Oral Immunotherapy for Children with Cow’s Milk Allergy: A Practical Approach
Abstract
1. Introduction
2. Materials and Methods
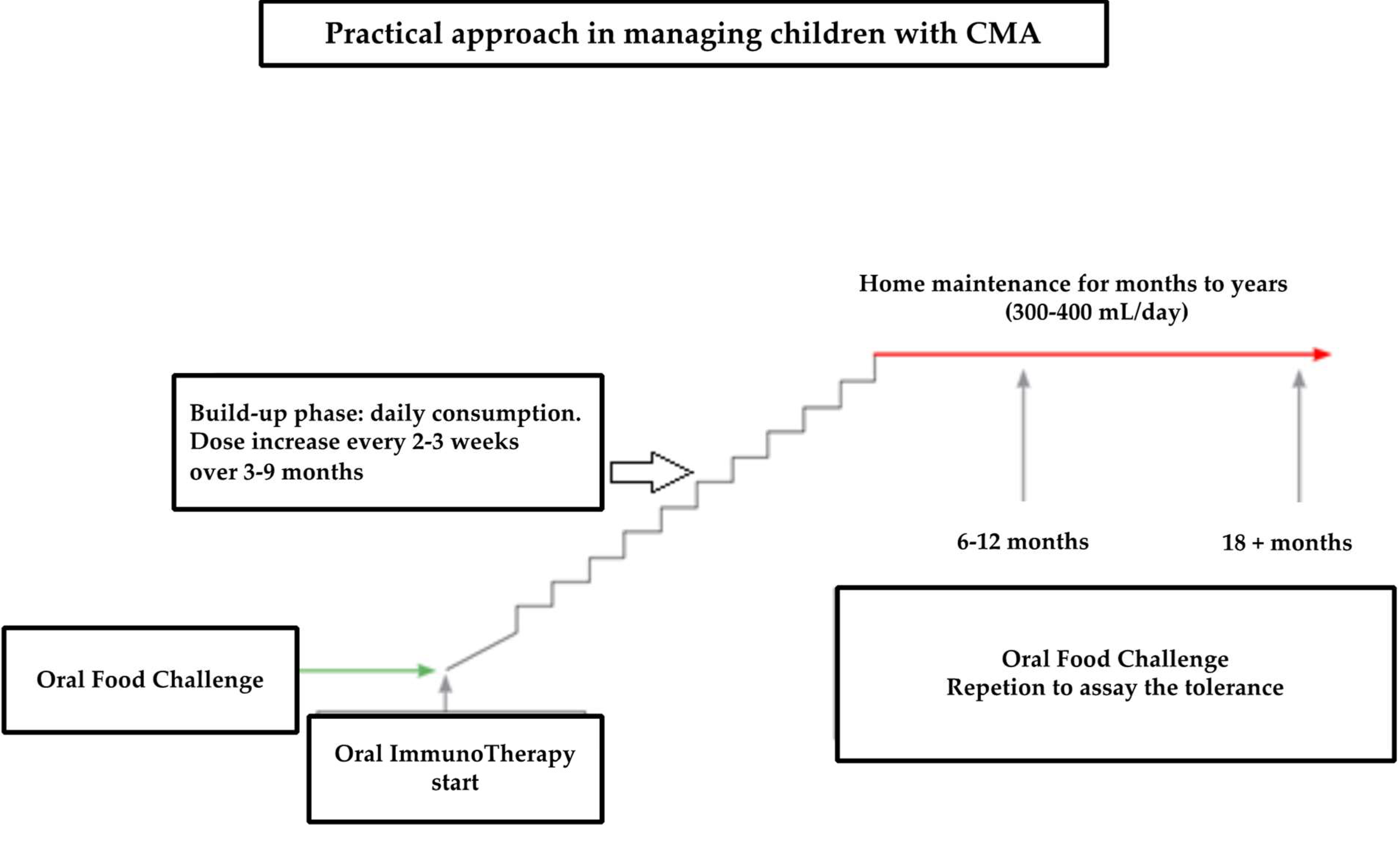
- A “low-dose and slow” schedule: patients started OFC by taking an initial dose of 0.05 mL (one drop) of CM; this dose contained 1.7 mg of CM protein. Alternatively, a quantity of biscuit (Plasmon biscuit) or cheese (Parmesan) containing the same amount of CM protein was used. The initial dose was increased by one drop at a time every 30 min if the previous dose was well tolerated. The procedure comprised a maximum of seven administrations per day. The challenge ended when a dose of 1 mL of CM was reached or if the subject presented with a clinical reaction. Usually, the challenge procedure lasts 2–3 days.
