The Relationship between Anthropometric Measurements and Vitamin D Levels and Insulin Resistance in Obese Children and Adolescents
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Güneş, H.; Güneş, H.; Temiz, F. The Relationship Between Epicardial Adipose Tissue and Insulin Resistance in Obese Children. Arq. Bras. Cardiol. 2020, 114, 675–682. [Google Scholar]
- Ferrannini, E. Insulin resistance is central to the burden of diabetes. Diab. Metab. Rev. 1997, 13, 81–86. [Google Scholar] [CrossRef]
- Khan, A.M.; Cheng, S.; Magnusson, M.; Larson, M.G.; Newton-Cheh, C.; McCabe, E.L.; Coviello, A.D.; Florez, J.C.; Fox, C.S.; Levy, D. Cardiac natriuretic peptides, obesity, and insulin resistance: Evidence from two community-based studies. J. Clin. Endocrinol. Metab. 2011, 96, 3242–3249. [Google Scholar] [CrossRef] [PubMed]
- Mıchalıszyn, S.F.; Arslanıan, S. Techniques to Assess Insulin Action in Youth. In Insulin Resistance Childhood Precursors of Adult Disease, 2nd ed.; Zeitler, P.S., Nadeau, K.J., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 19–37. [Google Scholar]
- Kurtoğlu, S.; Hatipoğlu, N.; Mazicioglu, M.M.; Kendirici, M.; Keskin, M.; Kondolot, M. Insulin Resistance in Obese Children and Adolescents: HOMA-IR Cut-Off Levels in the Prepubertal and Pubertal Periods—Original Article. J. Clin. Res. Pediatr. Endocrinol. 2010, 2, 100–106. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Turchiano, M.; Sweat, V.; Fierman, A.; Convit, A. Obesity, Metabolic Syndrome, and Insulin Resistance in Urban High School Students of Minority Race/Ethnicity. Arch. Pediatr. Adolesc. Med. 2012, 166, 1030–1036. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van der Aa, M.P.; Fazeli Farsani, S.; Knibbe, C.A.; De Boer, A.; Van Der Vorst, M.M. Population-based studies on the epidemiology of insulin resistance in children. J. Diabetes Res. 2015, 362375. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kwon, A.R.; Ahn, J.M.; Kim, Y.J.; Chae, H.W.; Kim, D.H.; Kim, H.-S. Relationship between serum 25-hydroxyvitamin D concentration and risks of metabolic syndrome in children and adolescents from Korean National Health and Nutrition Examination survey 2008–2010. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 46–52. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Luan, J.; Cooper, A.; Boucher, B.J.; Wareham, N.J. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance the medical research council ely prospective study 1990–2000. Diabetes 2008, 57, 2619–2625. [Google Scholar] [CrossRef]
