Internal Ileal Diversion as Treatment for Progressive Familial Intrahepatic Cholestasis Type 1-Associated Graft Inflammation and Steatosis after Liver Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Genetic Analysis
3. Case Reports
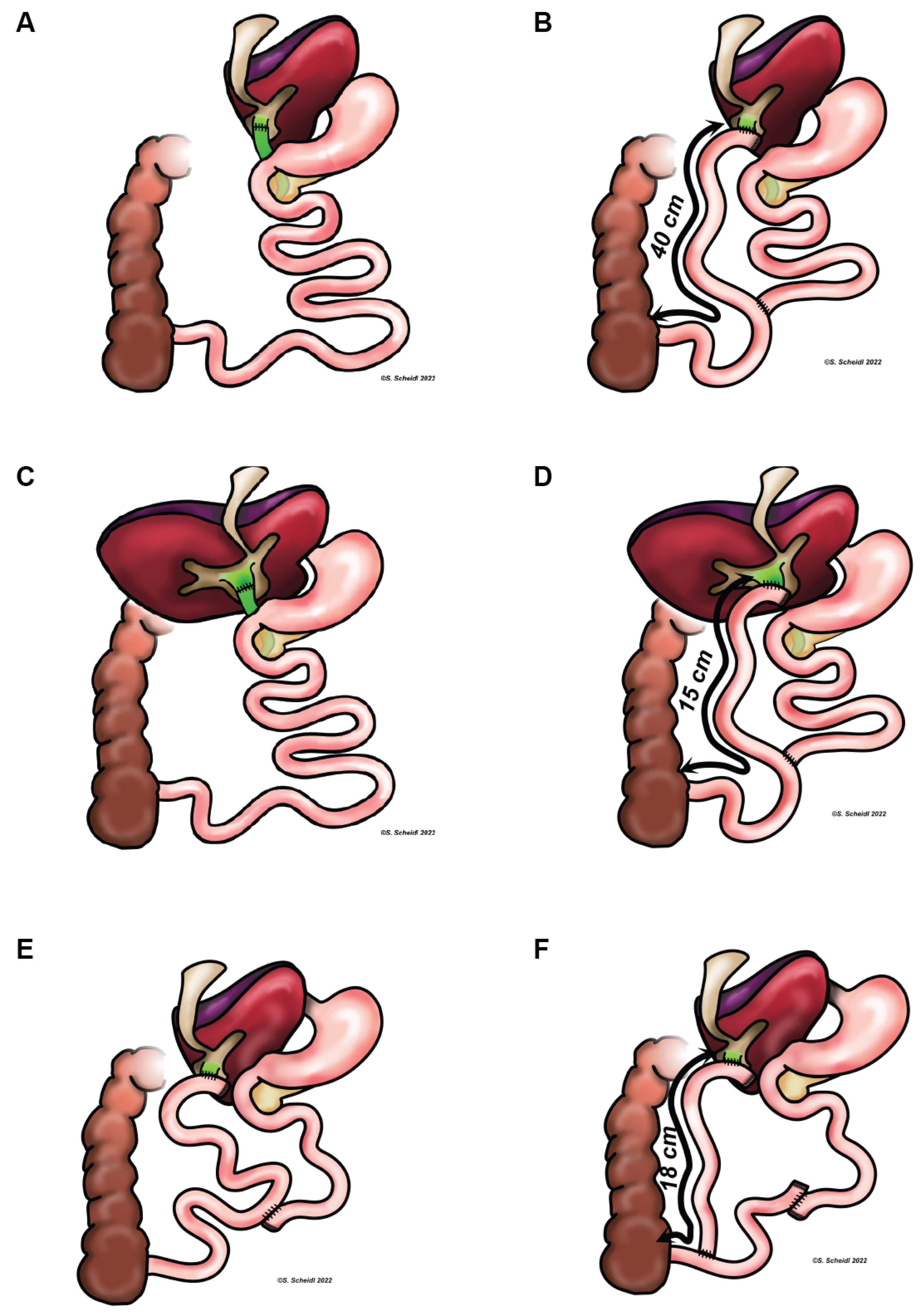
3.1. Case 1
3.2. Case 2
3.3. Case 3
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | alanine-aminotransferase |
| AST | aspartate-aminotransferase |
| ATP | adenosine triphosphate |
| GGT | γ-glutamyltransferase |
| IBAT | ileal bile acid transporter |
| LDLT | living donor liver transplantation |
| LT | liver transplantation |
| PEBD | partial external biliary diversion |
| PFIC | Progressive Familial Intrahepatic cholestasis |
| PIBD | partial internal biliary diversion |
| SBD | surgical biliary diversion |
| TIBD | total internal biliary diversion |
References
- Bull, L.N.; van Eijk, M.J.; Pawlikowska, L.; De Young, J.A.; Juijn, J.A.; Liao, M.; Klomp, L.W.; Lomri, N.; Berger, R.; Scharschmidt, B.R.; et al. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat. Genet. 1998, 18, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Clayton, R.J.; Iber, F.L.; Ruebner, B.H.; McKusick, V.A. Byler disease: Fatal familial intrahepatic cholestasis in an Amish kindred. Am. J. Dis. Child. 1969, 117, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Bull, L.N.; Thompson, R.J. Progressive Familial Intrahepatic Cholestasis. Clin. Liver Dis. 2018, 22, 657–669. [Google Scholar] [CrossRef] [PubMed]
- van Wessel, D.B.E.; Thompson, R.J.; Gonzales, E.; Jankowska, I.; Shneider, B.L.; Sokal, E.; Grammatikopoulos, T.; Kadaristiana, A.; Jacquemin, E.; Spraul, A.; et al. Impact of Genotype, Serum Bile Acids, and Surgical Biliary Diversion on Native Liver Survival in FIC1 Deficiency. Hepatology 2021, 74, 892–906. [Google Scholar] [CrossRef]
- Vitale, G.; Gitto, S.; Vukotic, R.; Raimondi, F.; Andreone, P. Familial intrahepatic cholestasis: New and wide perspectives. Dig. Liver Dis. 2019, 51, 922–933. [Google Scholar] [CrossRef]
- Stapelbroek, J.M.; Peters, T.A.; Van Beurden, D.H.A.; Curfs, J.H.A.J.; Joosten, A.; Beynon, A.J.; van Leeuwen, B.M.; van der Velden, L.M.; Bull, L.; Oude Elferink, R.P.; et al. ATP8B1 is essential for maintaining normal hearing. Proc. Natl. Acad. Sci. USA 2009, 106, 9709–9714. [Google Scholar] [CrossRef]
- Nagasaka, H.; Yorifuji, T.; Kosugiyama, K.; Egawa, H.; Kawai, M.; Murayama, K.; Hasegawa, M.; Sumazaki, R.; Tsubaki, J.; Kikuta, H.; et al. Resistance to parathyroid hormone in two patients with familial intrahepatic cholestasis: Possible involvement of the ATP8B1 gene in calcium regulation via parathyroid hormone. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 404–409. [Google Scholar] [CrossRef]