- A “rapid incremental” schedule: patients started from initial doses of 0.05 mL (one drop) of CM. The dose was doubled every 30 min if the last dose was well tolerated. The OFC end reached a cumulative dose of between 1 mL and 40 mL of CM. Usually, the challenge procedure lasts two days.
- An “ultra-rash” schedule: the starting dose was 1 mL of CM. The build-up phase was very quick as the dose was doubled every 30 min if the last dose was tolerated. For this schedule, only CM was used. The final dose was more than 40 mL of CM.
- “Low-dose and slow build-up”: the patient performed slow and gradual increases, starting with an initial dose of CM (or an equivalent quantity of CM proteins contained in a cookie or cheese), which was less than 33% of that obtained at the challenge. The dose was taken daily. The increase, usually of 10%, occurred monthly.
- “Rapid incremental build-up”: the initial dose ranged between 33% and 66% of that obtained at the challenge. This dose lasted for 1–2 weeks. Successively, the tolerated dose increased by 20% each week.
- “Ultra-rash build-up”: the initial dose was >66% of that obtained at the challenge. This dose was maintained for a week. Successively, the dose increased by 50% every week.
3. Result
3.1. General Characteristics
3.2. Clinical Characteristics
3.3. Biological Test before OFC
3.4. Characteristics of Patients Undergo to OFC
3.5. Reactions during OFC
3.6. Characteristics of Patients Undergo to OIT
3.7. Last Follow-Up Visit of Patients Undergo to OIT
3.8. Biological Test at the Last Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CM | Cow’s milk |
| CMA | Cow’s milk allergy |
| OFC | Oral food challenge |
| OIT | Oral immunotherapy |
| PC | Placebo-controlled |
References
- Roer, C.C.; Edenharter, G.; Reidmann, S.; Ehlers, I.; Worm, M.; Zuberbier, T.; Niggemann, B. Food allergy and non-allergic food hypersensitivity in children and adolescents. Clin. Exp. Allergy 2004, 34, 1534–1541. [Google Scholar] [CrossRef]
- Host, A.; Halken, S.; Jacobsen, H.P.; Christensen, A.E.; Herskind, A.M. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr. Allergy Immunol. 2002, 13, 23–28. [Google Scholar] [CrossRef]
- Saarinen, K.M.; Juntunen-Backman, K.; Jarvenpaa, A.L.; Kuitungen, P.; Lope, L.; Renlund, M.; Siivola, M.; Savilahti, E. Supplementary feeding in maternity hospitals and the risk of cow’s milk allergy: A prospective study of 6209 infants. J. Allergy Clin. Immunol. 1999, 104, 457–461. [Google Scholar] [CrossRef]
- Kvenshagen, B.; Halvorsen, R.; Jacobsen, M. Adverse reactions to milk in infants. Acta Paediatr. Int. J. Paediatr. 2008, 97, 196–200. [Google Scholar] [CrossRef]
- Venter, C.; Pereire, B.; Grundy, J.; Clayton, C.B.; Arshad, S.H.; Dean, T. Prevalence of sensitization reported and objectively assessed food hypersensitivity amongst six-year-old children: A population-based study. Pediatr. Allergy Immunol. 2006, 17, 356–363. [Google Scholar] [CrossRef]
