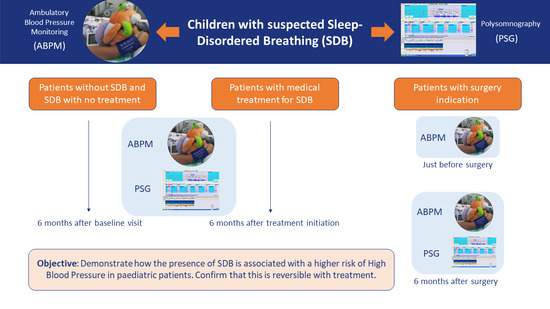
Prevalence of High Blood Pressure in Pediatric Patients with Sleep-Disordered Breathing, Reversibility after Treatment: The KIDS TRIAL Study Protocol
Abstract
1. Introduction
1.1. Obstructive Sleep Apnea in Children
1.2. OSA and Cardiovascular Consequences
1.3. Blood Pressure in SDB in Children
2. Methodology/Design
2.1. Primary Objective
2.2. Secondary Objectives
- Establish the relationship between the presence of HBP and the severity of OSA (apnea–hypopnea index—AHI, desaturation index—DI).
- Evaluate the variability along the circadian rhythm of the HBP patterns produced in pediatric patients with SDB.
- Establish the correlation between the diagnosis of HBP measured in the office and by ambulatory control of BP.
- Assess the organic damage produced:
- Evaluate the manifestation of subclinical organ damage through other markers such as: blood biomarkers (creatinine/glomerular filtration rate), urine (albuminuria/proteinuria), and echocardiography (left ventricular hypertrophy).
- Establish the pathophysiological mechanisms involved in the HBP/SDB relationship.
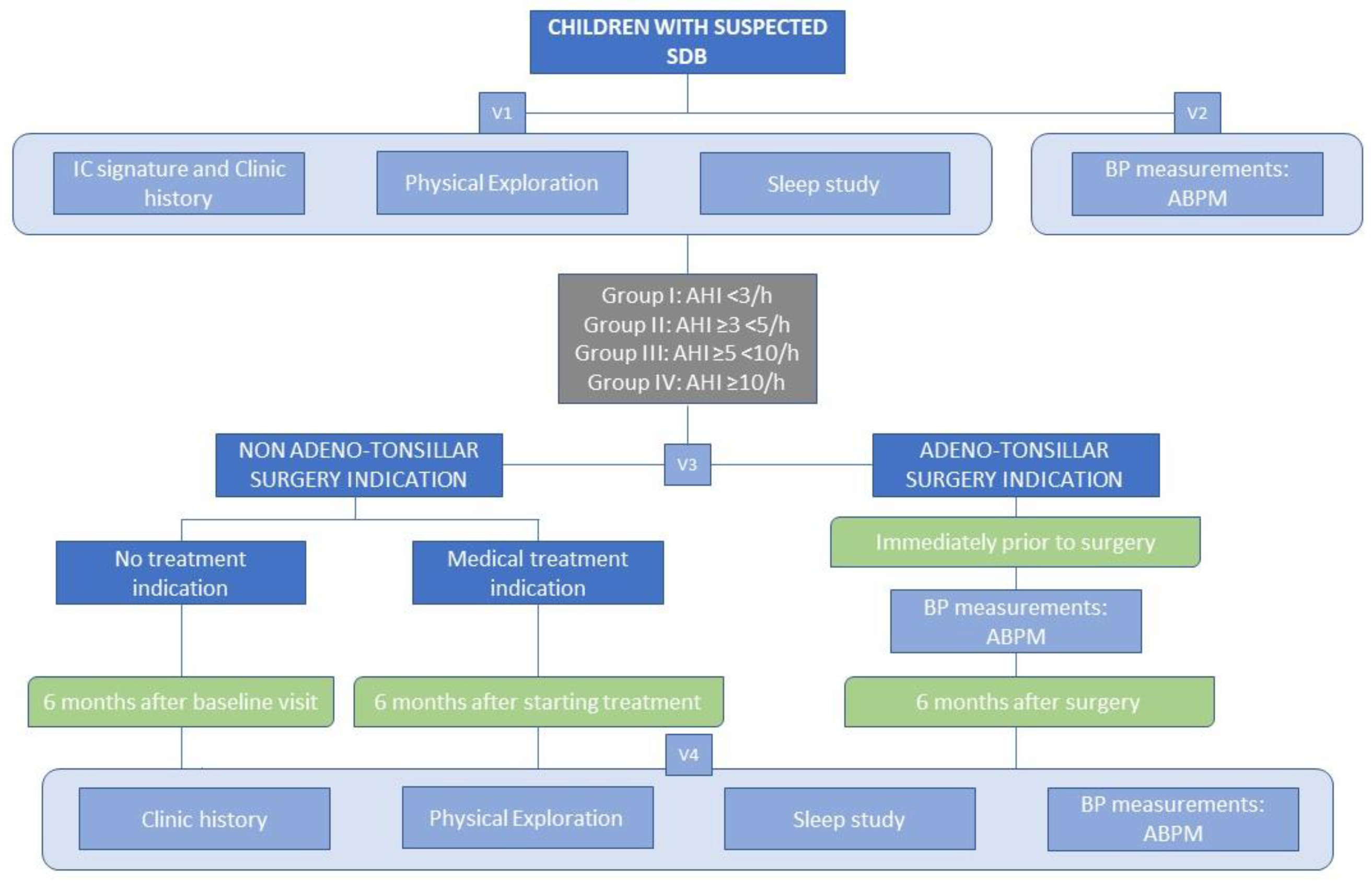
2.3. Design and Population
2.3.1. Inclusion Criteria
- Approval of the Ethics and Clinical Trials Committee (P02/18).
- Informed consent signed by parents and/or legal guardians.
- Children between 4 and 18 years old evaluated consecutively for suspected SDB.
2.3.2. Exclusion Criteria
- Associated comorbidities: cardiovascular disease (including cardiac malformation), cerebrovascular disease, or unstable severe or exacerbated respiratory disease that preclude the realization of the studies.
- Genetic diseases according to investigator criteria.
- Children with chronic insomnia and/or depressive syndrome.
- Children with malformation syndromes (including craniofacial malformations), Down syndrome, and neuromuscular diseases.
- Previous otorhinolaryngologic surgery and/or CPAP.
- Contraindication for realization of ABPM (arrhythmias, allergy to latex, or coagulation disorders).
2.4. Procedures
2.4.1. Full Polysomnography
2.4.2. Blood Pressure Measurement
2.5. Visits and Follow-Up
2.6. Study Variables and Data Collection
2.7. Sample Size Calculation
2.8. Ethical Considerations
