Preterm ETs Are Significantly Reduced Compared with Adults and Partially Reduced Compared with Term Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Collection of Umbilical Cord Blood
2.3. NET Assay by Microscopy
2.4. Microscopy Analysis of NETs
2.5. MET Assay
2.6. Microscopy Analysis of METs
2.7. NET FACS Preparation
2.8. Intracellular NE and MPO Staining in Neutrophils
2.9. Measurement of Arterial Cord Blood pH
2.10. Statistical Analysis
3. Results
3.1. Participant Characteristics
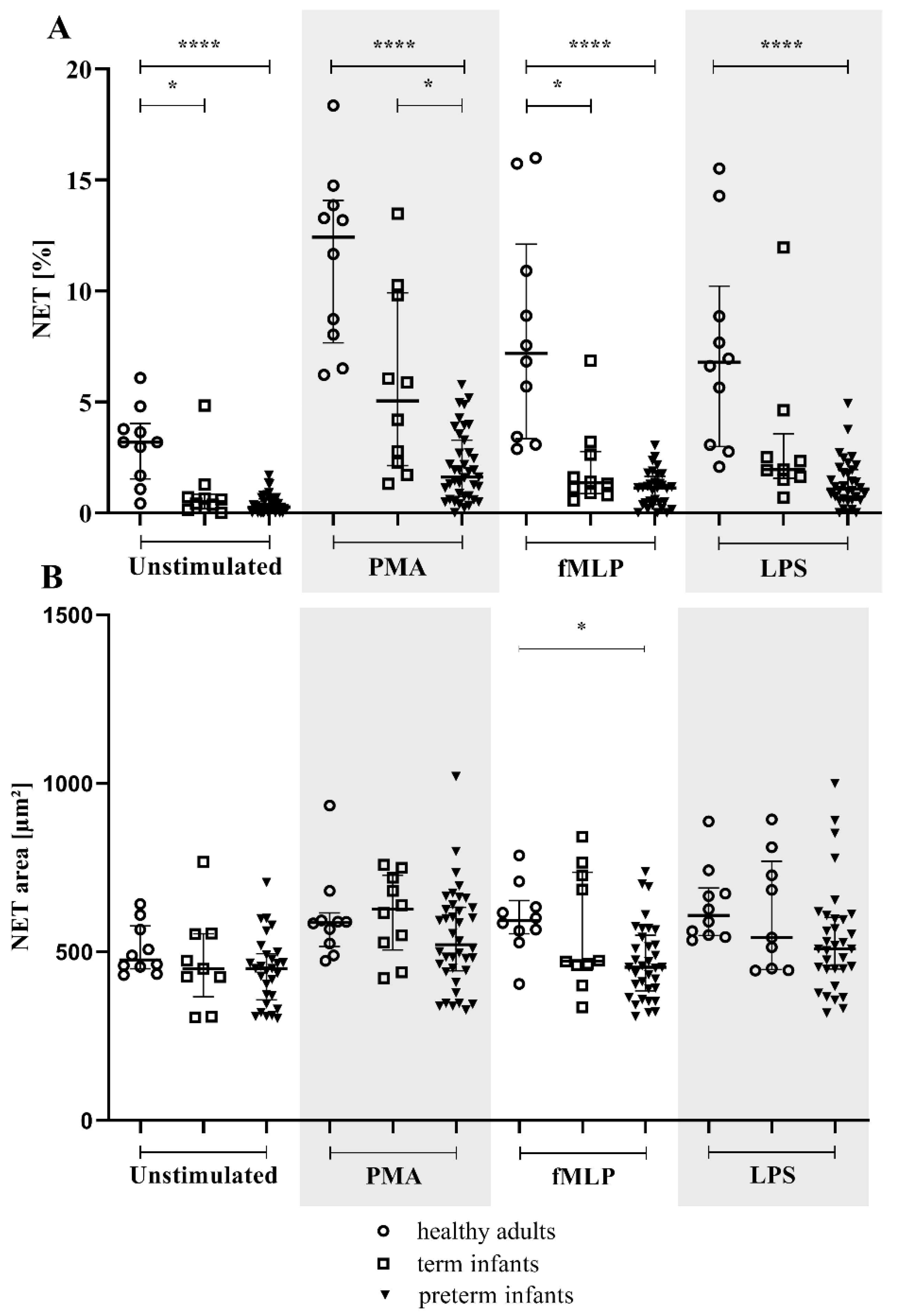
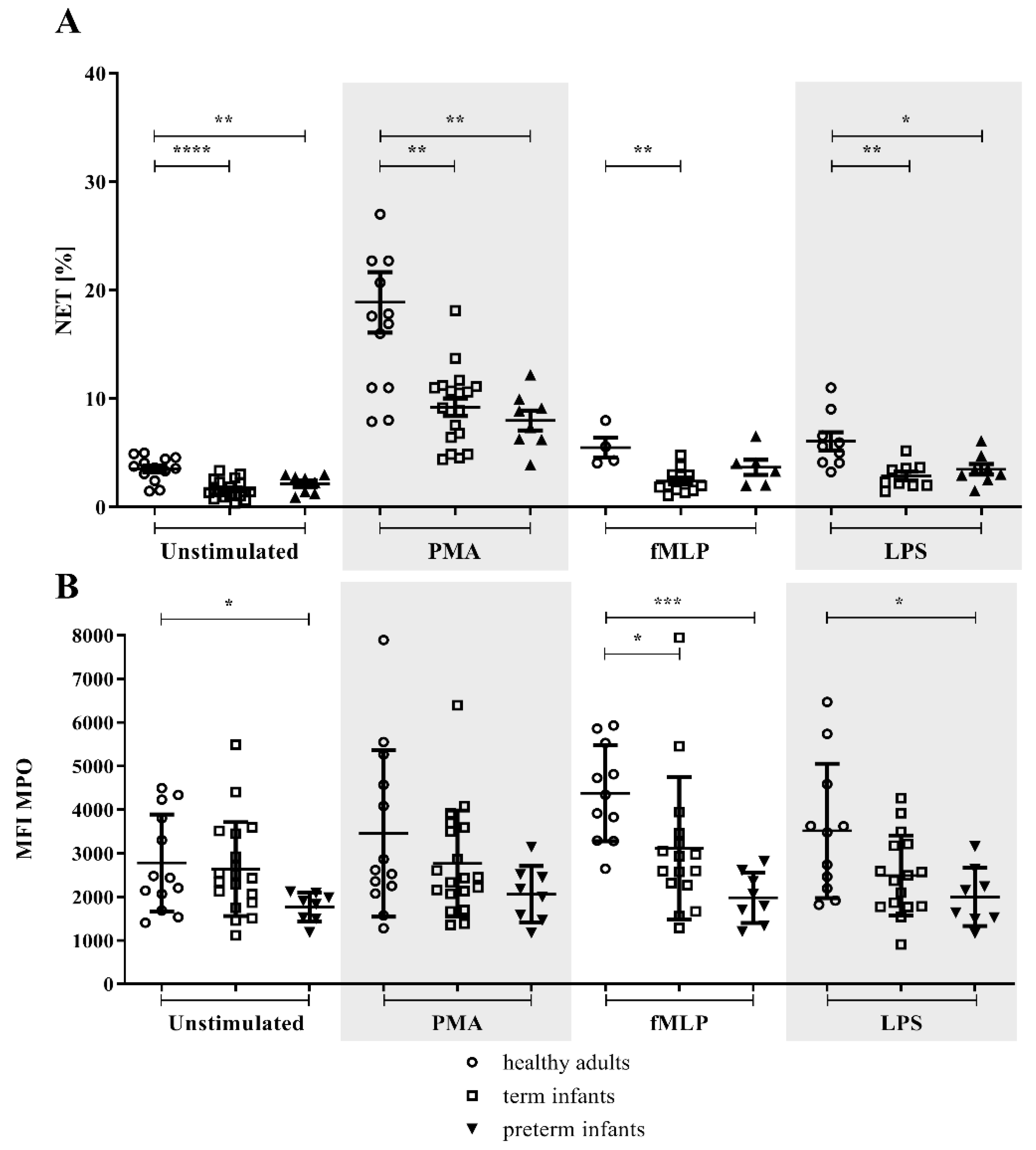
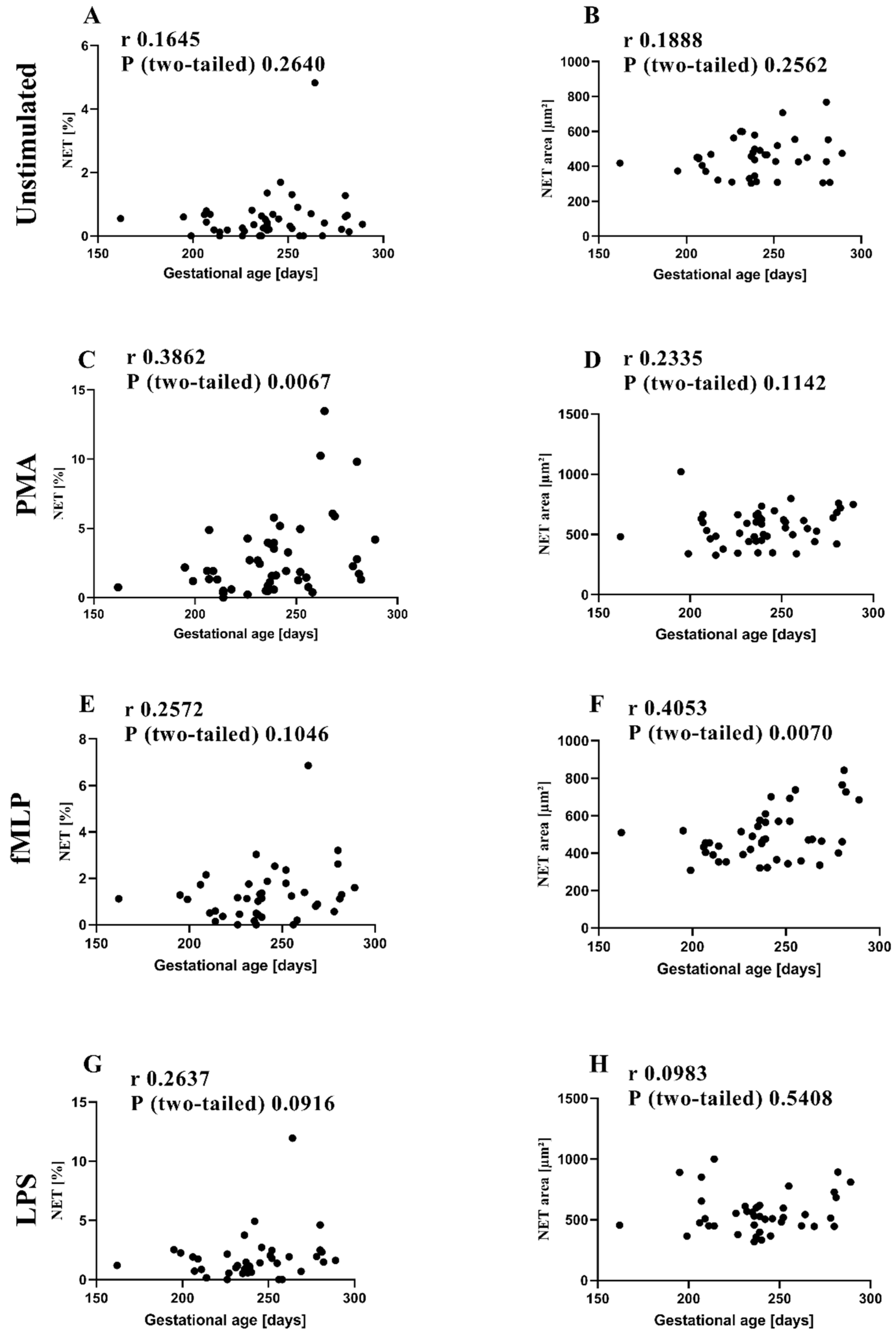
3.2. NETs and MPO within NETs in Preterm Infants in Comparison to Term Infants and Adults
3.3. MPO/NE in Neutrophils
3.4. METs in Preterm Infants in Comparison to Term Infants and Adults
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brook, B.; Harbeson, D.; Ben-Othman, R.; Viemann, D.; Kollmann, T.R. Newborn susceptibility to infection vs. disease depends on complex in vivo interactions of host and pathogen. Semin. Immunopathol. 2017, 39, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Weitkamp, J.-H.; Wynn, J.L. Why are preterm newborns at increased risk of infection? Arch. Dis. Child.-Fetal Neonatal Ed. 2018, 103, F391–F394. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, J.; Szamotulska, K.; Drewniak, N.; Mohangoo, A.; Chalmers, J.; Sakkeus, L.; Irgens, L.; Gatt, M.; Gissler, M.; Blondel, B.; et al. Preterm birth time trends in Europe: A study of 19 countries. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K. Births in the United States, 2018. NCHS Data Brief 2019, 346, 1–8. [Google Scholar]
- Harrison, M.S.; Goldenberg, R.L. Global burden of prematurity. Semin. Fetal Neonatal Med. 2016, 21, 74–79. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.H.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Förster-Waldl, E.; Sadeghi, K.; Tamandl, D.; Gerhold, B.; Hallwirth, U.; Rohrmeister, K.; Hayde, M.; Prusa, A.R.; Herkner, K.; Boltz-Nitulescu, G.; et al. Monocyte Toll-Like Receptor 4 Expression and LPS-Induced Cytokine Production Increase during Gestational Aging. Pediatr. Res. 2005, 58, 121–124. [Google Scholar] [CrossRef]