- Schrander, J.J.P.; Van den Bogart, J.P.; Forget, P.P.; Schrander-Strumpel, C.T.; Kuijten, R.H.; Kester, A.D. Cow’s milk protein intolerance in infants under 1 year of age: A prospective epidemiological study. Eur. J. Pediatr. 1993, 152, 640–644. [Google Scholar] [CrossRef]
- Colver, A.F.; Nevantaus, H.; Macdougall, C.F.; Cant, A.J. Severe food-allergic reactions in children across the UK and Ireland, 1998–2000. Acta Paediatr. Int. J. Paediatr. 2005, 94, 689–695. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Oliveira, M.B.P.P.; Mafra, I. Bovine Milk Allergens: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 137–164. [Google Scholar]
- Santos, A.; Dias, A.; Pinheiro, J.A. Predictive factors for the persistence of cow’s milk allergy. Pediatr. Allergy Immunol. 2010, 21, 1127–1134. [Google Scholar] [CrossRef]
- Skripak, J.M.; Matsui, E.C.; Mudd, K.; Wood, R.A. The natural history of IgE-mediated cow’s milk allergy. J. Allergy Clin. Immunol. 2007, 120, 1172–1177. [Google Scholar] [CrossRef]
- Ito, K.; Futamura, M.; Moverare, R.; Tanaka, A.; Kawabe, T.; Sakamoto, T.; Borres, M.P. The usefulness of casein-specific IgE and IgG4 antibodies in cow’s milk allergic children. Clin. Mol. Allergy 2012, 10, 1. [Google Scholar] [CrossRef]
- Ford, L.S.; Bloom, K.A.; Nowak-Wegrzyn, A.H.; Shreffel, W.G.; Masilamani, M.; Sampson, H.A. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow’s milk tolerance. J. Allergy Clin. Immunol. 2013, 131, 180–186. [Google Scholar] [CrossRef]
- Kim, J.S.; Nowak-Wegrzyn, A.; Sicherer, S.H.; Noone, S.; Moshier, E.L.; Sampson, H.A. Dietary baked milk accelerates the resolution of cow’s milk allergy in children. J. Allergy Clin. Immunol. 2011, 128, 125–131. [Google Scholar] [CrossRef]
- Shek, L.P.C.; Soderstrom, L.; Ahlstedt, S.; Beyer, K.; Sampson, H.A. Determination of food specific IgE levels over time can predict the development of tolerance in cow’s milk and hen’s egg allergy. J. Allergy Clin. Immunol. 2004, 114, 387–391. [Google Scholar] [CrossRef]
- Yanagida, N.; Sato, S.; Takahashi, K.; Nagakura, K.I.; Asaumi, T.; Ogura, K.; Ebisawa, M. Increasing specific immunoglobulin E levels correlate with the risk of anaphylaxis during an oral food challenge. Pediatr. Allergy Immunol. 2018, 29, 417–424. [Google Scholar] [CrossRef]
- Bock, S.A.; Muñoz-Furlong, A.; Sampson, H.A. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J. Allergy Clin. Immunol. 2007, 119, 1016–1018. [Google Scholar] [CrossRef]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef]
- Caubet, J.C.; Ford, L.S.; Sickles, L.; Jarvinen, K.M.; Sicherer, S.H.; Sampson, H.A.; Nowak-Wegrzyn, A. Clinical features and resolution of food protein-induced enterocolitis syndrome: 10-year experience. J. Allergy Clin. Immunol. 2014, 104, 382–389. [Google Scholar] [CrossRef]
