Evaluation of the Effectiveness of Functional Chewing Training Compared with Standard Treatment in a Population of Children with Cerebral Palsy: A Systematic Review of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Source and Search Strategy
2.3. Data Extraction
2.4. Risk of Bias
- Eligibility criteria were specified;
- Subjects were randomly allocated to groups (in crossover studies, subjects were randomly allocated to an order in which treatments were received);
- Allocation was concealed;
- The groups were similar at baseline regarding the most important prognostic indicators;
- All subjects were blinded;
- All therapists who administered the therapy were blinded;
- All assessors who measured at least one key outcome were blinded;
- Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups;
- All subjects for whom outcome measures were available received the treatment or control condition as allocated or, when this was not the case, data for at least one key outcome were analyzed by ‘intention to treat’;
- The results of between-group statistical comparisons were reported for at least one key outcome.
3. Results
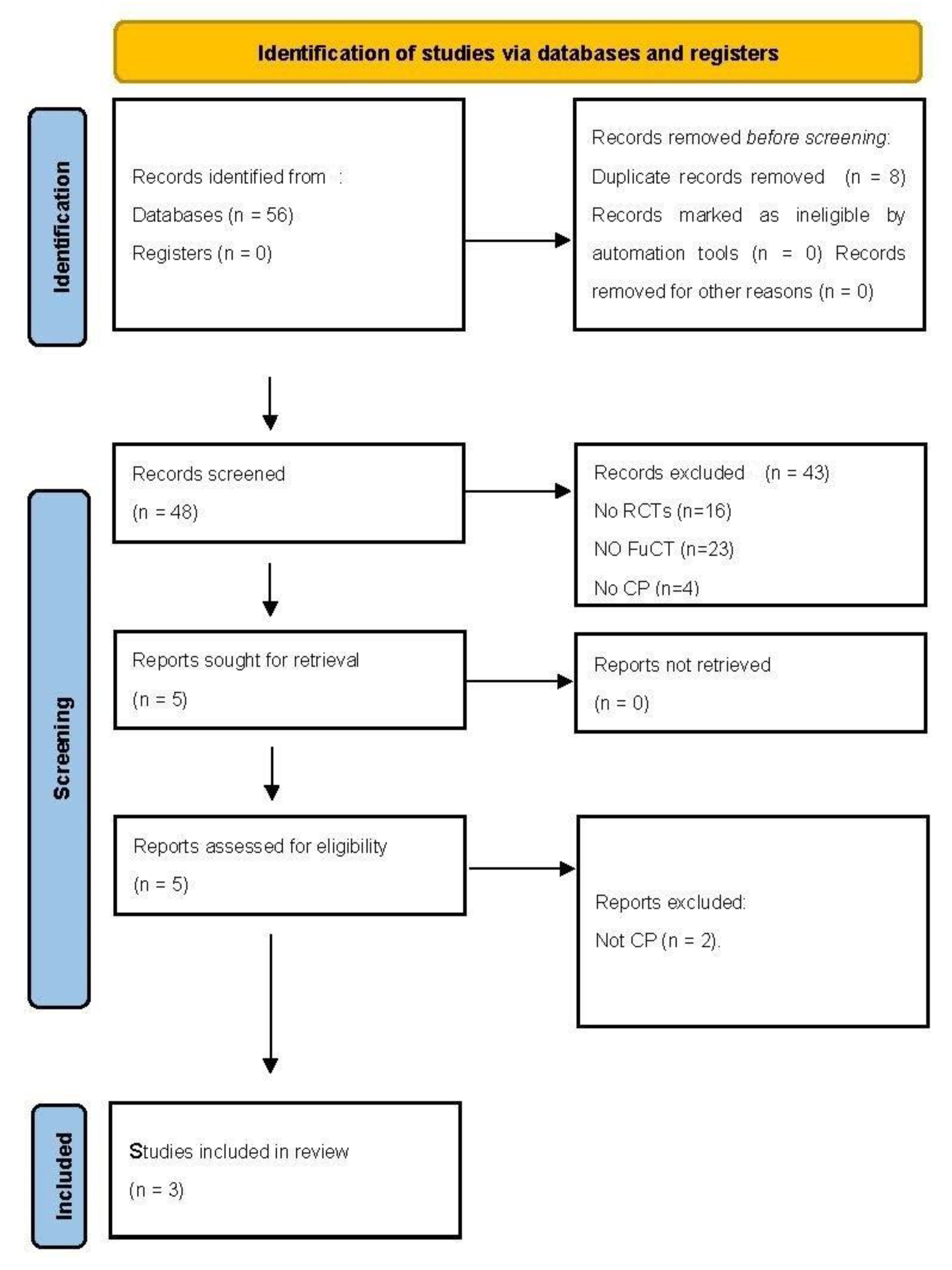
3.1. Study Selection
3.2. Participants
3.3. Study Characteristics
3.4. Intervention
- ➢
- Step I (positioning the child);
- ➢
- Step II (positioning the food);
- ➢
- Step III (sensory stimulation);
- ➢
- Step IV (chewing exercises);
- ➢
- Step V (adjustment of food consistency).