- Genel, F.; Atlihan, F.; Ozsu, E.; Ozbek, E. Monocyte HLA-DR expression as predictor of poor outcome in neonates with late onset neonatal sepsis. J. Infect. 2010, 60, 224–228. [Google Scholar] [CrossRef]
- Dreschers, S.; Saupp, P.; Hornef, M.; Prehn, A.; Platen, C.; Morschhäuser, J.; Orlikowsky, T.W. Reduced PICD in Monocytes Mounts Altered Neonate Immune Response to Candida albicans. PLoS ONE 2016, 11, e0166648. [Google Scholar] [CrossRef]
- Weinberger, B.; Laskin, D.L.; Mariano, T.M.; Sunil, V.R.; DeCoste, C.J.; Heck, D.E.; Gardner, C.R.; Laskin, J.D. Mechanisms underlying reduced responsiveness of neonatal neutrophils to distinct chemoattractants. J. Leukoc. Biol. 2001, 70, 969–976. [Google Scholar] [CrossRef]
- Branzk, N.; Papayannopoulos, V. Molecular mechanisms regulating NETosis in infection and disease. Semin. Immunopathol. 2013, 35, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Zychlinsky, A. NETs: A new strategy for using old weapons. Trends Immunol. 2009, 30, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Goldmann, O.; Medina, E. The expanding world of extracellular traps: Not only neutrophils but much more. Front. Immunol. 2012, 3, 420. [Google Scholar] [CrossRef]
- Halder, L.D.; Abdelfatah, M.A.; Jo, E.A.H.; Jacobsen, I.D.; Westermann, M.; Beyersdorf, N.; Lorkowski, S.; Zipfel, P.F.; Skerka, C. Factor H Binds to Extracellular DNA Traps Released from Human Blood Monocytes in Response to Candida albicans. Front. Immunol. 2016, 7, 671. [Google Scholar] [CrossRef] [PubMed]
- Haritha, V.H.; Seena, P.; Shaji, B.V.; Nithin, T.U.; Hazeena, V.N.; Anie, Y. Monocyte clearance of apoptotic neutrophils is unhindered in the presence of NETosis, but proteins of NET trigger ETosis in monocytes. Immunol. Lett. 2019, 207, 36–45. [Google Scholar] [CrossRef]