- Sorser, S.A.; Barawi, M.; Hagglund, K.; Almojaned, M.; Lyons, H. Eosinophilic esophagitis in children and adolescents: Epidemiology, clinical presentation and seasonal variation. J. Gastroenterol. 2013, 48, 81–85. [Google Scholar] [CrossRef]
- Cuomo, B.; Indirli, G.C.; Bianchi, A.M.; Arasi, S.; Caimmi, D.; Dondi, A.; La Grutta, S.; Panetta, V.; Verga, M.C.; Calvani, M. Specific IgE and skin prick tests to diagnose allergy to fresh and baked cow’s milk according to age: A systematic review. Ital. J. Pediatr. 2017, 43, 93. [Google Scholar] [CrossRef]
- Fiocchi, A.; Bognanni, A.; Brozek, J.; Ebisawa, M.; Schunemann, H. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines update–I–Plan and definitions. World Allergy Organ. J. 2022, 15, 100609. [Google Scholar] [CrossRef] [PubMed]
- Brozek, J.L.; Firmino, R.T.; Bognanni, A.; Arasi, S.; Ansotegui, I.; Assa’ad, A.H.; Bahna, S.L.; Canani, R.B.; Bozzola, M.; Chu, D.K.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guideline update–XIV–Recommendations on CMA immunotherapy. World Allergy Organ. J. 2022, 15, 100646. [Google Scholar] [CrossRef]
- Waserman, S.; Cruickshank, H.; Hildebrand, K.J.; Mack, D.; Bantock, L.; Bingemann, T.; Chu, D.K.; Cuello-Garcia, C.; Ebisawa, M.; Fahmy, D.; et al. Prevention and management of allergic reactions to food in child care centers and schools: Practice guidelines. J. Allergy Clin. Immunol. 2021, 147, 1561–1578. [Google Scholar]
- Fox, A.; Brown, T.; Walsh, J.; Venter, C.; Meyer, R.; Nowak-Wegrzyn, A.; Levin, M.; Spawls, H.; Beatson, J.; Lovis, M.T.; et al. An update to the Milk Allergy in Primary Care guideline. Clin. Transl. Allergy 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Stróżyk, A.; Ruszczyński, M.; Horvath, A.; Dahdah, L.; Fiocchi, A.; Nowak-Węgrzyn, A.; Shamir, R.; Spergel, J.; Vandenplas, Y.; Venter, C.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines update-IV-A quality appraisal with the AGREE II instrument. World Allergy Organ. J. 2022, 15, 100613. [Google Scholar] [CrossRef] [PubMed]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, S.L.; De Angelis, E.; Barni, S.; Pilolli, R.; Mori, F.; Novembre, E.; Monaci, L. Modulation of milk allergenicity by baking milk in foods: A proteomic investigation. Nutrients 2019, 11, 1536. [Google Scholar] [CrossRef]
- Chong, K.W.; Goh, S.H.; Saffari, S.E.; Loh, W.; Sia, I.; Seah, S.; Goh, A. IgE-mediated cow’s milk protein allergy in Singaporean children. Asian Pac. J. Allergy Immunol. 2022, 40, 65–67. [Google Scholar]
- Linhart, B.; Freidl, R.; Elisyutina, O.; Khaitov, M.; Karaulov, A.; Valenta, R. Molecular approach for diagnosis, therapy and prevention of cow’s milk allergy. Nutrients 2019, 11, 1492. [Google Scholar] [CrossRef]