- Oda, N.; Imamura, S.; Fujita, T.; Uchida, Y.; Inagaki, K.; Kakizawa, H.; Hayakawa, N.; Suzuki, A.; Takeda, J.; Horikawa, Y.; et al. The ratio of leptin to adiponectin can be used as an index of insulin resistance. Metabolism 2008, 57, 268–273. [Google Scholar] [CrossRef]
- Gün, E.; Uzun, H.; Bolu, S.; Arslanoğlu, İ.; Kocabay, K. Serum 25-hydroxyvitamin D is Associated with Insulin Resistance Independent of Obesity in Children Aged 5-17 Years. Prim. Care Diabetes 2020, 14, 741–746. [Google Scholar] [CrossRef]
- Delvin, E.E.; Lambert, M.; Levy, E.; O’Loughlin, J.; Mark, S.; Gray-Donald, K.; Paradis, G. Vitamin D Status Is Modestly Associated with Glycemia and Indicators of Lipid Metabolism in French-Canadian Children and Adolescents. J. Nutr. 2010, 140, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Rambhojan, C.; Larifla, L.; Clepier, J.; Bouaziz-Amar, E.; Velayoudom-Cephise, F.-L.; Blanchet-Deverly, A.; Armandr, C.; Plumasseau, J.; Lacorte, J.-M.; Foucan, L. Lydia F. Vitamin D Status, Insulin Resistance, Leptin-to-Adiponectin Ratio in Adolescents: Results of a 1-Year Lifestyle Intervention. Maced. J. Med. Sci. 2016, 4, 596–602. [Google Scholar]
- Mengen, E.; Kocaay, P. The Relation of Vitamin D Levels to Insulin Resistance and Hepatosteatosis in Obese Children. Turk. J. Pediatr. Dis. 2020, 14, 36–41. [Google Scholar] [CrossRef]
- Denova-Gutiérrez, E.; Muñoz-Aguirre, P.; López, D.; Flores, M.; Medeiros, M.; Tamborrel, N.; Clark, P. Low Serum Vitamin D Concentrations are Associated with Insulin Resistance in Mexican Children and Adolescents. Nutrients 2019, 11, 2109. [Google Scholar] [CrossRef] [PubMed]
- Moschonis, G.; Androutsos, O.; Hulshof, T.; Dracopoulou, M.; Chrousos, G.P.; Manios, Y. Vitamin D Insufficiency is Associated with Insulin Resistance Independently of Obesity in Primary School Children. The healthy growth study. Pediatr. Diabetes 2018, 19, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Garanty-Bogacka, B.; Syrenicz, M.; Goral, J.; Krupa, B.; Syrenicz, J.; Walczak, M.; Syrenicz, A. Serum 25-hydroxyvitamin D, (25-OH-D) in obese adolescents. Endokrynol. Pol. 2011, 62, 506–511. [Google Scholar]
- Khadgawat, R.; Thomas, T.; Gahlot, M.; Tandon, N.; Tangpricha, V.; Khandelwal, D.; Gupta, N. The Effect of Puberty on Interaction Between Vitamin D Status and Insulin Resistance in Obese Asian-Indian Children. Int. J. Endocrinol. 2012, 2012, 173581. [Google Scholar] [CrossRef]
- Torun, E.; Gönüllü, E.; Özgen, İ.T.; Cindemir, E.; Öktem, F. Vitamin D Deficiency and Insufficiency in Obese Children and Adolescents and Its Relationship with Insulin Resistance. Int. J. Endocrinol. 2013, 2013, 631845. [Google Scholar] [CrossRef]