- Loureiro, A.; Pais, C.; Sampaio, P. Relevance of Macrophage Extracellular Traps in C. albicans Killing. Front. Immunol. 2019, 10, 2767. [Google Scholar] [CrossRef]
- Lipp, P.; Ruhnau, J.; Lange, A.; Vogelgesang, A.; Dressel, A.; Heckmann, M. Less Neutrophil Extracellular Trap Formation in Term Newborns than in Adults. Neonatology 2017, 111, 182–188. [Google Scholar] [CrossRef]
- Yost, C.C.; Cody, M.J.; Harris, E.S.; Thornton, N.L.; McInturff, A.M.; Martinez, M.L.; Chandler, N.B.; Rodesch, C.K.; Albertine, K.H.; Petti, C.A.; et al. Impaired neutrophil extracellular trap (NET) formation: A novel innate immune deficiency of human neonates. Blood 2009, 113, 6419–6427. [Google Scholar] [CrossRef]
- Marcos, V.; Nussbaum, C.; Vitkov, L.; Hector, A.; Wiedenbauer, E.-M.; Roos, D.; Kuijpers, T.; Krautgartner, W.D.; Genzel-Boroviczény, O.; Sperandio, M.; et al. Delayed but functional neutrophil extracellular trap formation in neonates. Blood 2009, 114, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Sangaletti, S.; Tripodo, C.; Chiodoni, C.; Guarnotta, C.; Cappetti, B.; Casalini, P.; Piconese, S.; Parenza, M.; Guiducci, C.; Vitali, C.; et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood 2012, 120, 3007–3018. [Google Scholar] [CrossRef]
- Saito, Y.; Nishio, K.; Ogawa, Y.; Kimata, J.; Kinumi, T.; Yoshida, Y.; Noguchi, N.; Niki, E. Turning point in apoptosis/necrosis induced by hydrogen peroxide. Free Radic. Res. 2006, 40, 619–630. [Google Scholar] [CrossRef]
- Masuda, S.; Shimizu, S.; Matsuo, J.; Nishibata, Y.; Kusunoki, Y.; Hattanda, F.; Shida, H.; Nakazawa, D.; Tomaru, U.; Atsumi, T.; et al. Measurement of NET formation in vitro and in vivo by flow cytometry. Cytom. Part A 2017, 91, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Hegge, I.; Niepel, F.; Lange, A.; Vogelgesang, A.; Heckmann, M.; Ruhnau, J. Functional analysis of granulocyte and monocyte subpopulations in neonates. Mol. Cell. Pediatr. 