3.5. Outcome Measures
- The Behavioral Pediatrics Feeding Assessment Scale (BPFAS) is a 35-item standardized, reliable, and valid parent-completed screening tool. Each item is rated on a 5-point Likert scale based on the frequency with which particular behaviors occur [40].
- The Karaduman Chewing Performance Scale (KCPS) is a valid, reliable, quick, and clinically easy-to-use instrument to determine the level of chewing function in children [41].
- The Tongue Thrust Rating Scale (TTRS) is the first and only scale that is valid, reliable, quick, and clinically easy-to-use to define tongue thrust severity in children [42].
- The Drooling Severity and Frequency Scale (DSFS) was used to evaluate drooling severity and frequency [43]. Parents were asked to rate the severity and frequency of drooling.
3.6. Risk of Bias
3.7. Effects of FuCT on Chewing, Tongue Thrust and Drooling
4. Discussion
4.1. Limitations
4.2. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy. JAMA Pediatr. 2017, 171, 897. [Google Scholar] [CrossRef] [PubMed]
- Scarpato, E.; Staiano, A.; Molteni, M.; Terrone, G.; Mazzocchi, A.; Agostoni, C. Nutritional assessment and intervention in children with cerebral palsy: A practical approach. Int. J. Food Sci. Nutr. 2017, 68, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Speyer, R.; Cordier, R.; Kim, J.H.; Cocks, N.; Michou, E.; Wilkes-Gillan, S. Prevalence of drooling, swallowing, and feeding problems in cerebral palsy across the lifespan: A systematic review and meta-analyses. Dev. Med. Child Neurol. 2019, 61, 1249–1258. [Google Scholar] [CrossRef]
- Sullivan, P.B.; Juszczak, E.; Bachlet, A.M.; Lambert, B.; Vernon-Roberts, A.; Grant, H.W.; Eltumi, M.; McLean, L.; Alder, N.; Thomas, A.G. Gastrostomy tube feeding in children with cerebral palsy: A prospective, longitudinal study. Dev. Med. Child Neurol. 2005, 47, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Marquis, P.J.; Ruiz, N.A.; Lundy, M.S.; Dillard, R.G. Retention of primitive reflexes and delayed motor development in very low birth weight infants. J. Dev. Behav. Pediatr. 1984, 5, 124–126. [Google Scholar] [CrossRef]
- Allen, M.C.; Capute, A.J. The Evolution of Primitive Reflexes in Extremely Premature Infants. Pediatr. Res. 1986, 20, 1284–1289. [Google Scholar] [CrossRef]
- Blasco, P.A. Primitive Reflexes. Clin. Pediatr. 1994, 33, 388–397. [Google Scholar] [CrossRef]
- Andrew, M.J.; Parr, J.R.; Sullivan, P.B. Feeding difficulties in children with cerebral palsy. Arch. Dis. Child.-Educ. Pract. Ed. 2012, 97, 222–229. [Google Scholar] [CrossRef]
- Arvedson, J.C. Feeding children with cerebral palsy and swallowing difficulties. Eur. J. Clin. Nutr. 2013, 67, S9–S12. [Google Scholar] [CrossRef]
- Abraham, R.; Kamath, G.; Sodhi, J.S.; Sodhi, S.; Rita, C.; Sai Kalyan, S. Habit Breaking Appliance for Multiple Corrections. Case Rep. Dent. 2013, 2013, 1–5. [Google Scholar] [CrossRef][Green Version]
- Jann, G.R.; Ward, M.M.; Jann, H.W. A Longitudinal Study of Articulation, Deglutition, and Malocclusion. J. Speech Hear. Disord. 1964, 29, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.D.; Woda, A.; Peyron, M.A. Effect of Texture of Plastic and Elastic Model Foods on the Parameters of Mastication. J. Neurophysiol. 2006, 95, 3469–3479. [Google Scholar] [CrossRef] [PubMed]
- Alhaidary, A. Treatment of speech sound disorders in children: Nonspeech oral exercises. Int. J. Pediatr. Adolesc. Med. 2021, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Taş, S.A.; Çankaya, T. An investigation of the relationship of drooling with nutrition and head control in individuals with quadriparetic cerebral palsy. J. Phys. Ther. Sci. 2015, 27, 3487–3492. [Google Scholar] [CrossRef]