- Erdönmez, D.; Hatun, Ş.; Çizmecioğlu, F.M.; Keser, A. No Relationship Between Vitamin D Status and Insulin Resistance in a Group of High School Students. J. Clin. Res. Pediatr. Endocrinol. 2011, 3, 198–201. [Google Scholar] [PubMed]
- Reinehr, T.; De Sousa, G.; Alexy, U.; Kersting, M.; Andler, W. Vitamin D status and parathyroid hormone in obese children before and after weight loss. Eur. J. Endocrinol. 2007, 157, 225–232. [Google Scholar] [CrossRef]
- Elizabeth Glaser Pediatric Research Network Obesity Study Group; Lenders, C.M.; Feldman, H.A.; Von Scheven, E.; Merewood, A.; Sweeney, C.; Wilson, D.M.; Lee, P.D.K.; Abrams, S.H.; Gitelman, S.E.; et al. Relation of body fat indexes to vitamin D status and deficiency among obese adolescents. Am. J. Clin. Nutr. 2009, 90, 459–467. [Google Scholar] [CrossRef]
- Vanlint, S. Vitamin D and obesity. Nutrients 2013, 5, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Hedyeh, S.; Marjan, S.; Ali, S.; Leila, B.; Fatemeh, A.G.; Aida, F. Vitamin D Deficiency in Children and Adolescents: Role of Puberty and Obesity on Vitamin D Status. Nutr. Metab. Insights 2021, 14, 1–7. [Google Scholar]
- Veronica, M.T.; Cosimo, G.; Francesco, C. Insulin Resistance in Children. Front. Endocrinol. 2019, 10, 342. [Google Scholar]
- Preis, S.R.; Massaro, J.M.; Robins, S.J.; Hoffmann, U.; Vasan, R.S.; Irlbeck, T.; Meigs, J.B.; Sutherland, P.; D’Agostino, R.B., Sr.; O’Donnell, C.J.; et al. Abdominal Subcutaneous and Visceral Adipose Tissue and Insulin Resistance in the Framingham Heart Study. Obesity 2011, 18, 2191–2198. [Google Scholar] [CrossRef]
- Niu, Y.; Zhao, X.; He, H.; Mao, X.; Sheng, J.; Zou, J.; Tang, Q.; Cai, W. The effect of Different Adiposity Factors on Insulin Resistance in Obese Children and Adolescents. Clin. Endocrinol. (Oxf.) 2021, 94, 949–955. [Google Scholar] [CrossRef]
- Kindler, J.M.; Lobene, A.J.; Vogel, K.A.; Martin, B.R.; McCabe, L.D.; Peacock, M.; Warden, S.J.; McCabe, G.P.; Weaver, C.M. Adiposity, Insulin Resistance, and Bone Mass in Children and Adolescents. J. Clin. Endocrinol. Metab. 2019, 104, 892–899. [Google Scholar] [CrossRef]
- Abdelhamed, M.H.; Salah, S.; Lqudsi, K.K.A.; Jan, M.M.; Alahdal, D.K.; Alfaifi, S.A.; Jafar, N.M.; Alyahyawi, N.Y.; Al-Agha, A.A. Indices of Insulin Resistance and Adiposity Can Detect Obesity–Related Morbidty in Pediatrics. Saudi Med. J. 2022, 43, 161–168. [Google Scholar] [CrossRef]