- Li, L.; Deheragoda, M.; Lu, Y.; Gong, J.; Wang, J. Hypothyroidism Associated with ATP8B1 Deficiency. J. Pediatr. 2015, 167, 1334–1339. [Google Scholar] [CrossRef]
- Davit-Spraul, A.; Fabre, M.; Branchereau, S.; Baussan, C.; Gonzales, E.; Stieger, B.; Bernard, O.; Jacquemin, E. ATP8B1 and ABCB11 Analysis in 62 Children with Normal Gamma-Glutamyl Transferase Progressive Familial Intrahepatic Cholestasis (PFIC): Phenotypic Differences Between PFIC1 and PFIC2 and Natural History. Hepatology 2010, 51, 1645–1655. [Google Scholar] [CrossRef]
- Bull, L.N.; Pawlikowska, L.; Strautnieks, S.; Jankowska, I.; Czubkowski, P.; Dodge, J.L.; Emerick, K.; Wanty, C.; Wali, S.; Blanchard, S.; et al. Outcomes of surgical management of familial intrahepatic cholestasis 1 and bile salt export protein deficiencies. Hepatol. Commun. 2018, 2, 515–528. [Google Scholar] [CrossRef]
- Ismail, H.; Kaliciński, P.; Markiewicz, M.; Jankowska, I.; Pawłowska, J.; Kluge, P.; Eliadou, E.; Kamiński, A.; Szymczak, M.; Drewniak, T.; et al. Treatment of progressive familial intrahepatic cholestasis: Liver transplantation or partial external biliary diversion. Pediatr. Transplant. 1999, 3, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Hollands, C.M.; Rivera-Pedrogo, F.J.; Gonzalez-Vallina, R.; Loret-de-Mola, O.; Nahmad, M.; Burnweit, C.A. Ileal exclusion for Byler’s disease: An alternative surgical approach with promising early results for pruritus. J. Pediatr. Surg. 1998, 33, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Arnell, H.; Bergdahl, S.; Papadogiannakis, N.; Nemeth, A.; Fischler, B. Preoperative observations and short-term outcome after partial external biliary diversion in 13 patients with progressive familial intrahepatic cholestasis. J. Pediatr. Surg. 2008, 43, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Verkade, H.J.; Thompson, R.J.; Arnell, H.; Fischler, B.; Gillberg, P.-G.; Mattsson, J.P.; Torfgård, K.; Lindström, E. Systematic Review and Meta-analysis: Partial External Biliary Diversion in Progressive Familial Intrahepatic Cholestasis. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, C.; Superina, R. Surgical diversion of enterohepatic circulation in pediatric cholestasis. Semin. Pediatr. Surg. 2020, 29, 150946. [Google Scholar] [CrossRef]
- Karpen, S.J.; Kelly, D.; Mack, C.; Stein, P. Ileal bile acid transporter inhibition as an anticholestatic therapeutic target in biliary atresia and other cholestatic disorders. Hepatol. Int. 2020, 14, 677–689. [Google Scholar] [CrossRef]
- Deeks, E.D. Odevixibat: First Approval. Drugs 2021, 81, 1781–1786. [Google Scholar] [CrossRef]
- Egawa, H.; Yorifuji, T.; Sumazaki, R.; Kimura, A.; Hasegawa, M.; Tanaka, K. Intractable diarrhea after liver transplantation for Byler’s disease: Successful treatment with bile adsorptive resin. Liver Transplant. 2002, 8, 714–716. [Google Scholar] [CrossRef]