- Sığan, S.N.; Uzunhan, T.A.; Aydınlı, N.; Eraslan, E.; Ekici, B.; Çalışkan, M. Effects of oral motor therapy in children with cerebral palsy. Ann. Indian Acad. Neurol. 2013, 16, 342. [Google Scholar]
- Benfer, K.A.; Weir, K.A.; Bell, K.L.; Ware, R.S.; Davies, P.S.; Boyd, R.N. Food and fluid texture consumption in a population-based cohort of preschool children with cerebral palsy: Relationship to dietary intake. Dev. Med. Child Neurol. 2015, 57, 1056–1063. [Google Scholar] [CrossRef]
- Kuperminc, M.N.; Stevenson, R.D. Growth and nutrition disorders in children with cerebral palsy. Dev. Disabil. Res. Rev. 2008, 14, 137–146. [Google Scholar] [CrossRef]
- Serel Arslan, S.; Demir, N.; Karaduman, A.A. Effect of a new treatment protocol called Functional Chewing Training on chewing function in children with cerebral palsy: A double-blind randomised controlled trial. J. Oral Rehabil. 2017, 44, 43–50. [Google Scholar] [CrossRef]
- Walshe, M.; Smith, M.; Pennington, L. Interventions for drooling in children with cerebral palsy. In Cochrane Database of Systematic Reviews; Walshe, M., Ed.; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Arslan, S.S.; Demir, N.; Karaduman, A.A.; Tanyel, F.C.; Soyer, T. The functional chewing training for chewing dysfunction in children with repaired EA-TEF. J. Pediatr. Surg. 2020, 55, 635–638. [Google Scholar] [CrossRef]
- Fan, Q.L.; Wu, Z.F.; Yu, X.M.; Zeng, X.Y.; Peng, L.S.; Su, L.S.; Zhang, Y.P. Clinical effect of functional chewing training in treatment of oral motor dysfunction in children with cerebral palsy: A prospective randomized controlled clinical trial. Zhongguo Dang Dai Er Ke Za Zhi 2020, 22, 567–572. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Galeoto, G.; Guarino, D.; Marquez, M.A.; de Santis, R.; Valente, D.; Caporale, G.; Tofani, M. Construct validity, test-retest reliability, and the ability to detect change of the Canadian Occupational Performance Measure in a spinal cord injury population. Spinal Cord Ser. Cases 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Parente, M.; Tofani, M.; de Santis, R.; Esposito, G.; Santilli, V.; Galeoto, G. The role of the occupational therapist in disaster areas: Systematic review. Occup. Ther. Int. 2017, 2017, 6474761. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, G.; Leone, A.; Sansoni, J.; Tofani, M.; de Santis, R.; Valente, D.; Galeoto, G. Occupational Therapy’s efficacy in children with Asperger’s syndrome: A systematic review of randomized controlled trials Systematic review. Clin. Ter. 2019, 170, 382–387. [Google Scholar]
- Marquez, M.A.; de Santis, R.; Ammendola, V.; Antonacci, M.; Santilli, V.; Berardi, A.; Valente, D.; Galeoto, G. Cross-cultural adaptation and validation of the ‘spinal Cord Injury-Falls Concern Scale’ in the Italian population. Spinal Cord 2018, 56, 712–718. [Google Scholar] [CrossRef]
- Galeoto, G.; Turriziani, S.; Berardi, A.; Sansoni, J.; Santilli, V.; Mascio, M.; Paoloni, M. Levels of Cognitive Functioning Assessment Scale: Italian cross-cultural adaptation and validation. Ann. Ig. 2020, 32, 16–26. [Google Scholar] [CrossRef]
- Berardi, A.; Regoli, E.; Tofani, M.; Valente, D.; Fabbrini, G.; Fabbrini, A.; Ruggieri, M.; Panuccio, F.; Galeoto, G. Tools to assess the quality of life in patients with Parkinson’s disease: A systematic review. Expert Rev. Pharm. Outcomes Res. 2021, 21, 55–68. [Google Scholar] [CrossRef]