2019, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Metzler, K.D.; Fuchs, T.A.; Nauseef, W.M.; Reumaux, D.; Roesler, J.; Schulze, I.; Wahn, V.; Papayannopoulos, V.; Zychlinsky, A. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood 2011, 117, 953–959. [Google Scholar] [CrossRef]
- Gavillet, M.; Martinod, K.; Renella, R.; Harris, C.; Shapiro, N.I.; Wagner, D.D.; Williams, D.A. Flow cytometric assay for direct quantification of neutrophil extracellular traps in blood samples. Am. J. Hematol. 2015, 90, 1155–1158. [Google Scholar] [CrossRef]
- Byrd, A.S.; O’Brien, X.M.; Laforce-Nesbitt, S.S.; Parisi, V.E.; Hirakawa, M.P.; Bliss, J.M.; Reichner, J.S. NETosis in Neonates: Evidence of a Reactive Oxygen Species–Independent Pathway in Response to Fungal Challenge. J. Infect. Dis. 2015, 213, 634–639. [Google Scholar] [CrossRef]
- Hoppenbrouwers, T.; Autar, A.S.A.; Sultan, A.R.; Abraham, T.E.; Van Cappellen, W.A.; Houtsmuller, A.B.; Van Wamel, W.J.B.; Van Beusekom, H.M.M.; Van Neck, J.W.; De Maat, M.P.M. In vitro induction of NETosis: Comprehensive live imaging comparison and systematic review. PLoS ONE 2017, 12, e0176472. [Google Scholar] [CrossRef]
- Porzionato, A.; Zaramella, P.; Dedja, A.; Guidolin, D.; Van Wemmel, K.; Macchi, V.; Jurga, M.; Perilongo, G.; De Caro, R.; Baraldi, E.; et al. Intratracheal administration of clinical-grade mesenchymal stem cell-derived extracellular vesicles reduces lung injury in a rat model of bronchopulmonary dysplasia. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L6–L19. [Google Scholar] [CrossRef]