- Miyahawa-Hayashino, A.; Egawa, H.; Yorifuji, T.; Hasegawa, M.; Haga, H.; Tsuruyama, T.; Wen, M.C.; Sumazaki, R.; Manabe, T.; Uemoto, S. Allograft steatohepatitis in progressive familial intrahepatic cholestasis type 1 after living donor liver transplantation. Liver Transplant. 2009, 15, 610–618. [Google Scholar] [CrossRef]
- Berumen, J.; Feinberg, E.; Todo, T.; Bonham, C.A.; Concepcion, W.; Esquivel, C. Complications Following Liver Transplantation for Progressive Familial Intrahepatic Cholestasis. Dig. Dis. Sci. 2014, 59, 2649–2652. [Google Scholar] [CrossRef]
- Alrabadi, L.S.; Morotti, R.A.; Valentino, P.L.; Rodriguez-Davalos, M.I.; Ekong, U.D.; Emre, S.H. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: A single-center experience. Pediatr. Transplant. 2018, 22, e13184. [Google Scholar] [CrossRef] [PubMed]
- Henkel, S.A.F.; Salgado, C.M.; Reyes-Mugica, M.; Soltys, K.A.; Strauss, K.; Mazariegos, G.V.; Squires, R.H.; McKiernan, P.J.; Zhang, X.; Squires, J.E. Long-term liver transplant outcomes for progressive familial intrahepatic cholestasis type 1: The Pittsburgh experience. Pediatr. Transplant. 2021, 25, e14108. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Sonoda, M.; Ogawa, E.; Ito, S.; Togawa, T.; Hayashi, H.; Okajima, H.; Uemoto, S. Long-term Outcomes of Living-donor Liver Transplantation for Progressive Familial Intrahepatic Cholestasis Type 1. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Hang, C.; Jin, Y.; Luo, Y.; Feng, M.; Zhou, T.; Zhu, J.; Zhang, J.; Liu, Y.; Xia, Q. Long-Term Results of Pediatric Liver Transplantation for Progressive Familial Intrahepatic Cholestasis. J. Clin. Med. 2022, 11, 4684. [Google Scholar] [CrossRef]
- Mali, V.P.; Fukuda, A.; Shigeta, T.; Uchida, H.; Hirata, Y.; Rahayatri, T.H.; Kanazawa, H.; Sasaki, K.; de Ville de Goyet, J.; Kasahara, M. Total internal biliary diversion during liver transplantation for type 1 progressive familial intrahepatic cholestasis: A novel approach. Pediatr. Transplant. 2016, 20, 981–986. [Google Scholar] [CrossRef]
- Shanmugam, N.; Menon, J.; Vij, M.; Rammohan, A.; Rajalingam, R.; Rela, M. Total Internal Biliary Diversion for Post-Liver Transplant PFIC-1-Related Allograft Injury. J. Clin. Exp. Hepatol. 2022, 12, 212–215. [Google Scholar] [CrossRef]
- Jankowska, I.; Pawłowska, J.; Szymczak, M.; Ismail, H.; Broniszczak, D.; Cielecka-Kuszyk, J.; Socha, P.; Jarzębicka, D.; Czubkowski, P. A Report of 2 Infant Siblings with Progressive Intrahepatic Familial Cholestasis Type 1 and a Novel Homozygous Mutation in the ATP8B1 Gene Treated with Partial External Biliary Diversion and Liver Transplant. Am. J. Case Rep. 2021, 22, e932374. [Google Scholar] [CrossRef]