- Galeoto, G.; Scialpi, A.; Grassi, M.L.; Berardi, A.; Valente, D.; Tofani, M.; Paoloni, M. General Sleep Disturbance Scale: Translation, cultural adaptation, and psychometric properties of the Italian version. CRANIO® 2021, 39, 326–334. [Google Scholar] [CrossRef]
- Galeoto, G.; Berardi, A.; de Santis, R.; di Valentini, L.; Beccasio, R.; Marquez, M.A.; Giordano, M.L.; Guarino, D.; Tofani, M. Validation and cross-cultural adaptation of the Van Lieshout test in an Italian population with cervical spinal cord injury: A psychometric study. Spinal Cord Ser. Cases 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Ioncoli, M.; Berardi, A.; Tofani, M.; Panuccio, F.; Servadio, A.; Valente, D.; Galeoto, G. Crosscultural Validation of the Community Integration Questionnaire-Revised in an Italian Population. Occup. Ther. Int. 2020, 2020, 8916541. [Google Scholar] [CrossRef] [PubMed]
- La Torre, G.; Chiaradia, G.; Gianfagna, F.; Boccia, S.; de Laurentis, A.; Ricciardi, W. Quality assessment in meta-analisys. Ital. J. Public Health 2006, 3, 44–50. [Google Scholar]
- Hartling, L.; Ospina, M.; Liang, Y.; Dryden, D.M.; Hooton, N.; Seida, J.K.; Klassen, T.P. Risk of bias versus quality assessment of randomised controlled trials: Cross sectional study. BMJ 2009, 339, b4012. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Tofani, M.; Colalelli, F.; Valente, D.; Sellitto, G.; Ruotolo, I.; Galeoto, G. The psychometric properties of the Italian version of the PEDro Scale. Gazz. Med. Ital. Arch. Sci. Med. 2022, 181, 357–365. [Google Scholar] [CrossRef]
- Inal, Ö.; Serel Arslan, S.; Demir, N.; Tunca Yilmaz, Ö.; Karaduman, A.A. Effect of Functional Chewing Training on tongue thrust and drooling in children with cerebral palsy: A randomised controlled trial. J. Oral Rehabil. 2017, 44, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.; Bartlett, D. Gross Motor Function Classification System: Impact and utility. Dev. Med. Child Neurol. 2004, 46, 60–65. [Google Scholar] [CrossRef]
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Serel Arslan, S.; Demir, N.; Barak Dolgun, A.; Karaduman, A.A. Development of a new instrument for determining the level of chewing function in children. J. Oral Rehabil. 2016, 43, 488–495. [Google Scholar] [CrossRef]
- Serel Arslan, S.; Aydın, G.; Alemdaroğlu, İ.; Tunca Yılmaz, Ö.; Karaduman, A.A. Reliability and validity of the Karaduman Chewing Performance Scale in paediatric neuromuscular diseases: A system for classification of chewing disorders. J. Oral Rehabil. 2018, 45, 526–531. [Google Scholar] [CrossRef]
- Serel Arslan, S.; Demir, N.; Karaduman, A.A. Reliability and validity of a tool to measure the severity of tongue thrust in children: The Tongue Thrust Rating Scale. J. Oral Rehabil. 2017, 44, 119–124. [Google Scholar] [CrossRef]
- Rashnoo, P.; Daniel, S.J. Drooling quantification: Correlation of different techniques. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Sellers, D. Eating and Drinking Ability Classification System. Dysphagia 2019, 34, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Bodkin, A.W.; Robinson, C.; Perales, F.P. Reliability and Validity of the Gross Motor Function Classification System for Cerebral Palsy. Pediatr. Phys. Ther. 2003, 15, 247–252. [Google Scholar] [CrossRef]
- Hyun, S.E.; Yi, Y.G.; Shin, H.-I. Reliability and Validity of the Eating and Drinking Ability Classification System in Adults with Cerebral Palsy. Dysphagia 2021, 36, 351–361. [Google Scholar] [CrossRef] [PubMed]
| Database Search |
|---|
| PubMed |