- Thomazini, F.; de Carvalho, B.S.; de Araujo, P.X.; Franco, M.D.C. High Uric Acid Levels in Overweight and Obese Children and Their Relationship with Cardiometabolic Risk Factors: What is Missing in This Puzzle? J. Pediatr. Endocrinol. Metab. 2021, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Q.; Deng, J.; He, L.-P.; Ling, W.-H.; Su, Y.-X.; Chen, Y.-M. Comparison of Various Anthropometric and Body Fat Indices in Identifying Cardiometabolic Disturbances in Chinese Men and Women. PLoS ONE 2013, 8, e70893. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Tang, Q.; Zhao, X.; Zhao, X.; Mao, X.; Sheng, J.; Cai, W.; Feng, Y. Obesity-Induced Insulin Resistance is Mediated by High Uric Acid in Obese Children and Adolescents. Front. Endocrinol. 2021, 12, 773820. [Google Scholar] [CrossRef]

| Variables | Total n = 150 | HOMA-IR (+) n = 87 [58%] | HOMA-IR (–) n = 63 [42%] | Test | p | |
|---|---|---|---|---|---|---|
| Age(years) | 8.0 [6.0–12.0] | 8.0 [6.0–12.0] | 8.0 [6.0–11.0] | 2685.5 * | 0.833 | |
| Gender | Female | 78 [52%] | 45 [57.6%] | 33 [42.4%] | X2 = 0.006 | 0.937 |
| Male | 72 [48%] | 42 [58.3%] | 30 [41.7%] | |||
| Vitamin D (ng/mL) ** | [20.18 ± 7.83] | [17.72 ± 7.32] | [23.57 ± 7.27] | −4.834 | <0.001 | |
| Uric acid (mg/dL) | 4.75 [4.10–5.50] | 4.80 [4.00–5.70] | 4.70 [4.10–5.30] | 2606.5 * | 0.610 | |
| MCV(fL) | [80.28 ± 4.37] | [80.68 ± 4.35] | [79.73 ± 4.36] | 1.306 | 0.194 | |
| RDW (%) | 13.15 [12.60–13.70] | 13.20 [12.60–13.70] | 13.00 [12.40–13.60] | 2411.5 * | 0.210 | |
| NLR | 1.51 [1.18–1.98] | 1.49 [1.15–1.86] | 1.59 [1.19–2.01] | 2618.0 | 0.641 | |
| PMI (fl/nL) ** | [3231.33 ± 710.50] | [3397.23 ± 648.34] | [3140.32 ± 784.60] | 1338 | 0.183 | |
| Variables | Total n = 150 | HOMA-IR (+) n = 87 (58%) | HOMA-IR (–) n = 63 (42%) | Test | p |
|---|---|---|---|---|---|
| BMI (kg/m2) | 24.4 (21.97–27.47) | 26.0 (22.7–28.4) | 22.6 (20.9–25.6) | 1641.5 | <0.001 |
| BWH (%) | 135.0 (125.0–144.0) | 138.0 (130.0–151.0) | 127.0 (121.0–138.0) | 1413.5 | <0.001 |
| WC (cm) | 71.5 (55.0–89.0) | 84.0 (72.0–95.0) | 54.0 (50.0–60.0) | 303.0 | <0.001 |
| BFM (kg) | 15.05 (10.02–21.52) | 18.0 (12.5–25.1) | 10.8 (7.4–16.3) | 1286.0 | <0.001 |
| BFP (%) | 31.61 ± 8.44 | 35.11 ± 7.40 | 26.79 ± 7.38 | 6.801 | <0.001 |
| BMR (kcal) | 1394.0 (1200.0–1622.25) | 1445.0 (1277.0–1736.25) | 1286.0 (1124.0–1488.0) | 1750.0 | <0.001 |
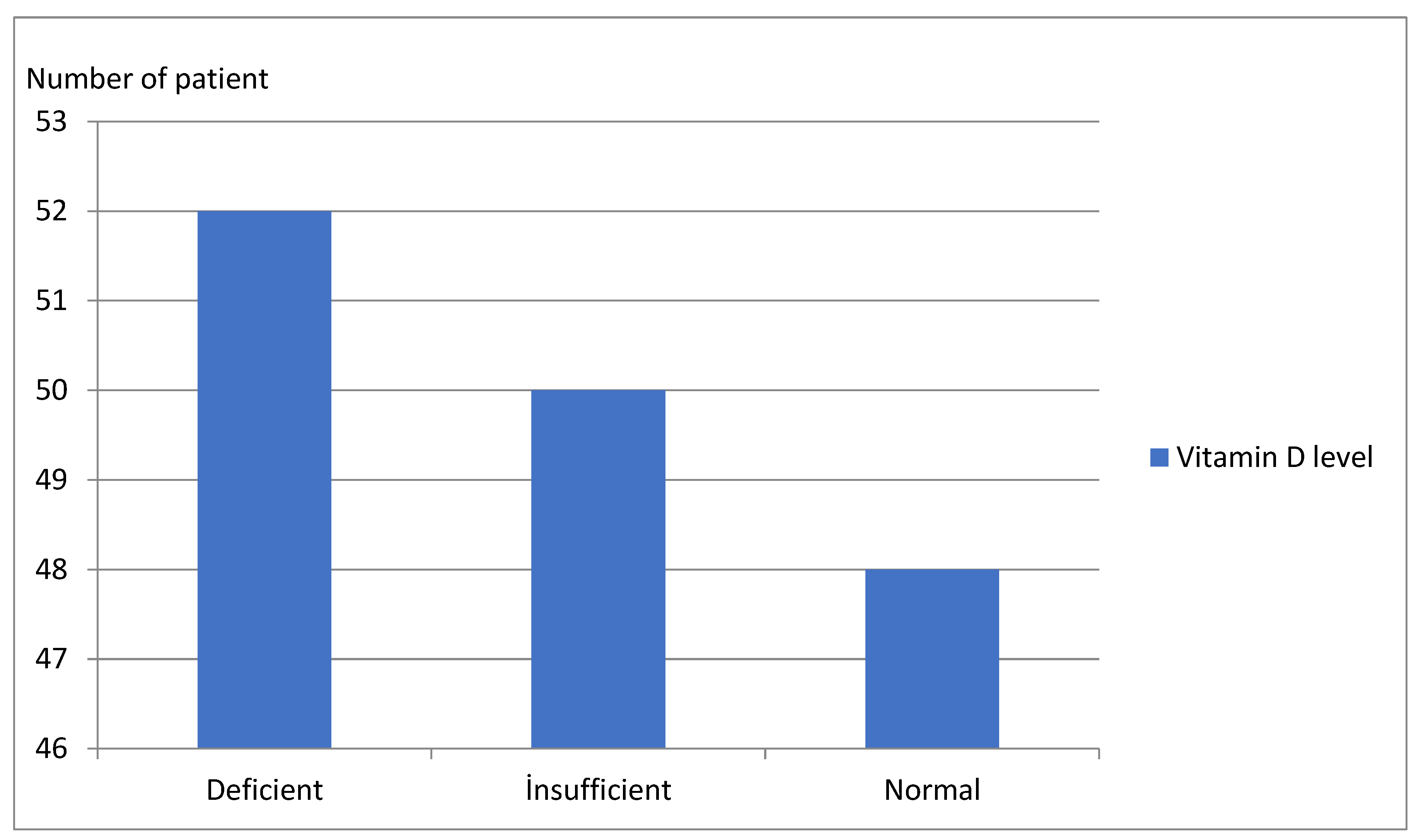
| Vitamin D Level (ng/mL) | <20 n = 52 (34.6%) | 20–30 n = 50 (33.4%) | ≥30 n = 48 (32%) | Test | p |
|---|---|---|---|---|---|
| BMI (kg/m2) | 27.95 (24.27–30.5) | 25.9 (22.2–27.7) | 23.0 (21.2–26.5) | 1641.5 | 0.003 |
