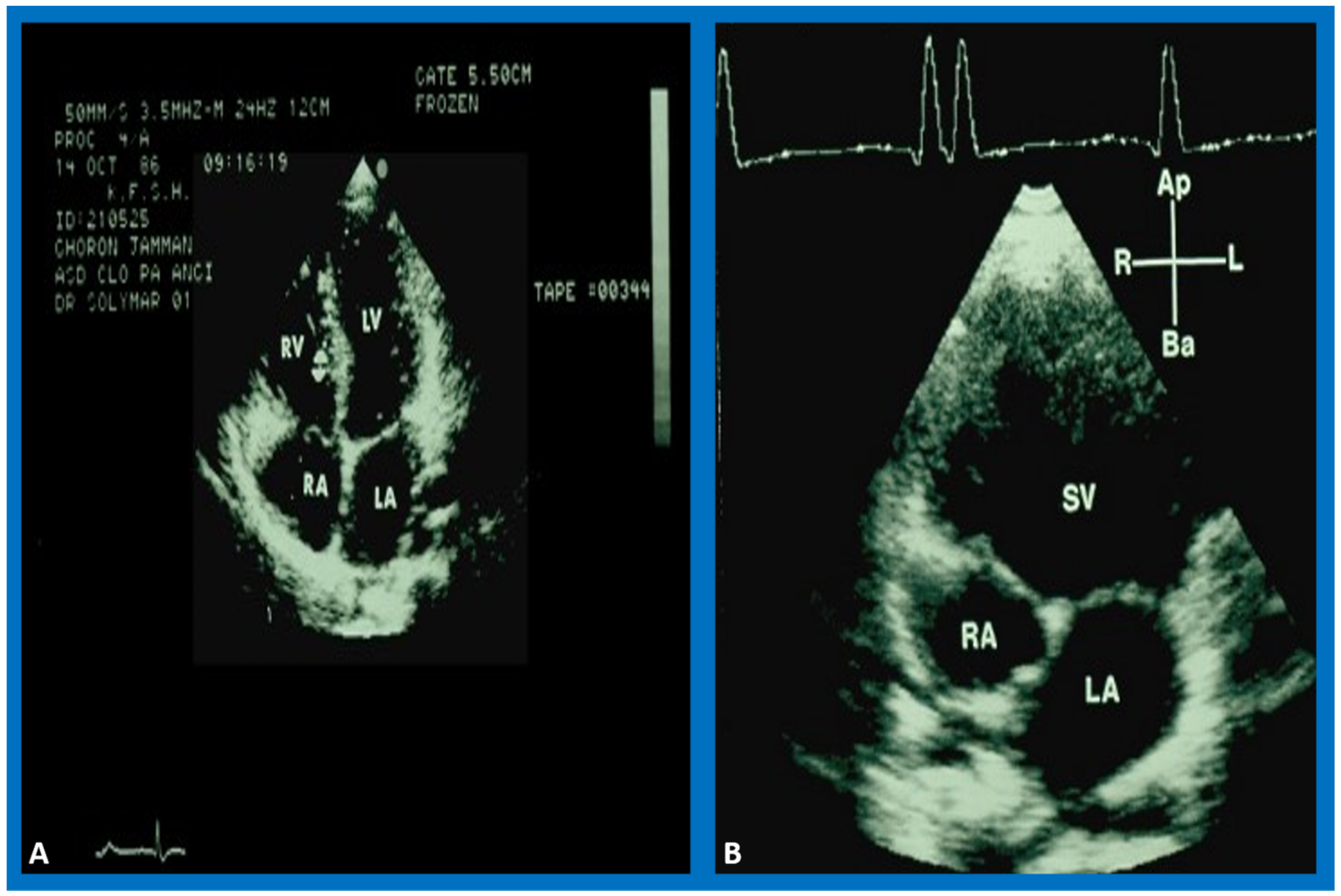
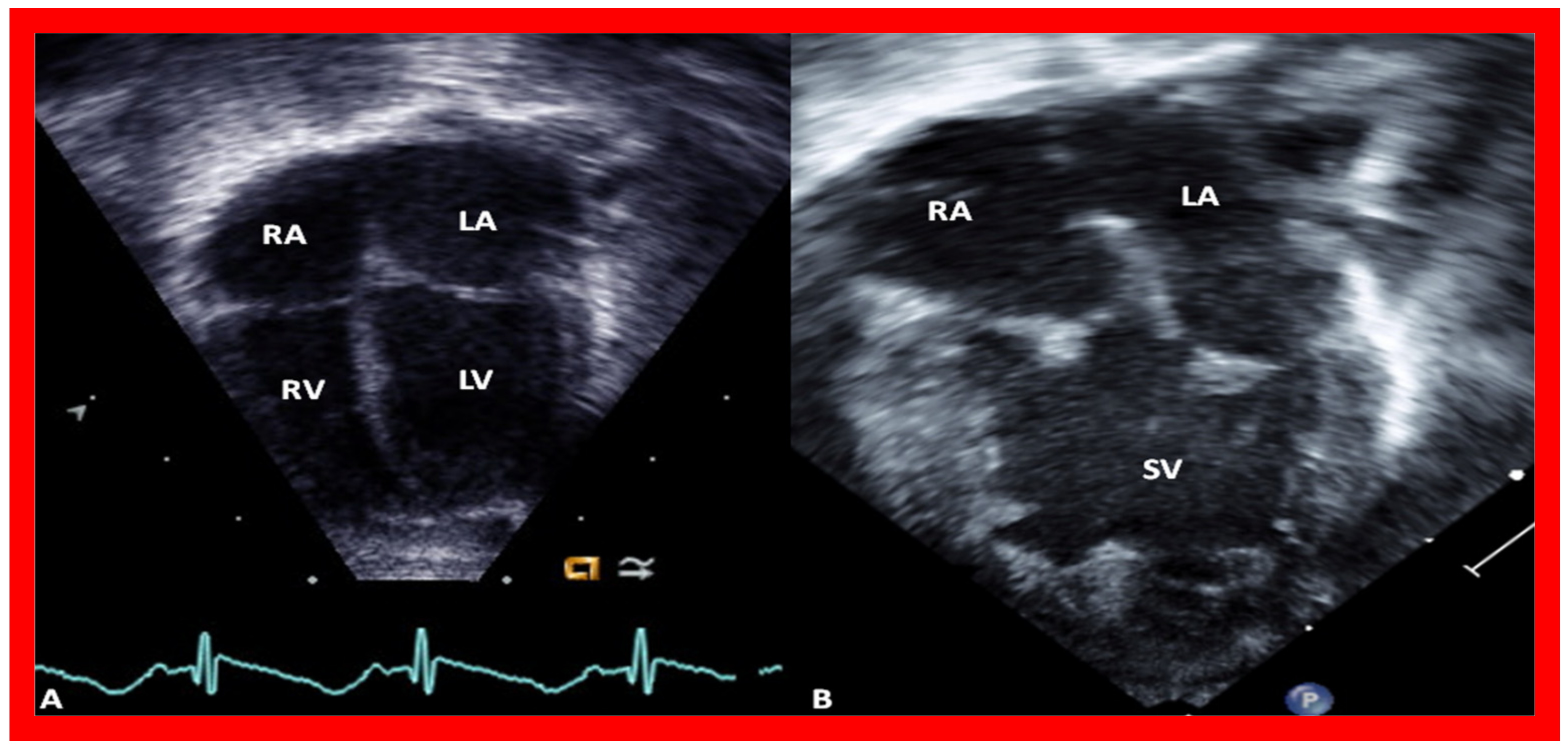
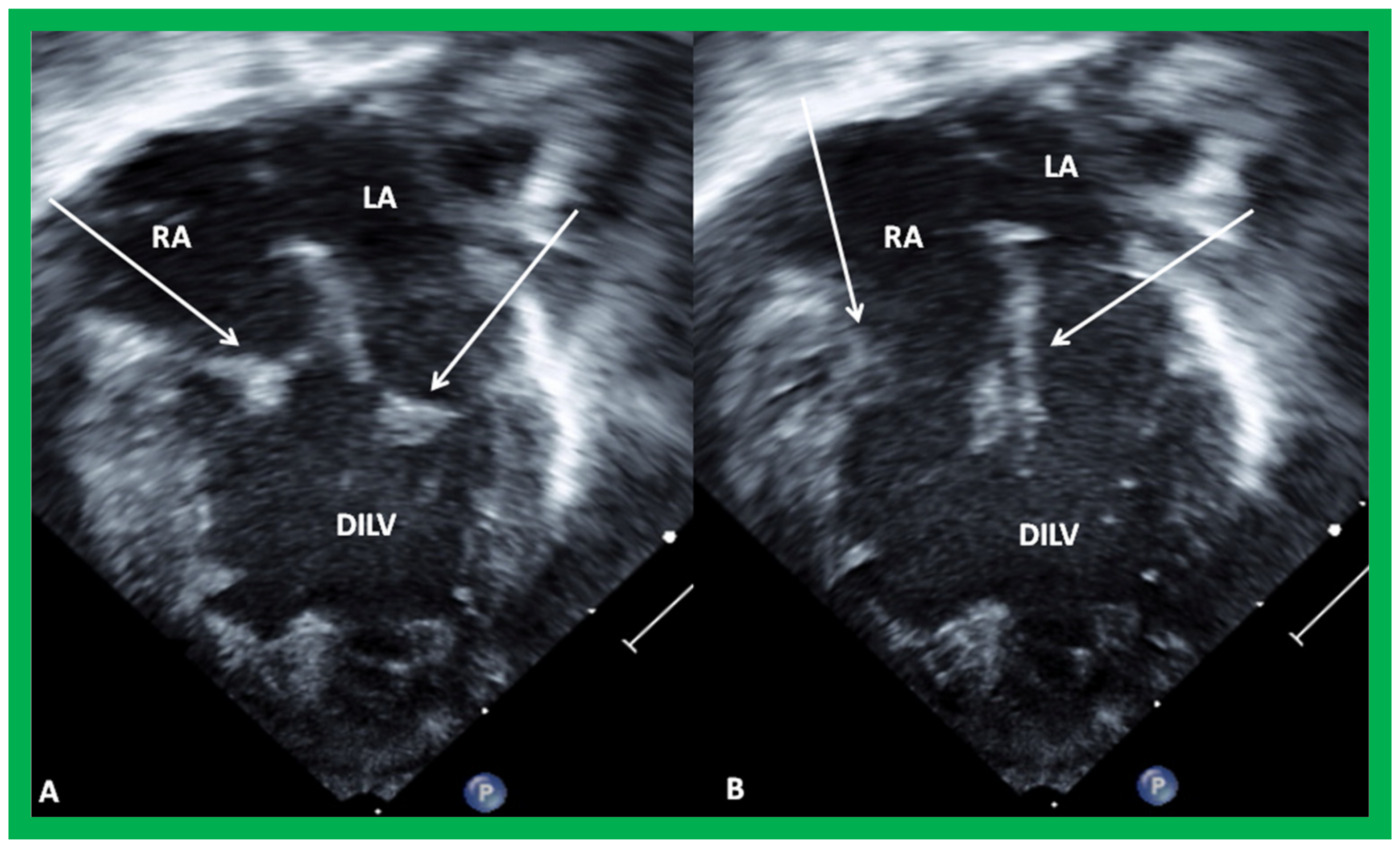
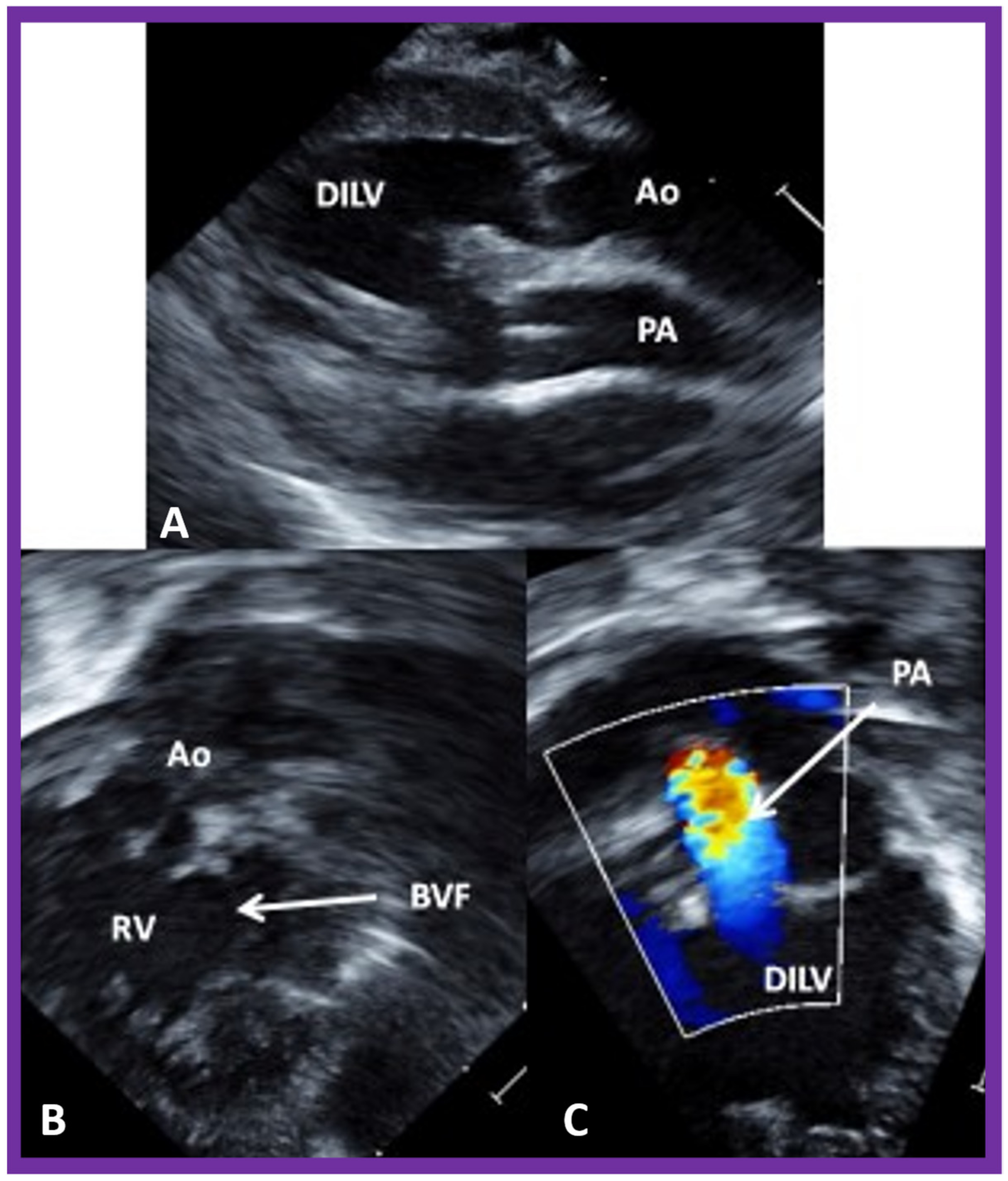
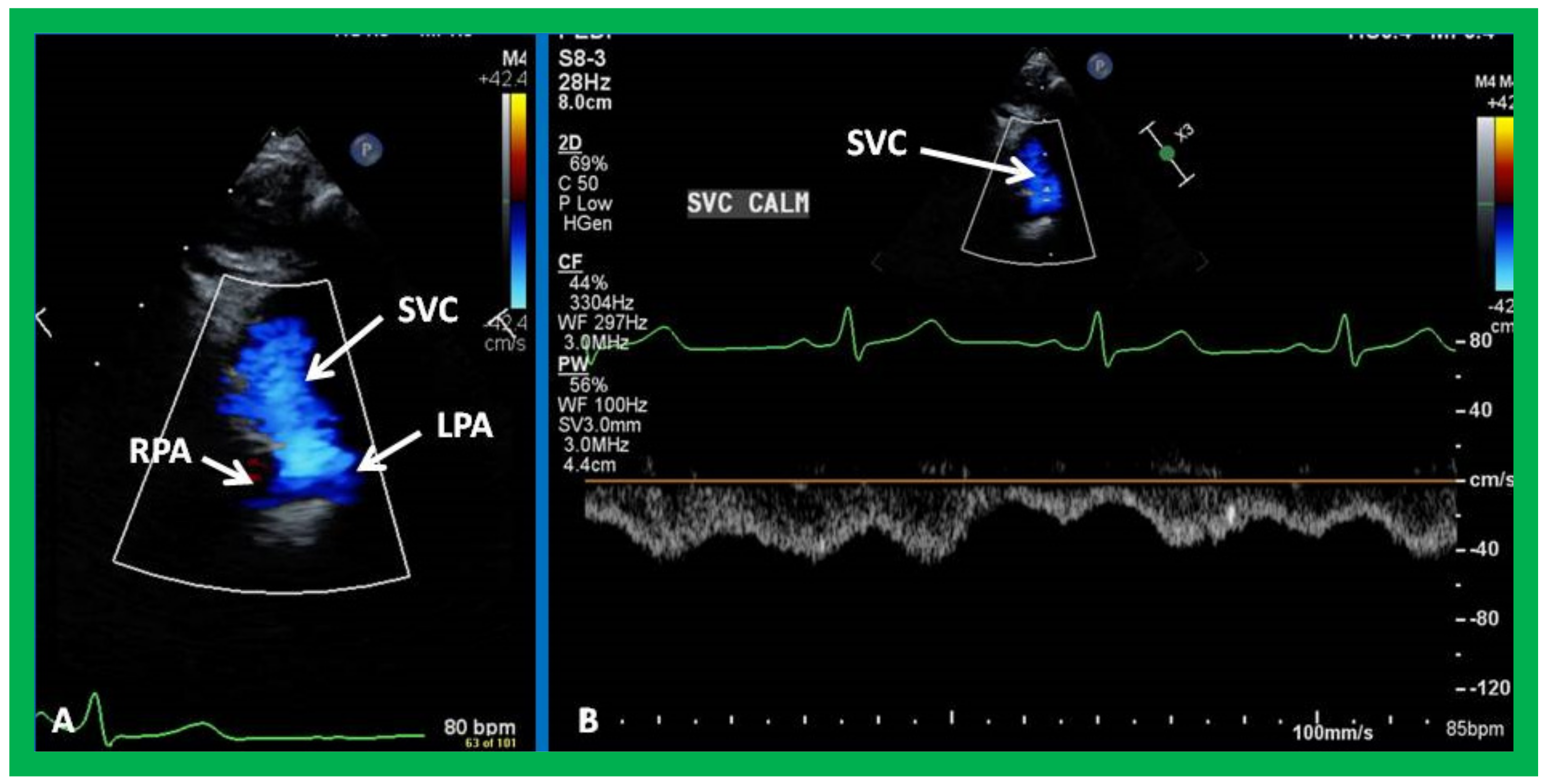
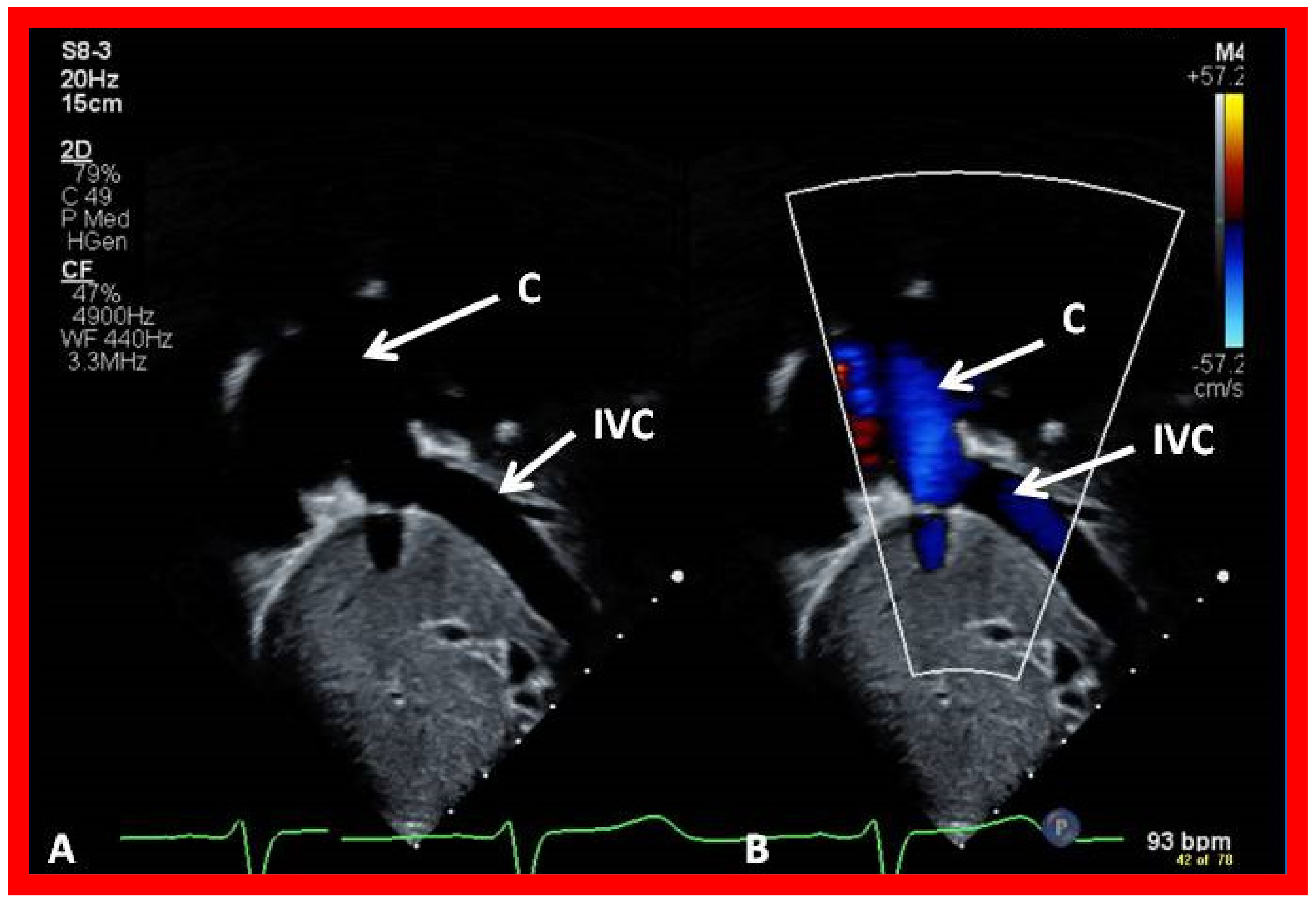
7. Echocardiogram
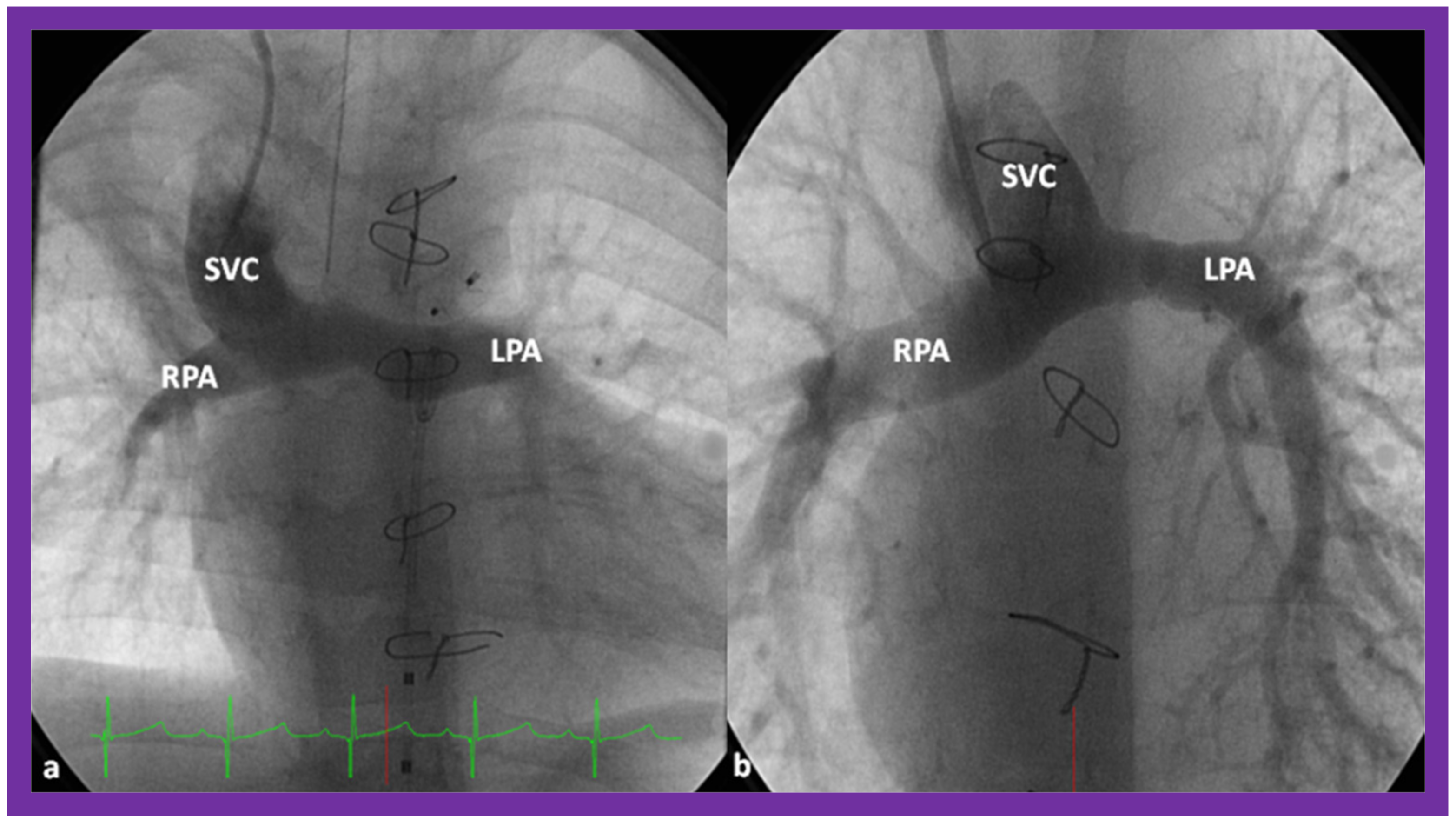
8. Other Imaging Studies
9. Comparison of Different Diagnostic Methods
10. Therapy
10.1. Stage I
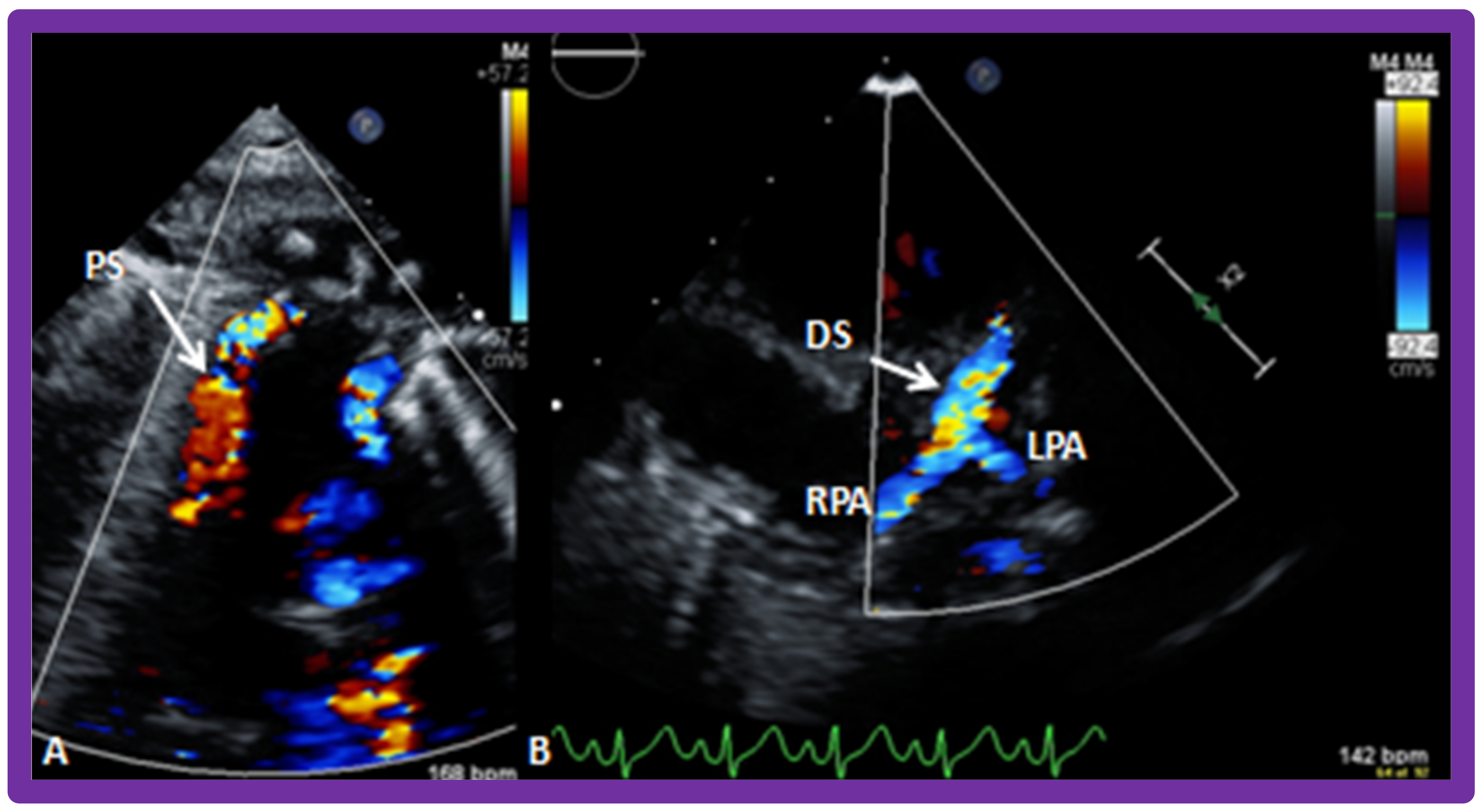
10.1.1. Reduced Pulmonary Blood Flow
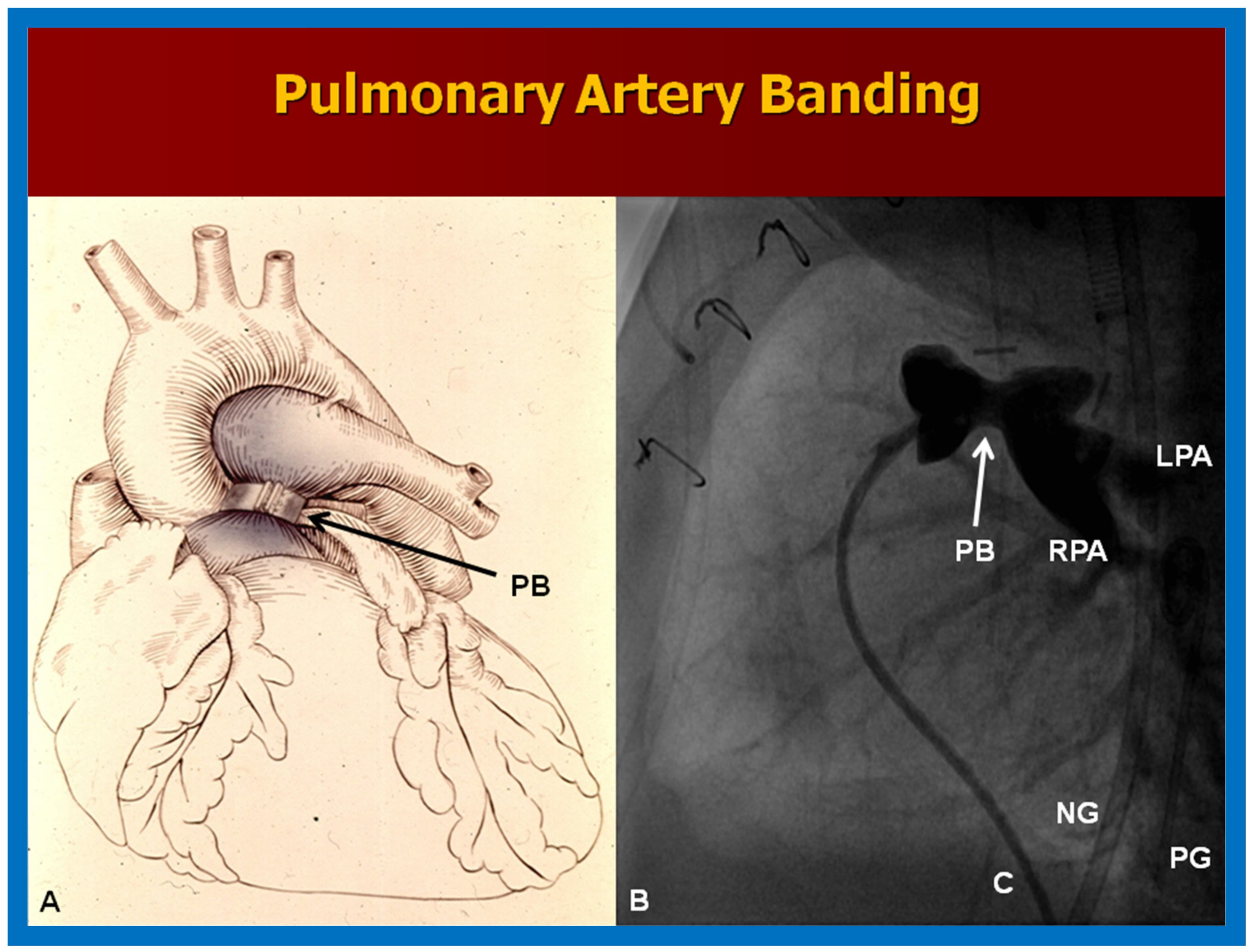
10.1.2. Elevated Pulmonary Blood Flow
10.1.3. Adequate Pulmonary Blood Flow
10.1.4. Inter-Atrial Obstruction
10.1.5. Inter-Ventricular Obstruction
10.1.6. Aortic Arch Obstruction
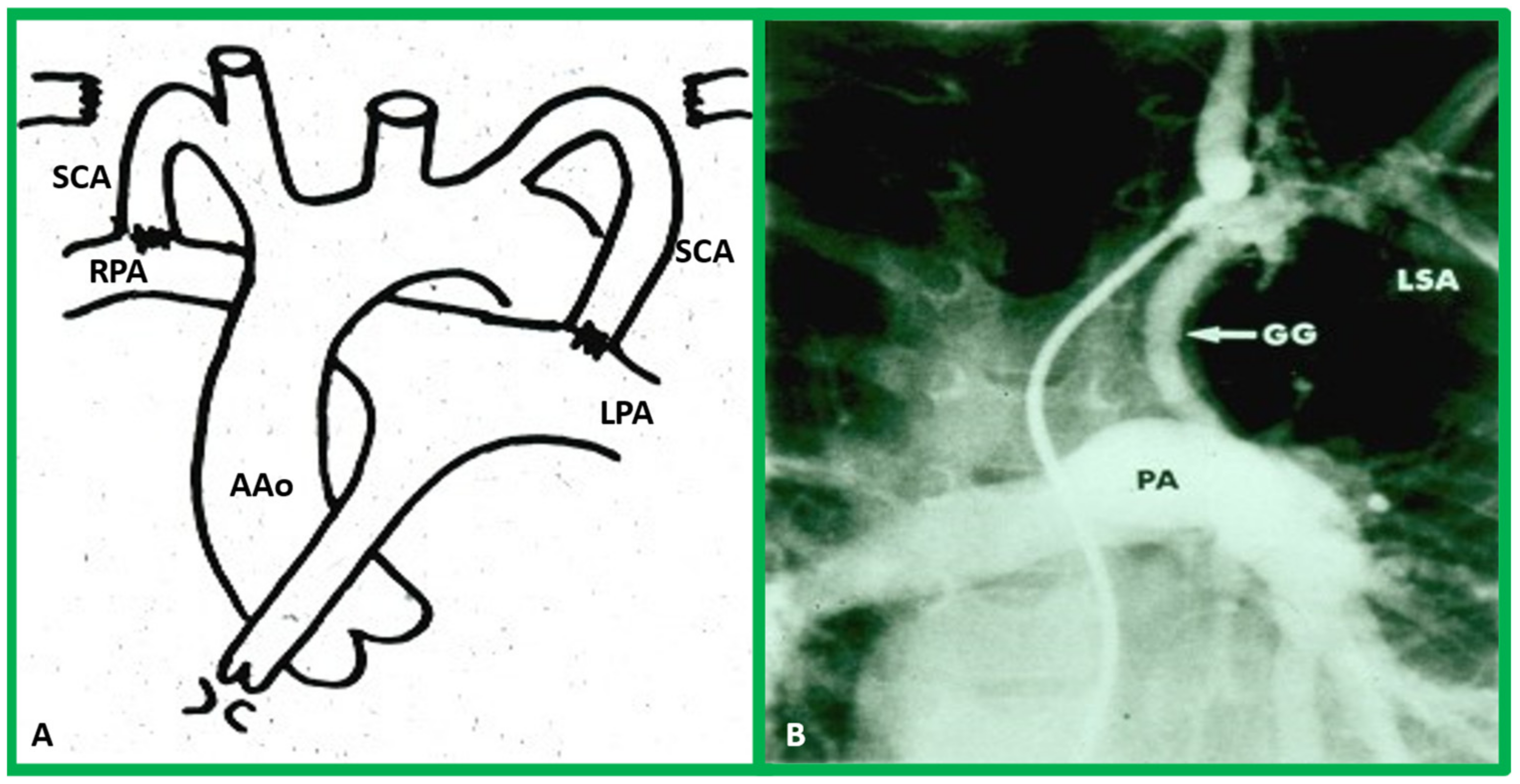
Aortic Arch Interruption
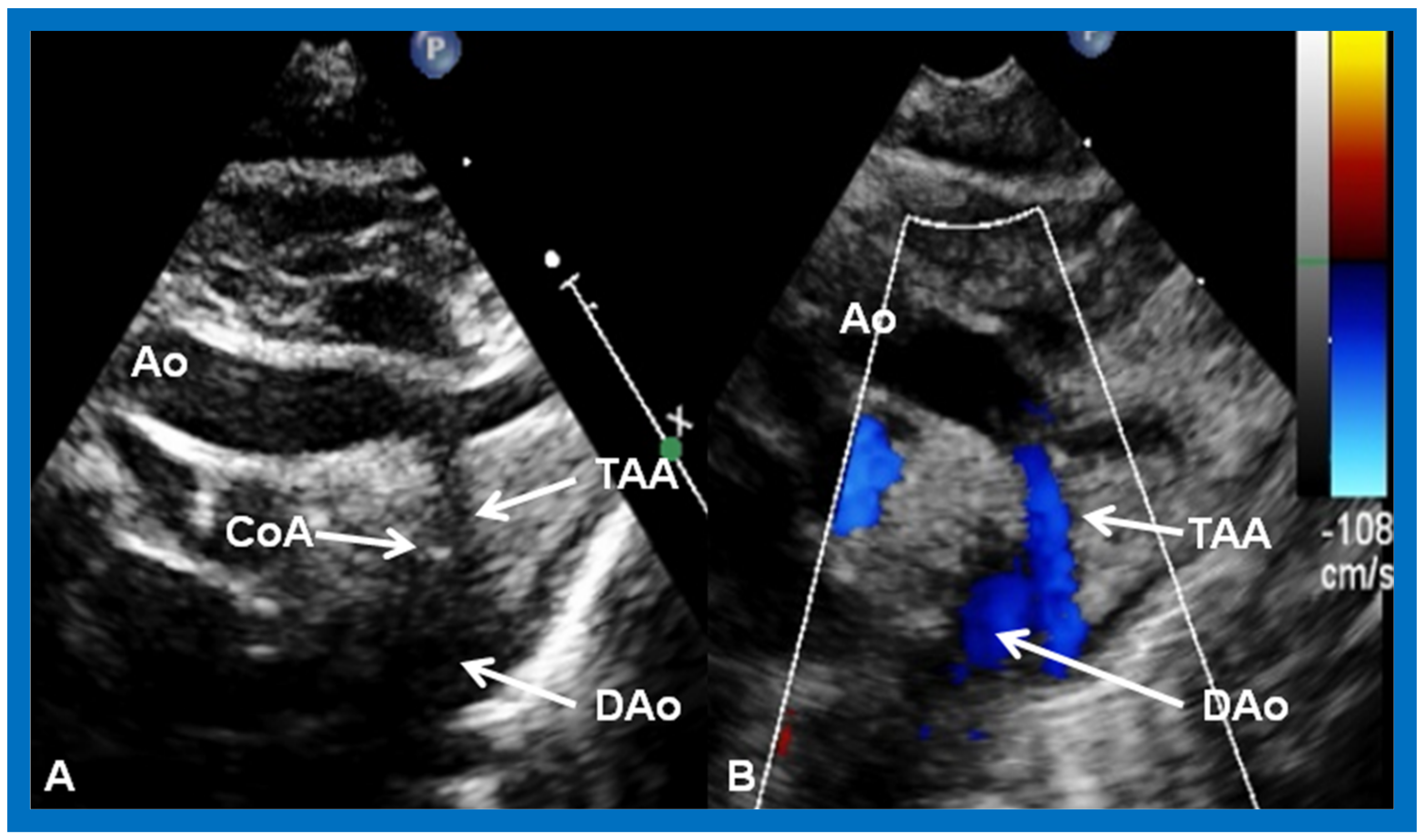
Aortic Coarctation
10.2. Stage II
10.3. Inter-Stage Issues
10.4. Stage III
10.4.1. Stage IIIA
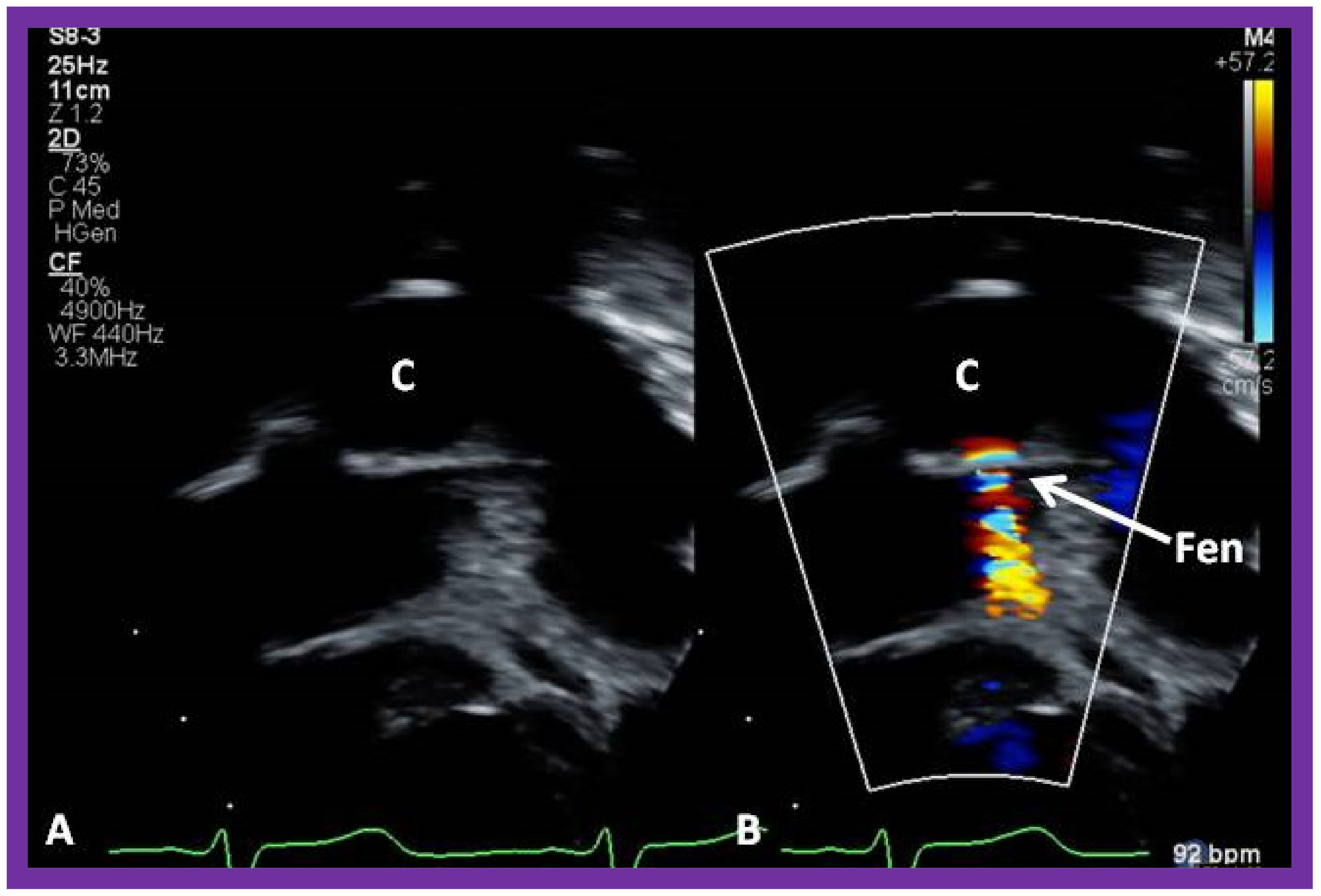
10.4.2. Stage IIIB
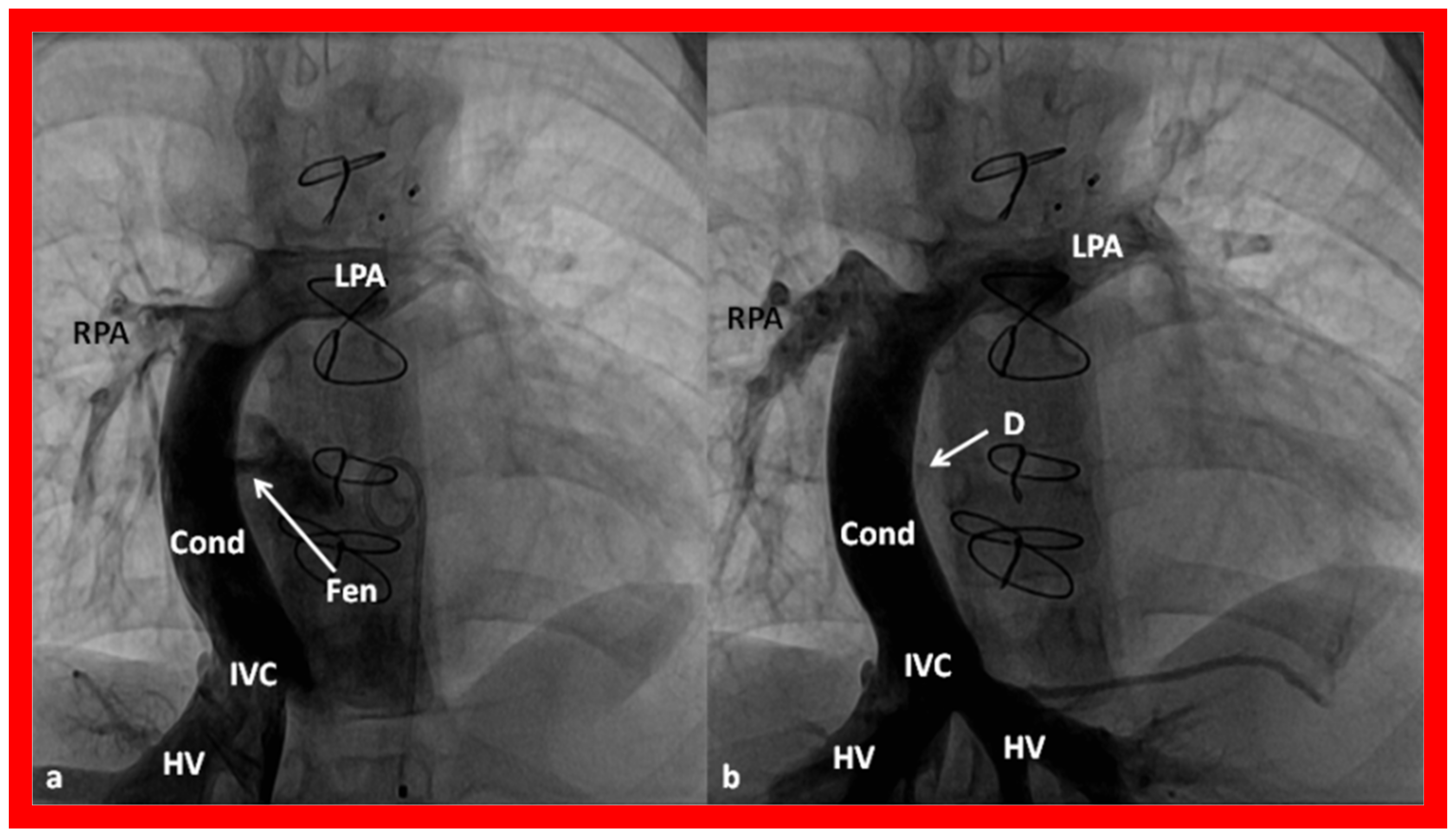
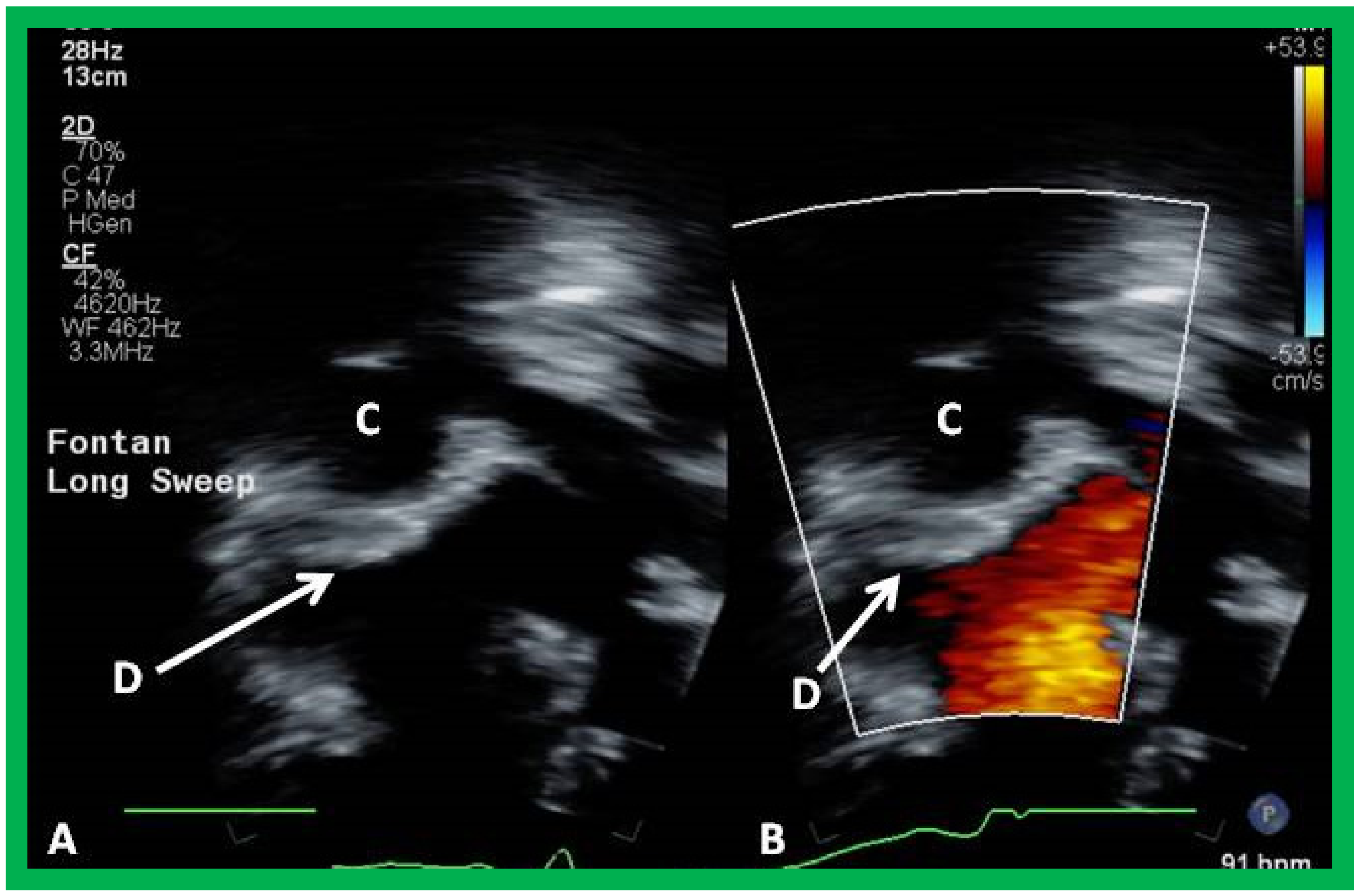
11. Post-Fontan Follow-Up
12. Summary and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, P.S. Diagnosis and management of cyanotic congenital heart disease: Part II. Indian J. Pediatr. 2009, 76, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S. Other cyanotic heart defects in the neonate. In Perinatal Cardiology: A Multidisciplinary Approach; Rao, P.S., Vidyasagar, D., Eds.; Cardiotext Publishing: Minneapolis, MN, USA, 2015; Chapter 37. [Google Scholar]
- Rao, P.S. Other cyanotic heart defects in the neonate. In A Multidisciplinary Approach to Perinatal Cardiology; Rao, P.S., Vidyasagar, D., Eds.; Cambridge Scholars Publishing: New Castle upon Tyne, UK, 2021; Volume 2, pp. 474–509. [Google Scholar]
- Rao, P.S. Cardiac malpositions including heterotaxy syndromes. In A Multidisciplinary Approach to Perinatal Cardiology; Rao, P.S., Vidyasagar, D., Eds.; Cambridge Scholars Publishing: New Castle upon Tyne, UK, 2021; Volume 2, pp. 432–466. [Google Scholar]