- Knisely, A.S.; Houwen, R.H.J. Liver Steatosis and Diarrhea After Liver Transplantation for Progressive Familial Intrahepatic Cholestasis Type 1: Can Biliary Diversion Solve These Problems? J. Pediatr. Gastroenterol. Nutr. 2021, 72, 341–342. [Google Scholar] [CrossRef]
- Lykavieris, P.; van Mil, S.; Cresteil, D.; Fabre, M.; Hadchouel, M.; Klomp, L.; Bernard, O.; Jacquemin, E. Progressive familial intrahepatic cholestasis type 1 and extrahepatic features: No catch-up of stature growth, exacerbation of diarrhea, and appearance of liver steatosis after liver transplantation. J. Hepatol. 2003, 39, 447–452. [Google Scholar] [CrossRef]
- Emond, J.C.; Whitington, P.F. Selective surgical management of progressive familial intrahepatic cholestasis (Byler’s disease). J. Pediatr. Surg. 1995, 30, 1635–1641. [Google Scholar] [CrossRef]
- Oya, Y.; Sugawara, Y.; Honda, M.; Yoshii, D.; Isono, K.; Hayashida, S.; Yamamoto, H.; Inomata, Y. Living Donor Liver Transplantation for Progressive Familial Intrahepatic Cholestasis Type 1: Two Reported Cases. Transplant. Proc. 2017, 49, 1123–1125. [Google Scholar] [CrossRef] [PubMed]
- Ohlendorf, J.; Goldschmidt, I.; Junge, N.; Laue, T.; Nasser, H.; Jäckel, E.; Mutschler, F.; Pfister, E.D.; Herebian, D.; Keitel, V.; et al. Ileal Bile Acid Transporter Inhibition Reduces Post-Transplant Diarrhea and Growth Failure in FIC1 Disease—A Case Report. Children 2022, 9, 669. [Google Scholar] [CrossRef] [PubMed]
| Patient 1 | Patient 2 | Patient 3 | ||
|---|---|---|---|---|
| general | sex | male | female | male |
| age first symptoms | 2 mo | 2 mo | 1 mo | |
| age at diagnosis | 5.5 yrs | 3 yrs | 2 mo | |
| ATP8B1 variant 1 | c.1214_1215del * | c.181_182ins179 * | c.1214_1215del * | |
| ATP8B1 variant 2 | c.(2931 + 1_2932-1)_ (3400 + 1_3401-1)del * | c.181_182ins179 * | c.(2931 + 1_2932-1)_ (3400 + 1_3401-1)del * | |
| pre-LT | indication | acute liver failure, INR 3.6, fibrinogen 195 | acute liver failure, INR 1.5, fibrinogen 87 | INR 2.5, fibrinogen 269, pruritus |
| age | 5 yrs | 2.5 yrs | 21 mo | |
| graft | LDLT (mother) | DD fullsize | LDLT (father) | |
| biliary anastomosis | E/E | E/E | H/J | |
| vitamin deficiency | D, E, K | / | D, K | |
| glycemia (mg/dL) | 76 | 81 | 96 | |
| cholesterol (mg/dL) | 93 | 151 | 133 | |
| triglycerides (mg/dL) | 126 | 233 | 196 | |
| post-LT | AST (U/L) | 488 | 208 | 326 |
| ALT (U/L) | 242 | 128 | 262 | |
| GGT (U/L) | 60 | 27 | 72 | |
| serum bile acids (μmol/L) | 10.3 | / | / | |
| glycemia (mg/dL) | 84 | 78 | 98 | |
| cholesterol (mg/dL) | 72 | 76 | 101 | |
| triglycerides (mg/dL) | 45 | 68 | 71 | |
| US | hepatic steatosis, portal vein stenosis 4 mm, splenomegaly | hepatic steatosis, splenomegaly | hepatic steatosis, splenomegaly | |