3. Relevance of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marcus, C.L.; Brooks, L.J.; Ward, S.D.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Lehmann, C.; Schechter, M.S.; Sheldon, S.; et al. Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome. Pediatrics 2012, 130, e714–e755. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society. Standards and Indications for Cardiopulmonary Sleep Studies in Children. American Thoracic Society. Am. J. Respir. Crit. Care Med. 1996, 153, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Blechner, M.; Williamson, A.A. Consequences of Obstructive Sleep Apnea in Children. Curr. Probl. Pediatr. Adolesc. Health Care 2016, 46, 19–26. [Google Scholar] [CrossRef]
- Luz Alonso-Álvarez, M.; Canet, T.; Cubell-Alarco, M.; Estivill, E.; Fernández-Julián, E.; Gozal, D.; Jurado-Luque, M.J.; Lluch-Roselló, M.A.; Martínez-Pérez, F.; Merino-Andreu, M.; et al. Documento de Consenso Del Síndrome de Apneas-Hipopneas Durante El Sueño En Niños (Versión Completa). Arch. Bronconeumol. 2011, 47, 2–18. [Google Scholar] [CrossRef]
- Lumeng, J.C.; Chervin, R.D. Epidemiology of Pediatric Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008, 5, 242–252. [Google Scholar] [CrossRef]
- Mediano, O.; González Mangado, N.; Montserrat, J.M.; Alonso-Álvarez, M.L.; Almendros, I.; Alonso-Fernández, A.; Barbé, F.; Borsini, E.; Caballero-Eraso, C.; Cano-Pumarega, I.; et al. Documento Internacional de Consenso Sobre Apnea Obstructiva Del Sueño. Arch. Bronconeumol. 2022, 58, 52–68. [Google Scholar] [CrossRef]
- Martínez-García, M.-A.; Capote, F.; Campos-Rodríguez, F.; Lloberes, P.; Díaz de Atauri, M.J.; Somoza, M.; Masa, J.F.; González, M.; Sacristán, L.; Barbé, F.; et al. Effect of CPAP on Blood Pressure in Patients with Obstructive Sleep Apnea and Resistant Hypertension. JAMA 2013, 310, 2407. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi Filho, G.; Genta, P.R.; Pedrosa, R.P.; Drager, L.F.; Martinez, D. Consequências Cardiovasculares Na SAOS. J. Bras. Pneumol. 2010, 36 (Suppl. 2), 38–42. [Google Scholar] [CrossRef]
- Barbé, F.; Durán-Cantolla, J.; Sánchez-de-la-Torre, M.; Martínez-Alonso, M.; Carmona, C.; Barceló, A.; Chiner, E.; Masa, J.F.; Gonzalez, M.; Marín, J.M.; et al. Effect of Continuous Positive Airway Pressure on the Incidence of Hypertension and Cardiovascular Events in Nonsleepy Patients with Obstructive Sleep Apnea: A Randomized Controlled Trial. JAMA 2012, 307, 2161–2168. [Google Scholar] [CrossRef]
- Labarca, G.; Dreyse, J.; Drake, L.; Jorquera, J.; Barbe, F. Efficacy of Continuous Positive Airway Pressure (CPAP) in the Prevention of Cardiovascular Events in Patients with Obstructive Sleep Apnea: Systematic Review and Meta-Analysis. Sleep Med. Rev. 2020, 52, 101312. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension Guidelines for the Management of High Blood Pressure in Children and Adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef] [PubMed]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics 2004, 114, 555–576. [Google Scholar] [CrossRef]