| (Cerebral palsy (MeSH)) AND (‘Functional Chewing Training’ OR ‘functional chewing treatment’ OR ‘functional mastication treatment’ OR ‘FuCT’ OR ‘functional mastication training’) |
| Scopus |
| (Cerebral palsy) AND (Functional Chewing Training) OR (functional chewing treatment) OR (functional mastication treatment) OR (FuCT) OR (functional mastication training) |
| CINAHL |
| (Cerebral palsy) AND (Functional Chewing Training) OR (functional chewing treatment) OR (functional mastication treatment) OR (FuCT) OR (functional mastication training) |
| Web of Science |
| (Cerebral palsy) AND (Functional Chewing Training) OR (functional chewing treatment) OR (functional mastication treatment) OR (FuCT) OR (functional mastication training) |
| Author/Year | Participants | Intervention | Control | Outcome Measure | Results |
|---|---|---|---|---|---|
| Arslan et al., (2017) [18] | FuCT group | The protocol aimed to ensure functional chewing improvement by stimulating and teaching the function. The FuCT is a holistic approach that includes therapy sessions (steps 1, 3, and 4) and daily rules (steps 1, 2, and 5). It takes 20 min to complete. FuCT was performed with five sets/day and for 5 days a week over a period of 12 weeks as a home program. | The control group received traditional oral motor exercises including passive and active lip and tongue exercises. Passive exercises included range of motion exercises. Exercises were performed with five sets/day and for 5 days a week over a period of 12 weeks as a home program. |
| After 12 weeks, the FuCT group showed improvement in chewing performance according to the KCPS (p < 0.001) and in feeding behaviors according to the BPFAS (p < 0.001). A significant improvement was found in the FuCT group as compared with the control group in KCPS score and in all BPFAS subscale scores, except the restriction score after 12 weeks of the intervention (p < 0.001). |
| N = 50 | |||||
| Age = 3.5 (±1.9) years | |||||
| Gender = 19 F/31 M | |||||
| Motor function level was not specified. | |||||
| Control group | |||||
| N = 30 | |||||
| Age = 3.4 ± 1.9 years | |||||
| Gender = 14 F/16 M | |||||
| Motor function level was not specified. | |||||
| Inal et al., (2017) [31] | FuCT group | Families were asked to perform FuCT exercises regularly for 12 weeks with five sets (1 set = 20 min) each day. | Group II received a traditional oral motor exercise program. Families were asked to perform the exercises regularly for 12 weeks with five sets (1 set = 20 min) each day. |
| After 12 weeks of treatment, the FuCT group showed improvement in chewing performance according to KCPS score (p = 0.001), in tongue thrust according to TTRS score (p = 0.046), and in drooling severity according to DSFS score (p = 0.002), but no improvement was found in terms of drooling frequency (p = 0.082). |
| N = 20 | |||||
| Age = 43.8 months F/56.2 months M | |||||
| Gender = 7 F/9 M | |||||
| GMFCS = L1 (0)/L2(1)/L3(4)/L4(0)/L5(11) | |||||
| Control group | |||||
| N = 20 | |||||
| Age = 37.5 months F/62.5 months M | |||||
| Gender = 6 F/10 M | |||||
| GMFCS = L1(0)/L2(1)/L3(6)/L4(0)/L5(9) | |||||
| Fan et al., (2020) [32] | FuCT group | The protocol aimed to improve chewing function, tongue function, and severity and frequency of drooling. Both groups received FuCT or oral motor training for 12 weeks, 5 times a day, and for 10 min each time. | The control group received traditional oral motor exercises. Families were asked to perform the exercises regularly for 12 weeks with five sets (1 set = 20 min) each day. |