- Rao, P. Subaortic obstruction after pulmonary artery banding in patients with tricuspid atresia and double-inlet left ventricle and ventriculoarterial discordance. J. Am. Coll. Cardiol. 1991, 18, 1585–1586. [Google Scholar] [CrossRef]
- Rao, P.S. Echocardiographic evaluation of neonates with suspected heart disease. In Perinatal Cardiology: A Multidisciplinary Approach.; Rao, P.S., Vidyasagar, D., Eds.; Cardiotext Publishing: Minneapolis, MN, USA, 2015; Chapter 11. [Google Scholar]
- Imai, Y.; Hoshino, S.; Koh, Y.S.; Nakazawa, M.; Momma, K. Ventricular septation procedure for univentricular connection of left ventricular type. Semin. Thorac. Cardiovasc. Surg. 1994, 6, 48–55. [Google Scholar] [PubMed]
- Fontan, F.; Baudet, E. Surgical repair of tricuspid atresia. Thorax 1971, 26, 240–248. [Google Scholar] [CrossRef]
- Kreutzer, G.; Bono, H.; Galindez, E. Una operacion para la correccion de la atresia tricuspidea. In Proceedings of the Ninth Argentinean Congress of Cardiology, Buenos Aires, Argentina, 31 October–6 November 1971. [Google Scholar]
- Chopra, P.S.; Rao, P. Corrective surgery for tricuspid atresia: Which modification of Fontan-Kreutzer procedure should be used? A review. Am. Heart J. 1992, 123, 758–767. [Google Scholar] [CrossRef]
- Rao, P.S.; Chopra, P.S. Modification of fontan-kreutzer procedure for tricuspid atresia: Can a choice be made? In Tricuspid Atresia, 2nd ed.; Rao, P.S., Ed.; Futura Publishing Co.: Mount Kisco, NY, USA, 1992; pp. 361–375. [Google Scholar]
- Rao, P.S. Fontan operation: Indications, short- and long-term outcomes. Indian J. Pediatr. 2015, 82, 1147–1156. [Google Scholar] [CrossRef]
- Rao, P.S. Pediatric tricuspid atresia. Medscape Drugs Diseases. Available online: http://emedicine.medscape.com/article/900832-overview (accessed on 29 July 2022).