- Tirosh, A.; Afek, A.; Rudich, A.; Percik, R.; Gordon, B.; Ayalon, N.; Derazne, E.; Tzur, D.; Gershnabel, D.; Grossman, E.; et al. Progression of Normotensive Adolescents to Hypertensive Adults. Hypertension 2010, 56, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Campana, E.M.G.; Brandão, A.A.; Pozzan, R.; França, M.D.F.; Fonseca, F.L.; Pizzi, O.L.; Magalhães, M.E.C.; de Freitas, E.V.; Brandão, A.P. Pressão Arterial Em Jovens Como Marcador de Risco Cardiovascular. Estudo Do Rio de Janeiro. Arq. Bras. Cardiol. 2009, 93, 657–665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Erlingsdottir, A.; Indridason, O.S.; Thorvaldsson, O.; Edvardsson, V.O. Blood Pressure in Children and Target-Organ Damage Later in Life. Pediatr. Nephrol. 2010, 25, 323–328. [Google Scholar] [CrossRef]
- Guilleminault, C.; Eldridge, F.L.; Simmons, F.B.; Dement, W.C. Sleep Apnea in Eight Children. Pediatrics 1976, 58, 23–30. [Google Scholar] [CrossRef]
- Zintzaras, E.; Kaditis, A.G. Sleep-Disordered Breathing and Blood Pressure in Children. Arch. Pediatr. Adolesc. Med. 2007, 161, 172. [Google Scholar] [CrossRef]
- Li, A.M.; Au, C.T.; Sung, R.Y.T.; Ho, C.; Ng, P.C.; Fok, T.F.; Wing, Y.K. Ambulatory Blood Pressure in Children with Obstructive Sleep Apnoea: A Community Based Study. Thorax 2008, 63, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-T.; Chiu, S.-N.; Weng, W.-C.; Lee, P.-L.; Hsu, W.-C. Analysis of 24-Hour Ambulatory Blood Pressure Monitoring in Children with Obstructive Sleep Apnea. Medicine 2015, 94, e1568. [Google Scholar] [CrossRef]
- Amin, R.; Anthony, L.; Somers, V.; Fenchel, M.; McConnell, K.; Jefferies, J.; Willging, P.; Kalra, M.; Daniels, S. Growth Velocity Predicts Recurrence of Sleep-Disordered Breathing 1 Year after Adenotonsillectomy. Am. J. Respir. Crit. Care Med. 2008, 177, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-L.; Kang, K.-T.; Chiu, S.-N.; Weng, W.-C.; Lee, P.-L.; Hsu, W.-C. Blood Pressure after Surgery among Obese and Nonobese Children with Obstructive Sleep Apnea. Otolaryngol.—Head Neck Surg. 2015, 152, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.-A.; Li, H.-Y.; Lin, Y.-S.; Fang, T.-J.; Huang, Y.-S.; Hsu, J.-F.; Wu, C.-M.; Huang, C.-G. Severity of Childhood Obstructive Sleep Apnea and Hypertension Improved after Adenotonsillectomy. Otolaryngol.—Head Neck Surg. 2015, 152, 553–560. [Google Scholar] [CrossRef]