| BWH (%) | 141.0 (129.25–154.0) | 135.0 (127.5–147.0) | 134.0 (121.0–140.0) | 1413.5 | 0.023 |
| WC (cm) | 87.5 (78.25–97.0) | 80.0 (66.5–92.5) | 60.0 (52.5–78.0) | 303.0 | <0.001 |
| BFM (kg) | 26.1 (10.77–36.2) | 16.8 (11.5–22.2) | 12.2 (7.75–19.2) | 1286.0 | 0.004 |
| BFP (%) * | 36.29 ± 10.62 | 31.95 ± 8.02 | 30.47 ± 8.26 | 2.584 | 0.079 |
| BMR (kcal) | 1548.5 (1421.0–1633.0) | 1408.0 (1237.5–1756.0) | 1334.0 (1159.5–1483.0) | 1750.0 | 0.019 |
| HOMA-IR | 6.8 (6.63–6.92) | 4.98 (3.38–5.78) | 1.99 (0.91–3.85) | 0.000 | <0.001 |
| BMI | BWH | WC | BFM | BFP | BMR | |
|---|---|---|---|---|---|---|
| r | 0.387 | 0.398 | 0.726 | 0.467 | 0.431 | 0.347 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Variables | β | SE | p | OR | 95% CI for OR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Constant | 33.002 | 10.443 | 0.002 | |||
| BMI | 1.126 | 0.393 | <0.001 | 1.374 | 2.033 | 9.498 |
| BWH | 1.229 | 0.080 | 0.004 | 1.555 | 0.680 | 0.930 |
| WC | 3.480 | 0.098 | <0.001 | 4.394 | 0.539 | 0.791 |
| BFP | 1.280 | 0.108 | 0.010 | 1.795 | 0.611 | 0.934 |
| Variables | AUC | Asymptotic Sig. | Asymptotic 95% CI | Cutoff | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| BMI | 0.701 | <0.001 | 0.616 | 0.785 | 24.15 | 66.7 | 65.1 |
| BWH | 0.742 | <0.001 | 0.662 | 0.822 | 133.5 | 66.7 | 65.1 |
| WC | 0.945 | <0.001 | 0.908 | 0.982 | 69.0 | 87.4 | 87.3 |
| BFM | 0.765 | <0.001 | 0.688 | 0.843 | 14.35 | 69.0 | 68.3 |
| BFP | 0.782 | <0.001 | 0.708 | 0.856 | 297.95 | 69.0 | 68.3 |
| BMR | 0.681 | <0.001 | 0.595 | 0.766 | 1377.5 | 64.4 | 63.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çığrı, E.; İnan, F.Ç. The Relationship between Anthropometric Measurements and Vitamin D Levels and Insulin Resistance in Obese Children and Adolescents. Children 2022, 9, 1837. https://doi.org/10.3390/children9121837
Çığrı E, İnan FÇ. The Relationship between Anthropometric Measurements and Vitamin D Levels and Insulin Resistance in Obese Children and Adolescents. Children. 2022; 9(12):1837. https://doi.org/10.3390/children9121837
Chicago/Turabian StyleÇığrı, Emrah, and Funda Çatan İnan. 2022. "The Relationship between Anthropometric Measurements and Vitamin D Levels and Insulin Resistance in Obese Children and Adolescents" Children 9, no. 12: 1837. https://doi.org/10.3390/children9121837
APA StyleÇığrı, E., & İnan, F. Ç. (2022). The Relationship between Anthropometric Measurements and Vitamin D Levels and Insulin Resistance in Obese Children and Adolescents. Children, 9(12), 1837. https://doi.org/10.3390/children9121837




