Parental Burden and Quality of Life in 5q-SMA Diagnosed by Newborn Screening
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Caregiver Outcome Measures
2.3. Analysis
3. Results
3.1. Participation
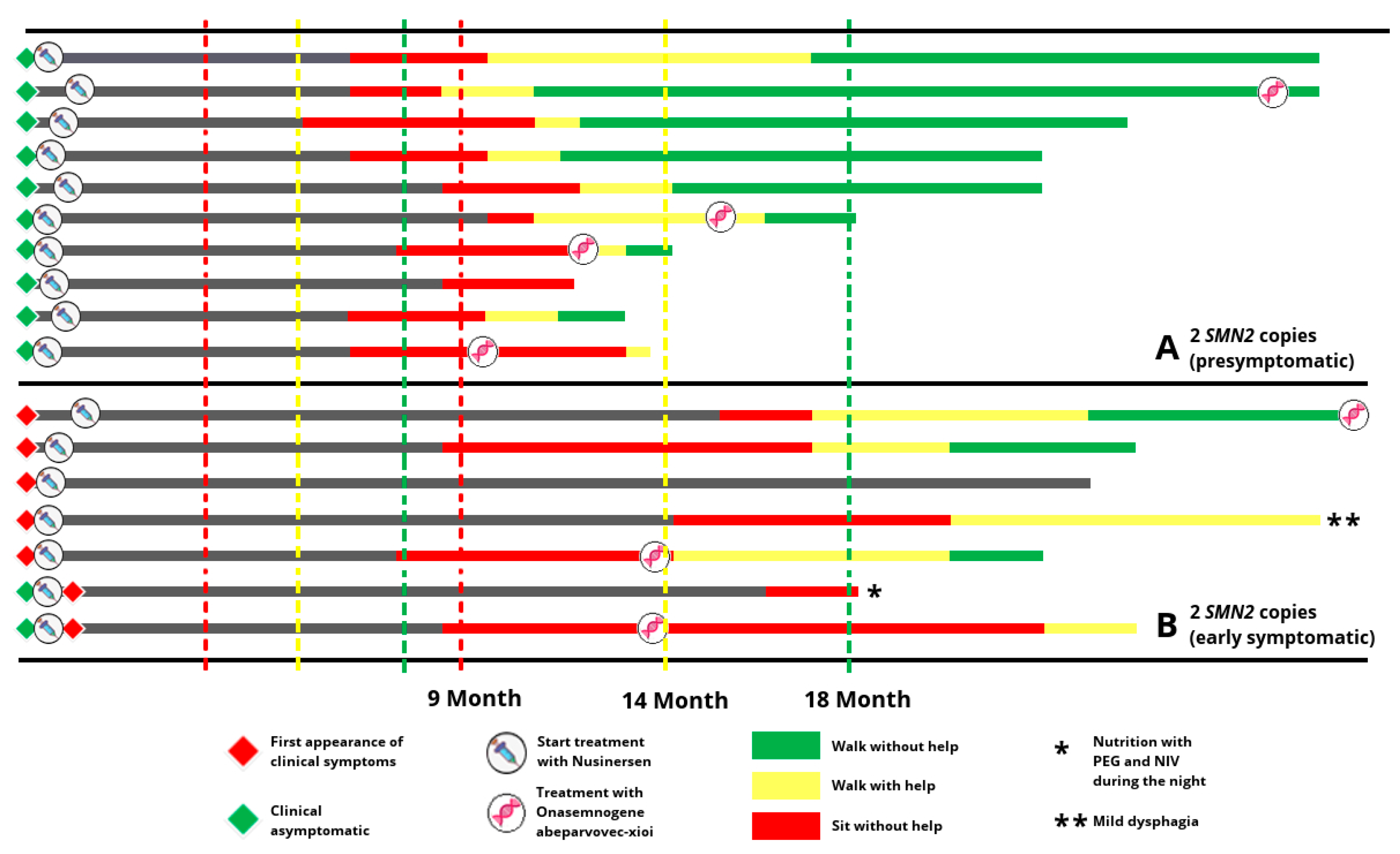
3.2. Characteristics and Motor Development of Infantile SMA Patients
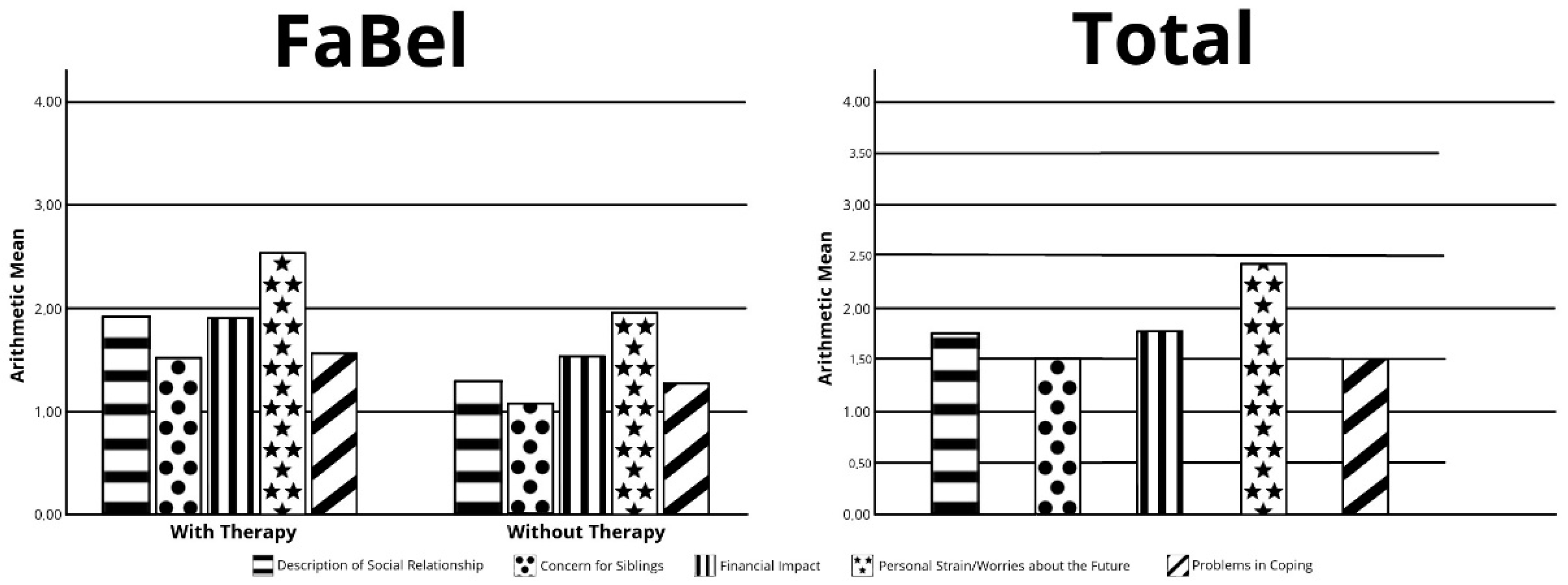
3.3. Family Burden Questionnaire (FaBel)
3.4. Quality-of-Life Inventory for Parents of Chronically Ill Children (ULQIE)
3.5. Work Productivity and Activity Impairment-Care Givers (WPAI-CG)
4. Discussion
5. Conclusions
Limitation of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. NMD 2018, 28, 103–115. [Google Scholar] [CrossRef]
- Lefebvre, S.; Burglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Meyer, O.H.; Simonds, A.K.; Schroth, M.K.; Graham, R.J.; Kirschner, J.; Iannaccone, S.T.; Crawford, T.O.; Woods, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul. Disord. NMD 2018, 28, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Wirth, B.; Brichta, L.; Schrank, B.; Lochmuller, H.; Blick, S.; Baasner, A.; Heller, R. Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number. Hum. Genet. 2006, 119, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Finkel, R.S.; Bertini, E.S.; Schroth, M.; Simonds, A.; Wong, B.; Aloysius, A.; Morrison, L.; Main, M.; Crawford, T.O.; et al. Consensus statement for standard of care in spinal muscular atrophy. J. Child Neurol. 2007, 22, 1027–1049. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Chiriboga, C.A.; Vajsar, J.; Day, J.W.; Montes, J.; De Vivo, D.C.; Yamashita, M.; Rigo, F.; Hung, G.; Schneider, E.; et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: A phase 2, open-label, dose-escalation study. Lancet 2017, 388, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Al-Zaidy, S.A.; Lehman, K.J.; McColly, M.; Lowes, L.P.; Alfano, L.N.; Reash, N.F.; Iammarino, M.A.; Church, K.R.; Kleyn, A.; et al. Five-Year Extension Results of the Phase 1 START Trial of Onasemnogene Abeparvovec in Spinal Muscular Atrophy. JAMA Neurol. 2021, 78, 834–841. [Google Scholar] [CrossRef]
- Hohenfellner, K.; Bergmann, C.; Fleige, T.; Janzen, N.; Burggraf, S.; Olgemoller, B.; Gahl, W.A.; Czibere, L.; Froschauer, S.; Roschinger, W.; et al. Molecular based Newborn Screening in Germany: Follow-up for cystinosis. Mol. Genet. Metab. Rep. 2019, 21, 100514. [Google Scholar] [CrossRef] [PubMed]