- Ng, D.K.; Wong, J.C.; Chan, C.; Leung, L.C.K.; Leung, S. Ambulatory Blood Pressure before and after Adenotonsillectomy in Children with Obstructive Sleep Apnea. Sleep Med. 2010, 11, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Apostolidou, M.T.; Alexopoulos, E.I.; Damani, E.; Liakos, N.; Chaidas, K.; Boultadakis, E.; Apostolidis, T.; Gourgoulianis, K.; Kaditis, A.G. Absence of Blood Pressure, Metabolic, and Inflammatory Marker Changes after Adenotonsillectomy for Sleep Apnea in Greek Children. Pediatr. Pulmonol. 2008, 43, 550–560. [Google Scholar] [CrossRef]
- Crisalli, J.A.; McConnell, K.; VanDyke, R.D.; Fenchel, M.C.; Somers, V.K.; Shamszumann, A.; Chini, B.; Daniels, S.R.; Amin, R.S. Baroreflex Sensitivity after Adenotonsillectomy in Children with Obstructive Sleep Apnea during Wakefulness and Sleep. Sleep 2012, 35, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-C.; Kang, K.-T.; Chiu, S.-N.; Weng, W.-C.; Lee, P.-L.; Lin, C.-Y. 24-Hour Ambulatory Blood Pressure after Adenotonsillectomy in Childhood Sleep Apnea. J. Pediatr. 2018, 199, 112–117. [Google Scholar] [CrossRef]
- Kang, K.; Chiu, S.; Weng, W.; Lee, P.; Hsu, W. Ambulatory Blood Pressure Variability after Adenotonsillectomy in Childhood Sleep Apnea. Laryngoscope 2022, 132, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-T.; Chiu, S.-N.; Lin, C.-Y.; Weng, W.-C.; Lee, P.-L.; Hsu, W.-C. Trajectory of Ambulatory Blood Pressure after Adenotonsillectomy in Children with Obstructive Sleep Apnea: Comparison at Three- and Six-Month Follow-Up. Sleep Med. 2020, 65, 127–133. [Google Scholar] [CrossRef]
- Kang, K.-T.; Chiu, S.-N.; Lin, C.-Y.; Weng, W.-C.; Lee, P.-L.; Hsu, W.-C. Effect of Adenotonsillectomy on Ambulatory Blood Pressure in Pediatric Obstructive Sleep Apnea: 6-Month Follow-up Study. Otolaryngol.—Head Neck Surg. 2019, 160, 911–921. [Google Scholar] [CrossRef]
- Lee, C.-H.; Kang, K.-T.; Chiu, S.-N.; Chang, I.-S.; Weng, W.-C.; Lee, P.-L.; Hsu, W.-C. Association of Adenotonsillectomy with Blood Pressure among Hypertensive and Nonhypertensive Children with Obstructive Sleep Apnea. JAMA Otolaryngol.—Head Neck Surg. 2018, 144, 300. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-de-la-Torre, A.; Soler, X.; Barbé, F.; Florés, M.; Maisel, A.; Malhotra, A.; Rue, M.; Bertran, S.; Aldomá, A.; Worner, F.; et al. Cardiac Troponin Values in Patients with Acute Coronary Syndrome and Sleep Apnea. Chest 2018, 153, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Duchnowski, P.; Hryniewiecki, T.; Koźma, M.; Mariusz, K.; Piotr, S. High-Sensitivity Troponin T Is a Prognostic Marker of Hemodynamic Instability in Patients Undergoing Valve Surgery. Biomark. Med. 2018, 12, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrial.gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT03696654 (accessed on 20 November 2022).
- Chervin, R.D.; Hedger, K.; Dillon, J.E.; Pituch, K.J. Pediatric Sleep Questionnaire (PSQ): Validity and Reliability of Scales for Sleep-Disordered Breathing, Snoring, Sleepiness, and Behavioral Problems. Sleep Med. 2000, 1, 21–32. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-García, M.; Solano-Pérez, E.; Romero-Peralta, S.; Viejo-Ayuso, M.E.; Silgado-Martínez, L.; Álvarez-Balado, L.; Mediano San Andrés, R.; Resano-Barrio, P.; García-Rio, F.; Cano-Pumarega, I.; et al. Prevalence of High Blood Pressure in Pediatric Patients with Sleep-Disordered Breathing, Reversibility after Treatment: The KIDS TRIAL Study Protocol. Children 2022, 9, 1849. https://doi.org/10.3390/children9121849
Castillo-García M, Solano-Pérez E, Romero-Peralta S, Viejo-Ayuso ME, Silgado-Martínez L, Álvarez-Balado L, Mediano San Andrés R, Resano-Barrio P, García-Rio F, Cano-Pumarega I, et al. Prevalence of High Blood Pressure in Pediatric Patients with Sleep-Disordered Breathing, Reversibility after Treatment: The KIDS TRIAL Study Protocol. Children. 2022; 9(12):1849. https://doi.org/10.3390/children9121849
Chicago/Turabian StyleCastillo-García, María, Esther Solano-Pérez, Sofía Romero-Peralta, María Esther Viejo-Ayuso, Laura Silgado-Martínez, Leticia Álvarez-Balado, Rosa Mediano San Andrés, Pilar Resano-Barrio, Francisco García-Rio, Irene Cano-Pumarega, and et al. 2022. "Prevalence of High Blood Pressure in Pediatric Patients with Sleep-Disordered Breathing, Reversibility after Treatment: The KIDS TRIAL Study Protocol" Children 9, no. 12: 1849. https://doi.org/10.3390/children9121849
APA StyleCastillo-García, M., Solano-Pérez, E., Romero-Peralta, S., Viejo-Ayuso, M. E., Silgado-Martínez, L., Álvarez-Balado, L., Mediano San Andrés, R., Resano-Barrio, P., García-Rio, F., Cano-Pumarega, I., Sánchez-de-la-Torre, M., Ortigado, A., López-Dueñas, A., Fidalgo, L., Rodríguez, Á., Mediano, O., & Network, S. S. (2022). Prevalence of High Blood Pressure in Pediatric Patients with Sleep-Disordered Breathing, Reversibility after Treatment: The KIDS TRIAL Study Protocol. Children, 9(12), 1849. https://doi.org/10.3390/children9121849