- Rao, P.S. Fontan operation: A comprehensive review. In Fontan Surgery; Khan, I., Ed.; InTechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Rao, P.S. Single ventricle—A comprehensive review. Children 2021, 8, 441. [Google Scholar] [CrossRef]
- De Leval, M.R.; Kilner, P.; Gewilling, M. Total cavopulmonary connection: A logical alternative to atriopulmonary connection for complex Fontan operation. J. Thorac. Cardiovasc. Surg. 1988, 96, 682–695. [Google Scholar] [CrossRef]
- Rao, P.S.; Covitz, W.; Chopra, P.S. Principles of palliative management of patients with tricuspid atresia. In Tricuspid Atresia, 2nd ed.; Rao, P.S., Ed.; Futura Publishing Co.: Mount Kisco, NY, USA, 1992; pp. 297–320. [Google Scholar]
- de Leval, M.; McKay, R.; Jones, M. Modified blalock-taussig shunt: Use of subclavian orifice as a flow regulator in prosthetic systemic-pulmonary artery shunts. J. Thorac. Cardiovasc. Surg. 1981, 18, 112–119. [Google Scholar] [CrossRef]
- Siblini, G.; Rao, P.S.; Singh, G.K.; Tinker, K.; Balfour, I.C. Transcatheter management of neonates with pulmonary atresia and intact ventricular septum. Catheter. Cardiovasc. Diagn. 1997, 42, 395–402. [Google Scholar] [CrossRef]
- Gibbs, J.L.; Orhan, U.; Blackburn, M.E.C. Fate of stented arterial duct. Circulation 1999, 99, 2621–2625. [Google Scholar] [CrossRef]
- Alwi, M.; Choo, K.; Latiff, H.A.; Kandavello, G.; Samion, H.; Mulyadi, M. Initial results and medium-term follow-up of stent implantation of patent ductus arteriosus in duct-dependent pulmonary circulation. J. Am. Coll. Cardiol. 2004, 44, 438–445. [Google Scholar] [CrossRef]
- Rao, P.S.; Brais, M. Balloon pulmonary valvuloplasty for congenital cyanotic heart defects. Am. Heart J. 1988, 115, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Wilson, A.D.; Thapar, M.K.; Brais, M. Balloon pulmonary valvuloplasty in the management of cyanotic congenital heart defects. Catheter. Cardiovasc. Diagn. 1992, 25, 16–24. [Google Scholar] [CrossRef]