- Vill, K.; Kölbel, H.; Schwartz, O.; Blaschek, A.; Olgemöller, B.; Harms, E.; Burggraf, S.; Röschinger, W.; Durner, J.; Gläser, D.; et al. One Year of Newborn Screening for SMA—Results of a German Pilot Project. J. Neuromuscul. Dis. 2019, 6, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Vill, K.; Schwartz, O.; Blaschek, A.; Glaser, D.; Nennstiel, U.; Wirth, B.; Burggraf, S.; Roschinger, W.; Becker, M.; Czibere, L.; et al. Newborn Screening for Spinal Muscular Atrophy in Germany: Clinical results after 2 years. Orphanet. J. Rare Dis. 2021, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Muller-Felber, W.; Vill, K.; Schwartz, O.; Glaser, D.; Nennstiel, U.; Wirth, B.; Burggraf, S.; Roschinger, W.; Becker, M.; Durner, J.; et al. Infants Diagnosed with Spinal Muscular Atrophy and 4 SMN2 Copies through Newborn Screening—Opportunity or Burden? J. Neuromuscul. Dis. 2020, 7, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Blaschek, A.; Kölbel, H.; Schwartz, O.; Kohler, C.; Glaser, D.; Eggermann, K.; Hannibal, I.; Schara-Schmidt, U.; Muller-Felber, W.; Vill, K. Newborn Screening for SMA—Can a Wait-and-See Strategy be Responsibly Justified in Patients With Four SMN2 Copies? J. Neuromuscul. Dis. 2022, 9, 597–605. [Google Scholar] [CrossRef]
- Glascock, J.; Sampson, J.; Haidet-Phillips, A.; Connolly, A.; Darras, B.; Day, J.; Finkel, R.; Howell, R.R.; Klinger, K.; Kuntz, N.; et al. Treatment Algorithm for Infants Diagnosed with Spinal Muscular Atrophy through Newborn Screening. J. Neuromuscul. Dis. 2018, 5, 145–158. [Google Scholar] [CrossRef]
- Ravens-Sieberer, U.; Morfeld, M.; Stein, R.E.; Jessop, D.J.; Bullinger, M.; Thyen, U. The testing and validation of the German version of the impact on family scale in families with children with disabilities. Psychother. Psychosom. Med. Psychol. 2001, 51, 384–393. [Google Scholar] [CrossRef]
- Reilly, M.C.; Zbrozek, A.S.; Dukes, E.M. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993, 4, 353–365. [Google Scholar] [CrossRef]
- Stein, R.E.; Riessman, C.K. The development of an impact-on-family scale: Preliminary findings. Med. Care 1980, 18, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Goldbeck, L.; Melches, J. Quality of life in families of children with congenital heart disease. Qual. Life Res. 2005, 14, 1915–1924. [Google Scholar] [CrossRef]
- Schwartz, O.; Kölbel, H.; Blaschek, A.; Gläser, D.; Burggraf, S.; Röschinger, W.; Schara, U.; Müller-Felber, W.; Vill, K. Spinal Muscular Atrophy—Is Newborn Screening Too Late for Children with Two SMN2 Copies? J. Neuromuscul. Dis. 2022, 9, 389–396. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.; Jukes, T.; Goobie, S.; DiRaimo, J.; Moran, G.; Potter, B.K.; Chakraborty, P.; Rupar, C.A.; Gannavarapu, S.; Prasad, C. Psychosocial impact on mothers receiving expanded newborn screening results. Eur. J. Hum. Genet. EJHG 2018, 26, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.O.; Rivera-Cruz, G.; Chau, M.H.K.; Dong, Z.; Choy, K.W.; Shen, J.; Amr, S.; Giersch, A.B.S.; Morton, C.C. The Burden and Benefits of Knowledge: Ethical Considerations Surrounding Population-Based Newborn Genome Screening for Hearing. Int. J. Neonatal. Screen 2022, 8, 36. [Google Scholar] [CrossRef]