- Campbell, R.A.; Campbell, H.D.; Bircher, J.S.; de Araujo, C.V.; Denorme, F.; Crandell, J.L.; Rustad, J.L.; Monts, J.; Cody, M.J.; Kosaka, Y.; et al. Placental HTRA1 cleaves α1-antitrypsin to generate a NET-inhibitory peptide. Blood 2021, 138, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Yost, C.C.; Schwertz, H.; Cody, M.J.; Wallace, J.A.; Campbell, R.A.; Vieira-de-Abreu, A.; Araujo, C.V.; Schubert, S.; Harris, E.S.; Rowley, J.W.; et al. Neonatal NET-inhibitory factor and related peptides inhibit neutrophil extracellular trap formation. J. Clin. Investig. 2016, 126, 3783–3798. [Google Scholar] [CrossRef] [PubMed]
- Denorme, F.; Rustad, J.L.; Portier, I.; Crandell, J.L.; de Araujo, C.V.; Cody, M.J.; Campbell, R.A.; Yost, C.C. Neutrophil extracellular trap inhibition improves survival in neonatal mouse infectious peritonitis. Pediatr. Res. 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Granger, V.; Faille, D.; Marani, V.; Noël, B.; Gallais, Y.; Szely, N.; Flament, H.; Pallardy, M.; Chollet-Martin, S.; De Chaisemartin, L. Human blood monocytes are able to form extracellular traps. J. Leukoc. Biol. 2017, 102, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Thang, C.M.; Thanh, L.Q.; Dai, V.T.T.; Phan, V.T.; Nhu, B.T.H.; Trang, D.N.X.; Trinh, P.T.P.; Nguyen, T.V.; Toan, N.T.; et al. Immune Profiling of Cord Blood From Preterm and Term Infants Reveals Distinct Differences in Pro-Inflammatory Responses. Front. Immunol. 2021, 12, 777927. [Google Scholar] [CrossRef]
- Sullivan, G.; Galdi, P.; Borbye-Lorenzen, N.; Stoye, D.Q.; Lamb, G.J.; Evans, M.J.; Skogstrand, K.; Chandran, S.; Boardman, J.P. Preterm Birth Is Associated With Immune Dysregulation Which Persists in Infants Exposed to Histologic Chorioamnionitis. Front. Immunol. 2021, 12, 722489. [Google Scholar] [CrossRef]
- Webster, S.J.; Daigneault, M.; Bewley, M.A.; Preston, J.A.; Marriott, H.M.; Walmsley, S.R.; Read, R.C.; Whyte, M.K.B.; Dockrell, D.H. Distinct Cell Death Programs in Monocytes Regulate Innate Responses Following Challenge with Common Causes of Invasive Bacterial Disease. J. Immunol. 2010, 185, 2968–2979. [Google Scholar] [CrossRef]