- Rao, P.S. Pulmonary valve disease: Pulmonary valve in cyanotic heart defects with pulmonary oligemia. In Interventions in Structural, Valvular and Congenital Heart Disease; Sievert, H., Qureshi, S.A., Wilson, N., Hijazi, Z., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 297–308. [Google Scholar]
- Blalock, A.; Taussig, H.B. The surgical treatment of malformations of the heart in which there is pulmonary stenosis or atresia. J. Am. Med. Assoc. 1945, 128, 189–194. [Google Scholar] [CrossRef]
- Rao, P.S. Echocardiography in the diagnosis and management of tricuspid atresia. Appl. Sci. 2021, 11, 9472. [Google Scholar] [CrossRef]
- Rao, P.S. Congenital heart disease. In Conn’s Current Therapy; Rakel, R.E., Ed.; Saunders Co.: Philadelphia, PA, USA, 1989; pp. 201–213. [Google Scholar]
- Muller, W.H.; Danimann, J.F. The treatment of certain congenital malformations of the heart by the creation of pulmonic stenosis to reduce pulmonary hypertension and excessive pulmonary blood flow; a preliminary report. Surg. Gynecol. Obstet. 1952, 95, 213–219. [Google Scholar]
- Rashkind, W.J.; Miller, W.W. Creation of an atrial septal defect without thoracotomy. J. Am. Med. Assoc. 1966, 196, 991–992. [Google Scholar] [CrossRef]
- Rao, P.S. Neonatal catheter interventions. In Cardiac Catheterization and Imaging (From Pediatrics to Geriatrics); Vijayalakshmi, I.B., Ed.; Jaypee Publications: New Delhi, India, 2015; pp. 388–432. [Google Scholar]
- Rao, P.S.; Kulangara, R.J.; Moore, H.V.; Strong, W.B. Syndrome of single ventricle without pulmonary stenosis but with left atrio-ventricular vale atresia and interatrial obstruction. J. Thorac. Cardiovasc. Surg. 1981, 81, 127–132. [Google Scholar] [CrossRef]
- Stansel, H. A new operation for d-Loop transposition of the great vessels. Ann. Thorac. Surg. 1975, 19, 565–567. [Google Scholar] [CrossRef]