| After a 12-week training, the FuCT group showed significant improvements in masticatory function, tongue thrust severity, and drooling severity (p < 0.05), but no improvement in drooling frequency (p >0.05), while the oral motor training group had no improvement in masticatory function, tongue thrust severity, or drooling severity or frequency (p > 0.05). After the 12-week training, the FuCT group had more significant improvements in tongue thrust severity and drooling severity and frequency than the oral motor training group (p < 0.05). |
| N = 24 | |||||
| Age = 5.5 years | |||||
| Gender = 11 F/13 M | |||||
| GMFCS = L1(1)/L2(3)/L3(6)/L4(2)/L5 (12) | |||||
| Control group | |||||
| N = 24 | |||||
| Age = 5.1 years | |||||
| Gender = 8 F/16 M | |||||
| GMFCS = L1(1)/L2(4)/L3(5)/L4(2)/L5 (12) |
| Jadad Scale Item | |||
|---|---|---|---|
| Author | Randomization | Blinding | Account of Patients |
| Arslan et al., (2017) 4/5 [18] | 2 (Randomized and split between the FuCT group and the control group using randomized sampling, which was computer-generated with a basic random number generator; the allocation ratio was 5:3) | 1 (This study was designed as a double-blind RCT of FuCT in patients with CP as compared with traditional oral motor exercises) | 1 (Deducted from the tables) |
| Inal et al., (2017) 4/5 [31] | 2 (Of the 40 participants, 20 were randomized to the FuCT group and 20 to the traditional oral motor exercise group with block randomization methods. The Random Allocation Software 2.0 program was used to randomize two groups with an equal number to the block randomization system) | 1 (Evaluations were performed in a standardized manner at baseline and after 12 weeks of treatment by an experienced physical therapist blinded to the group allocation of the children) | 1 (Flow chart) |
| Fan et al., (2020) 2/5 [32] | 1 (Casual randomization) | 0 (Blinding was not mentioned) | 1 (Deducted from the tables) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banzato, A.; Cerchiari, A.; Pezzola, S.; Ranucci, M.; Scarfò, E.; Berardi, A.; Tofani, M.; Galeoto, G. Evaluation of the Effectiveness of Functional Chewing Training Compared with Standard Treatment in a Population of Children with Cerebral Palsy: A Systematic Review of Randomized Controlled Trials. Children 2022, 9, 1876. https://doi.org/10.3390/children9121876
Banzato A, Cerchiari A, Pezzola S, Ranucci M, Scarfò E, Berardi A, Tofani M, Galeoto G. Evaluation of the Effectiveness of Functional Chewing Training Compared with Standard Treatment in a Population of Children with Cerebral Palsy: A Systematic Review of Randomized Controlled Trials. Children. 2022; 9(12):1876. https://doi.org/10.3390/children9121876
Chicago/Turabian StyleBanzato, Alessandra, Antonella Cerchiari, Sofia Pezzola, Michela Ranucci, Eleonora Scarfò, Anna Berardi, Marco Tofani, and Giovanni Galeoto. 2022. "Evaluation of the Effectiveness of Functional Chewing Training Compared with Standard Treatment in a Population of Children with Cerebral Palsy: A Systematic Review of Randomized Controlled Trials" Children 9, no. 12: 1876. https://doi.org/10.3390/children9121876
APA StyleBanzato, A., Cerchiari, A., Pezzola, S., Ranucci, M., Scarfò, E., Berardi, A., Tofani, M., & Galeoto, G. (2022). Evaluation of the Effectiveness of Functional Chewing Training Compared with Standard Treatment in a Population of Children with Cerebral Palsy: A Systematic Review of Randomized Controlled Trials. Children, 9(12), 1876. https://doi.org/10.3390/children9121876