| ultrasound elastography | - | 4.93 kPa | 10.5 | |
| Bx | steatosis (85%) with periportal spearing, no steatohepatitis | Steatosis (>90%) with focal and mild steatohepatitis | mild to moderate portal-tract fibrosis, mild lobular chronic inflammation, steatosis (20%) | |
| diarrhea (per day) | 5–8 | 4–7 | 10–12 | |
| vitamin deficiency | D, A, K | D, K | D, K | |
| post-LT SBD | age | 9 yrs | 6 yrs | 3 yrs |
| AST (U/L) | 47 | 47 | 37 | |
| ALT (U/L) | 74 | 32 | 60 | |
| GGT (U/L) | 19 | 46 | 78 | |
| serum bile acids (μmol/L) | 11.8 | 8.9 | 16.6 | |
| glycemia (mg/dL) | 79 | 69 | 78 | |
| cholesterol (mg/dL) | 113 | 106 | 101 | |
| triglycerides (mg/dL) | 89 | 107 | 83 | |
| US | hyperechogenic parenchyma, no splenomegaly | homogeneous parenchyma, no splenomegaly | homogeneous parenchyma, no splenomegaly | |
| ultrasound elastography | 2.9 kPa | 3 kPa | 1.75 kPa | |
| diarrhea (per day) | 6–8 | 3 | 8–10 | |
| vitamin deficiency | A, D, E, K | A, D, E, K | D, K | |
| fecal elastase (μg/g) | 293 | 436 | 162 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavallar, A.M.; Messner, F.; Scheidl, S.; Oberhuber, R.; Schneeberger, S.; Aldrian, D.; Berchtold, V.; Sanal, M.; Entenmann, A.; Straub, S.; et al. Internal Ileal Diversion as Treatment for Progressive Familial Intrahepatic Cholestasis Type 1-Associated Graft Inflammation and Steatosis after Liver Transplantation. Children 2022, 9, 1964. https://doi.org/10.3390/children9121964
Kavallar AM, Messner F, Scheidl S, Oberhuber R, Schneeberger S, Aldrian D, Berchtold V, Sanal M, Entenmann A, Straub S, et al. Internal Ileal Diversion as Treatment for Progressive Familial Intrahepatic Cholestasis Type 1-Associated Graft Inflammation and Steatosis after Liver Transplantation. Children. 2022; 9(12):1964. https://doi.org/10.3390/children9121964
Chicago/Turabian StyleKavallar, Anna M., Franka Messner, Stefan Scheidl, Rupert Oberhuber, Stefan Schneeberger, Denise Aldrian, Valeria Berchtold, Murat Sanal, Andreas Entenmann, Simon Straub, and et al. 2022. "Internal Ileal Diversion as Treatment for Progressive Familial Intrahepatic Cholestasis Type 1-Associated Graft Inflammation and Steatosis after Liver Transplantation" Children 9, no. 12: 1964. https://doi.org/10.3390/children9121964
APA StyleKavallar, A. M., Messner, F., Scheidl, S., Oberhuber, R., Schneeberger, S., Aldrian, D., Berchtold, V., Sanal, M., Entenmann, A., Straub, S., Gasser, A., Janecke, A. R., Müller, T., & Vogel, G. F. (2022). Internal Ileal Diversion as Treatment for Progressive Familial Intrahepatic Cholestasis Type 1-Associated Graft Inflammation and Steatosis after Liver Transplantation. Children, 9(12), 1964. https://doi.org/10.3390/children9121964