- Kaye, M.P. Anatomic correction of transposition of great arteries. Mayo Clin. Proc. 1975, 50, 638–640. [Google Scholar] [PubMed]
- Damus, P.S. Correspondence. Ann. Thorac. Surg. 1975, 20, 724. [Google Scholar] [CrossRef]
- Celoria, G.C.; Patton, R.B. Congenital absence of the aortic arch. Am. Heart J. 1959, 58, 407–413. [Google Scholar] [CrossRef]
- Sharma, S.K.; Rao, P.S. Interrupted aortic arch. In The Encyclopedic Reference of Molecular Mechanisms of Disease; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- McCrindle, B.W.; Tchervenkov, C.I.; Konstantinov, I.E.; Williams, W.G.; Neirotti, R.A.; Jacobs, M.L.; Blackstone, E.H. Risk factors associated with mortality and interventions in 472 neonates with interrupted aortic arch: A congenital heart surgeons society study. J. Thorac. Cardiovasc. Surg. 2005, 129, 343–350. [Google Scholar] [CrossRef]
- Rao, P.S. Coarctation of the aorta. In Secondary Forms of Hypertension; Ram, C.V.S., Ed.; Seminars in Nephrology; Kurtzman, N.A., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1995; Volume 15, pp. 81–105. [Google Scholar]
- Crafoord, O.; Nylin, G. Congenital coarctation of the aorta and its surgical treatment. J. Thorac. Cardiovasc. Surg. 1945, 14, 347–361. [Google Scholar] [CrossRef]
- Gross, R.E. Surgical correction for coarctation of the aorta. Surgery 1945, 18, 673–678. [Google Scholar]
- van Heurn, L.W.; Wong, C.M.; Spiegelhalter, D.J. Surgical treatment of aortic coarctation in infants younger than three months: 1985 to 1990. Success of extended end-to-end arch aortoplasty. J. Thorac. Cardiovasc. Surg. 1994, 107, 74–85. [Google Scholar] [CrossRef]
- Rao, P.S. Current status of balloon angioplasty for neonatal and infant aortic coarctation. Prog. Pediatr. Cardiol. 2001, 14, 35–44. [Google Scholar] [CrossRef]
- Rao, P.S.; Jureidini, S.B.; Balfour, I.C.; Singh, G.K.; Chen, S.-C. Severe aortic coarctation in infants less than 3 months: Successful palliation by balloon angioplasty. J. Invasive Cardiol. 2003, 15, 203–208. [Google Scholar]