- Cox, L.E.; Walstein, K.; Völlger, L.; Reuner, F.; Bick, A.; Dötsch, A.; Engler, A.; Peters, J.; von Köckritz-Blickwede, M.; Schäfer, S.T. Neutrophil extracellular trap formation and nuclease activity in septic patients. BMC Anesthesiol. 2020, 20, 15. [Google Scholar] [CrossRef]
- Stiel, C.U.; Ebenebe, C.U.; Trochimiuk, M.; Raluy, L.P.; Vincent, D.; Singer, D.; Reinshagen, K.; Boettcher, M. Markers of NETosis Do Not Predict Neonatal Early Onset Sepsis: A Pilot Study. Front. Pediatr. 2020, 7, 555. [Google Scholar] [CrossRef]
- Colón, D.F.; Wanderley, C.W.; Franchin, M.; Silva, C.M.; Hiroki, C.H.; Castanheira, F.V.S.; Donate, P.B.; Lopes, A.H.; Volpon, L.C.; Kavaguti, S.K.; et al. Neutrophil extracellular traps (NETs) exacerbate severity of infant sepsis. Crit. Care 2019, 23, 113. [Google Scholar] [CrossRef]
- Levy, O.; Martin, S.; Eichenwald, E.; Ganz, T.; Valore, E.; Carroll, S.F.; Lee, K.; Goldmann, D.; Thorne, G.M. Impaired Innate Immunity in the Newborn: Newborn Neutrophils Are Deficient in Bactericidal/Permeability-Increasing Protein. Pediatrics 1999, 104, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Depreester, E.; Meyer, E.; Demeyere, K.; Van Eetvelde, M.; Hostens, M.; Opsomer, G. Flow cytometric assessment of myeloperoxidase in bovine blood neutrophils and monocytes. J. Dairy Sci. 2017, 100, 7638–7647. [Google Scholar] [CrossRef] [PubMed]

| Population | N | Female | Gestational Age, Weeks | Birth Weight, g | Arterial Umbilical pH | APGAR 5-min | Cesarean Section | Spontaneous Delivery | |
|---|---|---|---|---|---|---|---|---|---|
| NET microscopy | Preterm | 39 | 20 (39) | 33 + 5 (23 + 1–36 + 3) | 1950 (600–3150) | 7.34 (7.14–7.45) | 9 (6–10) | 26 (39) | 13 (39) |
| Term | 10 | 5 (10) | 39 + 5 (37 + 3–40 + 2) | 3190 (2500–4970) | 7.28 (7.13–7.34) | 10 (9–10) | 6 (10) | 4 (10) | |
| Adults | 10 | 8 (10) | n.a. * | n.a. | n.a. | n.a. | n.a. | n.a. | |
| NET FACS | Preterm | 8 | 3 (8) | 33 + 4 (31 + 0–35 + 2) | 2262.5 (1480–2450) | 7.33 (7.29–7.36) | 9 (8–10) | 5 (8) | 3 (8) |