- Andrews, S.M.; Porter, K.A.; Bailey, D.B., Jr.; Peay, H.L. Preparing newborn screening for the future: A collaborative stakeholder engagement exploring challenges and opportunities to modernizing the newborn screening system. BMC Pediatr. 2022, 22, 90. [Google Scholar] [CrossRef]
- Prakash, S.; Penn, J.D.; Jackson, K.E.; Dean, L.W. Newborn screening for Pompe disease: Parental experiences and follow-up care for a late-onset diagnosis. J. Genet. Couns. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Crossen, K.; Berry, L.; Myers, M.F.; Leslie, N.; Goueli, C. A Qualitative Study: Mothers’ Experiences of Their Child’s Late-Onset Pompe Disease Diagnosis Following Newborn Screening. Int. J. Neonatal. Screen 2022, 8, 43. [Google Scholar] [CrossRef]
- Kariyawasam, D.S.T.; D’Silva, A.M.; Vetsch, J.; Wakefield, C.E.; Wiley, V.; Farrar, M.A. "We needed this": Perspectives of parents and healthcare professionals involved in a pilot newborn screening program for spinal muscular atrophy. EClinicalMedicine 2021, 33, 100742. [Google Scholar] [CrossRef]
- Thyen, U.; Sperner, J.; Morfeld, M.; Meyer, C.; Ravens-Sieberer, U. Unmet health care needs and impact on families with children with disabilities in Germany. Ambul. Pediatr. 2003, 3, 74–81. [Google Scholar] [CrossRef]
- Gramer, G.; Haege, G.; Glahn, E.M.; Hoffmann, G.F.; Lindner, M.; Burgard, P. Living with an inborn error of metabolism detected by newborn screening-parents’ perspectives on child development and impact on family life. J. Inherit. Metab. Dis. 2014, 37, 189–195. [Google Scholar] [CrossRef]
- Weaver, M.S.; Hanna, R.; Hetzel, S.; Patterson, K.; Yuroff, A.; Sund, S.; Schultz, M.; Schroth, M.; Halanski, M.A. A Prospective, Crossover Survey Study of Child- and Proxy-Reported Quality of Life According to Spinal Muscular Atrophy Type and Medical Interventions. J. Child Neurol. 2020, 35, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Landfeldt, E.; Edström, J.; Sejersen, T.; Tulinius, M.; Lochmüller, H.; Kirschner, J. Quality of life of patients with spinal muscular atrophy: A systematic review. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2019, 23, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Ma, Y.; Qian, R.; Xia, Y.; Yuan, C.; Bai, G.; Mao, S. Quality of life of children with spinal muscular atrophy and their caregivers from the perspective of caregivers: A Chinese cross-sectional study. Orphanet J. Rare Dis. 2021, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.; Johannsen, L.; Inhestern, L.; Bergelt, C. Parents as informal caregivers of children and adolescents with spinal muscular atrophy: A systematic review of quantitative and qualitative data on the psychosocial situation, caregiver burden, and family needs. Orphanet J. Rare Dis. 2022, 17, 274. [Google Scholar] [CrossRef]
- Goldbeck, L. The impact of newly diagnosed chronic paediatric conditions on parental quality of life. Qual. Life Res. 2006, 15, 1121–1131. [Google Scholar] [CrossRef]
- Yang, X.; Yoo, H.K.; Amin, S.; Cheng, W.Y.; Sundaresan, S.; Zhang, L.; Duh, M.S. Burden Among Caregivers of Pediatric Patients with Neurofibromatosis Type 1 (NF1) and Plexiform Neurofibroma (PN) in the United States: A Cross-Sectional Study. Neurol. Ther. 2022, 11, 1221–1233. [Google Scholar] [CrossRef]
- King-Stephens, D.; Wheless, J.; Krogh, C.; Bettles, M.; Niemira, J.; Stolper, R.; Benitez, A.; Fournier, M.; Spalding, W.; Lu, M. Burden of disease in patients with a history of status epilepticus and their caregivers. Epilepsy Behav. 2020, 112, 107374. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.A.; Martínez, Ó.; Amayra, I.; López-Paz, J.F.; Al-Rashaida, M.; Lázaro, E.; Caballero, P.; Pérez, M.; Berrocoso, S.; García, M.; et al. Diseases Costs and Impact of the Caring Role on Informal Carers of Children with Neuromuscular Disease. Int. J. Env. Res. Public Health 2021, 18, 2991. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kölbel, H.; Modler, L.; Blaschek, A.; Schara-Schmidt, U.; Vill, K.; Schwartz, O.; Müller-Felber, W. Parental Burden and Quality of Life in 5q-SMA Diagnosed by Newborn Screening. Children 2022, 9, 1829. https://doi.org/10.3390/children9121829
Kölbel H, Modler L, Blaschek A, Schara-Schmidt U, Vill K, Schwartz O, Müller-Felber W. Parental Burden and Quality of Life in 5q-SMA Diagnosed by Newborn Screening. Children. 2022; 9(12):1829. https://doi.org/10.3390/children9121829
Chicago/Turabian StyleKölbel, Heike, Laura Modler, Astrid Blaschek, Ulrike Schara-Schmidt, Katharina Vill, Oliver Schwartz, and Wolfgang Müller-Felber. 2022. "Parental Burden and Quality of Life in 5q-SMA Diagnosed by Newborn Screening" Children 9, no. 12: 1829. https://doi.org/10.3390/children9121829
APA StyleKölbel, H., Modler, L., Blaschek, A., Schara-Schmidt, U., Vill, K., Schwartz, O., & Müller-Felber, W. (2022). Parental Burden and Quality of Life in 5q-SMA Diagnosed by Newborn Screening. Children, 9(12), 1829. https://doi.org/10.3390/children9121829