- Rao, P.S. Neonatal (and infant) coarctation of the aorta: Management challenges. In Research and Reports in Neonatology; Dovepress: Macclesfield, UK, 2020; Volume 10, pp. 11–22. [Google Scholar] [CrossRef]
- Hopkins, R.A.; Armstrong, S.E.; Serwer, G.A. Physiological rationale for a bidirectional cavopulmonary shunt. A versatile complement to the Fontan principle. J. Thorac. Cardiovasc. Surg. 1985, 90, 391–398. [Google Scholar] [CrossRef]
- Yarrabolu, T.R.; Douglas, W.I. Single ventricle physiology. In A Multidisciplinary Approach to Perinatal Cardiology; Rao, P.S., Vidyasagar, D., Eds.; Cambridge Scholars Publishing: New Castle upon Tyne, UK, 2021; Volume 2, pp. 616–645. [Google Scholar]
- Tweddell, J.S.; Hoffman, G.M.; Mussatto, K.A.; Fedderly, R.T.; Berger, S.; Jaquiss, R.D.B.; Ghanayem, N.S.; Frisbee, S.J.; Litwin, S.B. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: Lessons learned from 115 consecutive patients. Circulation 2002, 106, 82–89. [Google Scholar] [CrossRef]
- Yates, M.C.; Rao, P.S. Pediatric cardiac emergencies. Emerg. Med. 2013, 3, 164. [Google Scholar] [CrossRef]
- Bartram, U.; Grünenfelder, J.; Van Praagh, R. Causes of death after the modified norwood procedure: A study of 122 postmortem cases. Ann. Thorac. Surg. 1997, 64, 1795–1802. [Google Scholar] [CrossRef]
- Cashen, K.; Gupta, P.; Lieh-Lai, M.; Mastropietro, C. Infants with single ventricle physiology in the emergency department: Are physicians prepared? J. Pediatr. 2011, 159, 273–277.e1. [Google Scholar] [CrossRef]
- Puga, F.J.; Chiavarelli, M.; Hagler, D.J. Modifications of the fontan operation applicable to patients with left atrioventricular valve atresia or single atrioventricular valve. Circulation 1987, 76, III 53–III 60. [Google Scholar]
- Jonas, R.A.; Castaneda, A.R. Modified fontan procedure: Atrial baffle and systemic venous to pulmonary artery anastomotic techniques. J. Card. Surg. 1988, 3, 91–96. [Google Scholar] [CrossRef]