| Term | 19 | 10 (19) | 40 + 0 (39 + 0–41 + 5) | 3680 (2640–4485) | 7.28 (7.13–7.36) | 10 (8–10) | 11(19) | 8 (19) | |
| Adults | 13 | 8 (13) | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |
| MET microscopy | Preterm | 8 | 2 (8) | 32 + 4 (26 + 2–36 + 1) | 1917.5 (990–2950) | 7.33 (7.28–7.42) | 9 (8–10) | 6 (8) | 2 (8) |
| Term | 16 | 10 (16) | 40 + 0 (37 + 4–41 + 5) | 3495 (2640–4830) | 7.31 (7.14–7.36) | 10 (8–10) | 10 (16) | 6 (16) | |
| Adults | 10 | 5 (5) | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |
| NE/MPO FACS | Term | 10 | 5 (10) | 40 + 0 (40 + 0–40 + 0) | 3483 (2720–3915) | 7.32 (7.23–7.38) | 9.5 (8–10) | 7 (10) | 3 (10) |
| Adults | 10 | 6 (10) | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wirkner, A.; Vogelgesang, A.; Hegge, I.; Lange, A.; Olbertz, D.M.; Gerber, B.; Heckmann, M.; Ruhnau, J. Preterm ETs Are Significantly Reduced Compared with Adults and Partially Reduced Compared with Term Infants. Children 2022, 9, 1522. https://doi.org/10.3390/children9101522
Wirkner A, Vogelgesang A, Hegge I, Lange A, Olbertz DM, Gerber B, Heckmann M, Ruhnau J. Preterm ETs Are Significantly Reduced Compared with Adults and Partially Reduced Compared with Term Infants. Children. 2022; 9(10):1522. https://doi.org/10.3390/children9101522
Chicago/Turabian StyleWirkner, Aila, Antje Vogelgesang, Ines Hegge, Anja Lange, Dirk Manfred Olbertz, Bernd Gerber, Matthias Heckmann, and Johanna Ruhnau. 2022. "Preterm ETs Are Significantly Reduced Compared with Adults and Partially Reduced Compared with Term Infants" Children 9, no. 10: 1522. https://doi.org/10.3390/children9101522
APA StyleWirkner, A., Vogelgesang, A., Hegge, I., Lange, A., Olbertz, D. M., Gerber, B., Heckmann, M., & Ruhnau, J. (2022). Preterm ETs Are Significantly Reduced Compared with Adults and Partially Reduced Compared with Term Infants. Children, 9(10), 1522. https://doi.org/10.3390/children9101522