- De Jong, N.W.; van Splunter, M.E.; Emons, J.A.M.; Hettinga, K.A. Introduction of Heated Cow’s Milk Protein in Challenge-Proven Cow’s Milk Allergic Children: The iAGE Study. Nutrients 2022, 14, 629. [Google Scholar] [CrossRef]
- Chatchatee, P.; Nowak-Wegrzyn, A.; Lange, L. Tolerance development in cow’s milk–allergic infants receiving amino acid-based formula: A randomized controlled trial. J. Allergy Clin. Immunol. 2022, 149, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.A.; Fiocchi, A.; Baars, T.; Jordakieva, G.; Nowak-Wegrzyn, A.; Pali-Schöll, I.; Passanisi, S.; Pranger, C.L.; Roth-Walter, F.; Takkinen, K.; et al. Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines update-III-Cow’s milk allergens and mechanisms triggering immune activation. World Allergy Organ. J. 2022, 15, 100668. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.M.; Agrawal, A.; Gandhi, D.; Gupta, R.S. The US population-level burden of cow’s milk allergy. World Allergy Organ. J. 2022, 15, 100644. [Google Scholar] [CrossRef] [PubMed]
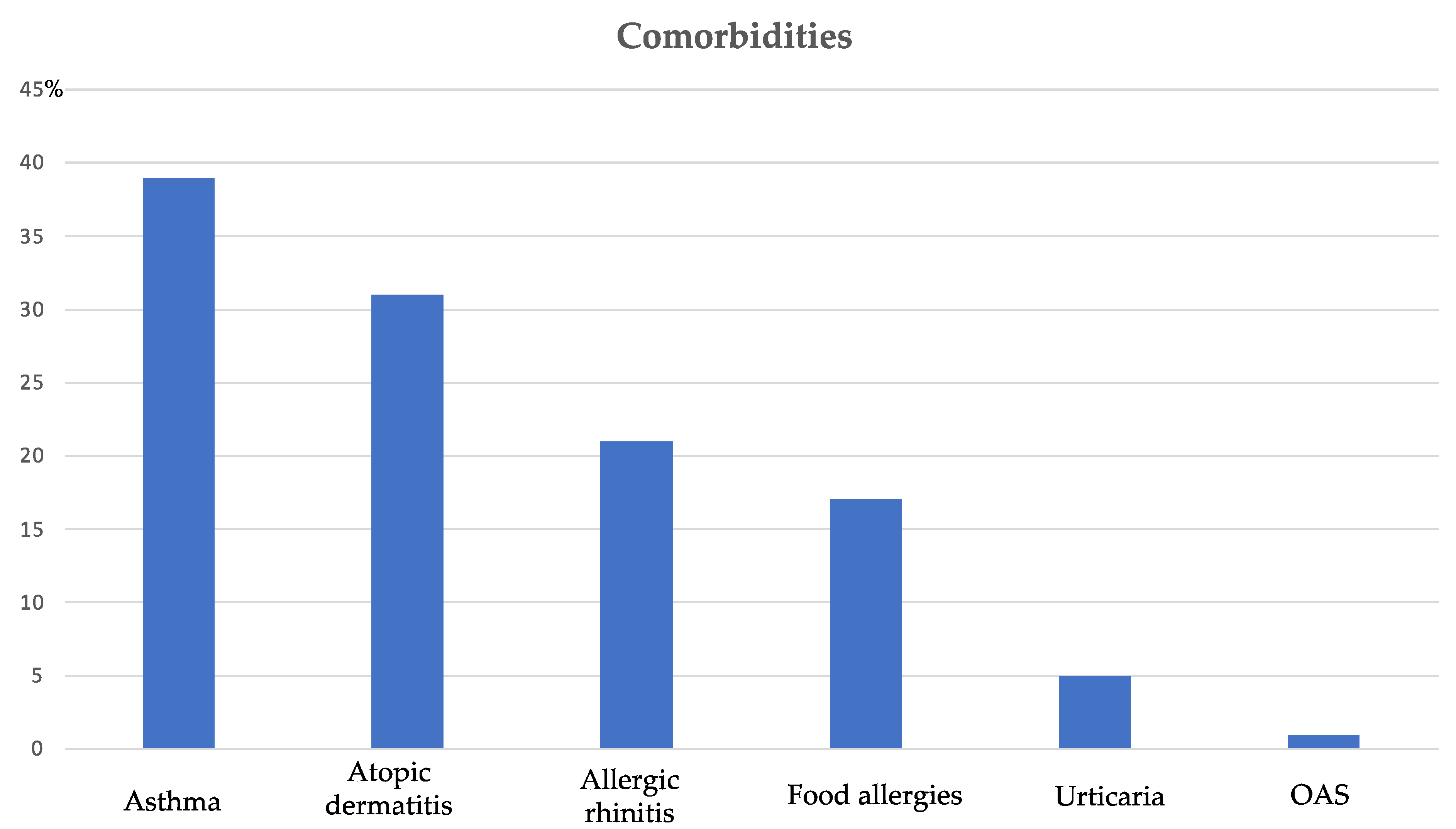
| Eosinophilia (>500/μL) | 3 (4.0%) |
| IgE Total (kU/mL) | 628.9 ± 1146.20 |
| IgE to casein (kUA/mL) | 10.6 ± 20.20 |
| IgE to α-lactoalbumin (kUA/mL) | 6.1 ± 15.04 |
| IgE to β-lattoglobulina (kUA/mL) | 4.2 ± 9.33 |
| IgE to raw milk (kUA/mL) | 14.1 ± 23.62 |
| Eosinophilia (>500/μL) | 1 (2.7%) |
| IgE Total (kU/mL) | 820 ± 967.85 |
| IgE to casein (kUA/mL) | 11.9 ± 25.41 |
| IgE to α-lactoalbumin (kUA/mL) | 6.1 ± 17.26 |
| IgE to β-lattoglobulina (kUA/mL) | 3.3 ± 9.9 |
| IgE to raw milk (kUA/mL) | 15.8 ± 25.36 |
| No Reaction | Reaction | p | |
|---|---|---|---|
| IgE Total (kU/mL) | 646.6 ± 1212.07 | 499.6 ± 655.88 | 0.97 |
| IgE to casein (kUA/mL) | 5.2 ± 11.82 | 38.6 ± 34.11 | <0.001 |
| IgE to α-lactoalbumin (kUA/mL) | 3.3 ± 5.69 | 26.9 ± 35.78 | <0.01 |
| IgE to β-lattoglobulina (kUA/mL) | 3.3 ± 5.69 | 13.6 ± 21.86 | 0.051 |
| IgE to raw milk (kUA/mL) | 9.9 ± 19.68 | 42.3 ± 34.63 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosca, M.A.; Olcese, R.; Marinelli, G.; Schiavetti, I.; Ciprandi, G. Oral Immunotherapy for Children with Cow’s Milk Allergy: A Practical Approach. Children 2022, 9, 1872. https://doi.org/10.3390/children9121872
Tosca MA, Olcese R, Marinelli G, Schiavetti I, Ciprandi G. Oral Immunotherapy for Children with Cow’s Milk Allergy: A Practical Approach. Children. 2022; 9(12):1872. https://doi.org/10.3390/children9121872
Chicago/Turabian StyleTosca, Maria Angela, Roberta Olcese, Guido Marinelli, Irene Schiavetti, and Giorgio Ciprandi. 2022. "Oral Immunotherapy for Children with Cow’s Milk Allergy: A Practical Approach" Children 9, no. 12: 1872. https://doi.org/10.3390/children9121872
APA StyleTosca, M. A., Olcese, R., Marinelli, G., Schiavetti, I., & Ciprandi, G. (2022). Oral Immunotherapy for Children with Cow’s Milk Allergy: A Practical Approach. Children, 9(12), 1872. https://doi.org/10.3390/children9121872