- Marcelletti, C.; Corno, A.; Giannico, S.; Marino, B. Inferior vena cava-pulmonary artery extra-cardiac conduit. A new form of right heart bypass. J. Thorac. Cardiovasc. Surg. 1990, 100, 228–232. [Google Scholar] [CrossRef]
- Marcelletti, C.; Iorio, F.S.; Abella, R.F. Late results of extra-cardiac Fontan repair. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annual. 1999, 2, 131–141. [Google Scholar] [CrossRef]
- Bridges, N.D.; Lock, J.E.; Castaneda, A.R. Baffle fenestration with subsequent transcatheter closure. Modification of the Fontan operation for patients at increased risk. Circulation 1990, 82, 1681–1689. [Google Scholar] [CrossRef]
- Laks, H.; Pearl, J.M.; Haas, G.S.; Drinkwater, D.C.; Milgalter, E.; Jarmakani, J.M.; Isabel-Jones, J.; George, B.L.; Williams, R.G. Partial fontan: Advantages of an adjustable interatrial communication. Ann. Thorac. Surg. 1991, 52, 1084–1095. [Google Scholar] [CrossRef]
- Rao, P.; Chandar, J.S.; Sideris, E.B. Role of inverted buttoned device in transcatheter occlusion of atrial septal defects or patent foramen ovale with right-to-left shunting associated with previously operated complex congenital cardiac anomalies. Am. J. Cardiol. 1997, 80, 914–921. [Google Scholar] [CrossRef]
- Goff, D.A.; Blume, E.D.; Gauvreau, K.; Mayer, J.E.; Lock, J.E.; Jenkins, K.J. Clinical advances in complex valvular disease: Outcome of fenestrated Fontan patients after closure: The first 10 years. Circulation 2000, 102, 2094–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudjemline, Y.; Bonnet, D.; Sidi, D.; Agnoletti, G. Closure of extracardiac Fontan fenestration by using the Amplatzer duct oc-cluder. Arch. Mal. Coeur Vaiss. 2005, 98, 449–454. [Google Scholar] [PubMed]
- Monagle, P.; Cochrane, A.; Roberts, R.; Manlhiot, C.; Weintraub, R.; Szechtman, B.; Hughes, M.; Andrew, M.; McCrindle, B.W.; Fontan Anticoagulation Study Group. A multicenter, randomized trial comparing heparin/warfarin and acetylsalicylic acid as primary thromboprophylaxis for 2 years after the fontan procedure in children. J. Am. Coll. Cardiol. 2011, 58, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S. Protein-losing enteropathy following the fontan operation (editorial). J. Invasive Cardiol. 2007, 19, 447–448. [Google Scholar] [PubMed]
- Wang-Giuffre, E.W.; Doshi, U.H. Cardiopulmonary exercise test as a tool in surveillance after Fontan operation. Vessel Plus 2022, 6, 29. [Google Scholar] [CrossRef]
- Rao, P.S. What an adult cardiologist should know about cyanotic congenital heart disease? J. Cardiovasc. Dis. Diagn. 2013, 1, 104. [Google Scholar] [CrossRef]
- Brancaccio, G.; Carotti, A.; D’Argenio, P. Protein-losing enteropathy after Fontan surgery: Resolution after cardiac trans-plantation. J. Heart Lung Transpl. 2003, 22, 484–486. [Google Scholar] [CrossRef]
- Gamba, A.; Merlo, M.; Fiocchi, R.; Terzi, A.; Mammana, C.; Sebastiani, R.; Ferrazzi, P. Heart transplantation in patients with previous Fontan operations. J. Thorac. Cardiovasc. Surg. 2004, 127, 555–562. [Google Scholar] [CrossRef]
- Jayakumar, K.A.; Addonizio, L.J.; Kichuk-Chrisant, M.R.; Galantowicz, M.E.; Lamour, J.M.; Quaegebeur, J.M.; Hsu, D.T. Cardiac transplantation after the Fontan or Glenn procedure. J. Am. Coll. Cardiol. 2004, 44, 2065–2072. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, P.S. Double-Inlet Left Ventricle. Children 2022, 9, 1274. https://doi.org/10.3390/children9091274
Rao PS. Double-Inlet Left Ventricle. Children. 2022; 9(9):1274. https://doi.org/10.3390/children9091274
Chicago/Turabian StyleRao, P. Syamasundar. 2022. "Double-Inlet Left Ventricle" Children 9, no. 9: 1274. https://doi.org/10.3390/children9091274
APA StyleRao, P. S. (2022). Double-Inlet Left Ventricle. Children, 9(9), 1274. https://doi.org/10.3390/children9091274





