Airway Management in Pediatric Patients: Cuff-Solved Problem?
Abstract
1. Introduction
2. Airway Anatomy
3. Uncuffed vs. Cuffed Tubes
4. Tube Size
5. Airway Management in Pediatric Anesthesiology
6. Airway Management in Neonatal Intensive Care (NICU) and Pediatric Intensive Care (PICU)
7. Cuffed Tracheostomy Tubes
8. Cuff-Related Complications
9. Optimal Pediatric Airway Management
10. EBM View and Future Perspectives
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Eckenhoff, J.E. Some Anatomic Considerations of the Infant Larynx Influencing Endotracheal Anesthesia. Anesthesiology 1951, 12, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, E.K. The Shape of the Pediatric Larynx: Cylindrical or Funnel Shaped? Anesth. Analg. 2009, 108, 1379–1381. [Google Scholar] [CrossRef]
- Tobias, J.D. Pediatric Airway Anatomy May Not Be What We Thought: Implications for Clinical Practice and the Use of Cuffed Endotracheal Tubes. Pediatr. Anesth. 2015, 25, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Dullenkopf, A. Cuffed Tracheal Tubes in Children: Past, Present and Future. Expert Rev. Med. Devices 2007, 4, 73–82. [Google Scholar] [CrossRef]
- Chambers, N.A.; Ramgolam, A.; Sommerfield, D.; Zhang, G.; Ledowski, T.; Thurm, M.; Lethbridge, M.; Hegarty, M.; von Ungern-Sternberg, B.S. Cuffed vs. Uncuffed Tracheal Tubes in Children: A Randomised Controlled Trial Comparing Leak, Tidal Volume and Complications. Anaesthesia 2018, 73, 160–168. [Google Scholar] [CrossRef]
- Weiss, M.; Dullenkopf, A.; Fischer, J.E.; Keller, C.; Gerber, A.C.; European Paediatric Endotracheal Intubation Study Group. Prospective Randomized Controlled Multi-Centre Trial of Cuffed or Uncuffed Endotracheal Tubes in Small Children. Br. J. Anaesth. 2009, 103, 867–873. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Pan, G.; Li, X.; Shi, T.; He, W. Cuffed Versus Uncuffed Endotracheal Tubes in Pediatrics: A Meta-Analysis. Open Med. Wars. Pol. 2018, 13, 366–373. [Google Scholar] [CrossRef]
- Shah, A.; Carlisle, J.B. Cuffed Tracheal Tubes: Guilty Now Proven Innocent. Anaesthesia 2019, 74, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Litman, R.S.; Weissend, E.E.; Shibata, D.; Westesson, P.-L. Developmental Changes of Laryngeal Dimensions in Unparalyzed, Sedated Children. Anesthesiology 2003, 98, 41–45. [Google Scholar] [CrossRef]
- Wani, T.M.; Rafiq, M.; Akhter, N.; AlGhamdi, F.S.; Tobias, J.D. Upper Airway in Infants-a Computed Tomography-Based Analysis. Paediatr. Anaesth. 2017, 27, 501–505. [Google Scholar] [CrossRef]
- Mizuguchi, S.; Motomura, Y.; Maki, J.; Baba, R.; Ichimiya, Y.; Tokuda, K.; Kaku, N.; Takada, H.; Maehara, Y.; Ohga, S. Tracheal Size and Morphology on the Reconstructed CT Imaging. Pediatr. Crit. Care Med. 2019, 20, e366–e371. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E.; Rao, S.C.; Minutillo, C.; Hullett, B.; Bulsara, M.K. Cuffed Endotracheal Tubes in Infants Less than 3 kg: A Retrospective Cohort Study. Pediatr. Anesth. 2018, 28, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, D.P.; Bowman, S.M.; Klein, M.B.; Archer, D.; Sharar, S.R. Perioperative Use of Cuffed Endotracheal Tubes Is Advantageous in Young Pediatric Burn Patients. Burns 2010, 36, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Khine, H.H.; Corddry, D.H.; Kettrick, R.G.; Martin, T.M.; McCloskey, J.J.; Rose, J.B.; Theroux, M.C.; Zagnoev, M. Comparison of Cuffed and Uncuffed Endotracheal Tubes in Young Children during General Anesthesia. Anesthesiology 1997, 86, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Dullenkopf, A.; Gerber, A.C.; Weiss, M. Fit and Seal Characteristics of a New Paediatric Tracheal Tube with High Volume-Low Pressure Polyurethane Cuff. Acta Anaesthesiol. Scand. 2005, 49, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Salgo, B.; Schmitz, A.; Henze, G.; Stutz, K.; Dullenkopf, A.; Neff, S.; Gerber, A.C.; Weiss, M. Evaluation of a New Recommendation for Improved Cuffed Tracheal Tube Size Selection in Infants and Small Children. Acta Anaesthesiol. Scand. 2006, 50, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Eschertzhuber, S.; Salgo, B.; Schmitz, A.; Roth, W.; Frotzler, A.; Keller, C.H.; Gerber, A.C.; Weiss, M. Cuffed Endotracheal Tubes in Children Reduce Sevoflurane and Medical Gas Consumption and Related Costs. Acta Anaesthesiol. Scand. 2010, 54, 855–858. [Google Scholar] [CrossRef]
- Murat, I. Cuffed Tubes in Children: A 3-Year Experience in a Single Institution. Paediatr. Anaesth. 2001, 11, 748–749. [Google Scholar] [CrossRef]
- Thomas, R.E.; Erickson, S.; Hullett, B.; Minutillo, C.; Lethbridge, M.; Vijayasekaran, S.; Agrawal, S.; Bulsara, M.K.; Rao, S.C. Comparison of the Efficacy and Safety of Cuffed versus Uncuffed Endotracheal Tubes for Infants in the Intensive Care Setting: A Pilot, Unblinded RCT. Arch. Dis. Child.-Fetal Neonatal Ed. 2021, 106, 614–620. [Google Scholar] [CrossRef]
- Thomas, R.; Rao, S.; Minutillo, C. Cuffed Endotracheal Tubes in Neonates and Infants: A Survey of Practice. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F181–F182. [Google Scholar] [CrossRef]
- Flynn, P.E.; Black, A.E.; Mitchell, V. The Use of Cuffed Tracheal Tubes for Paediatric Tracheal Intubation, a Survey of Specialist Practice in the United Kingdom. Eur. J. Anaesthesiol. 2008, 25, 685–688. [Google Scholar] [CrossRef]
- Nishisaki, A.; Turner, D.A.; Brown, C.A.; Walls, R.M.; Nadkarni, V.M.; National Emergency Airway Registry for Children (NEAR4KIDS); Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. A National Emergency Airway Registry for Children: Landscape of Tracheal Intubation in 15 PICUs. Crit. Care Med. 2013, 41, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.P.B.B.; Bacci, S.L.L.D.S.; Pereira, J.M.; Johnston, C.; Azevedo, V.M.G.O. Frequency and Characterization of the Use of Cuffed Tracheal Tubes in Neonatal and Pediatric Intensive Care Units in Brazil. Rev. Bras. Ter. Intensiva 2020, 32. [Google Scholar] [CrossRef] [PubMed]
- Van de Voorde, P.; Turner, N.M.; Djakow, J.; de Lucas, N.; Martinez-Mejias, A.; Biarent, D.; Bingham, R.; Brissaud, O.; Hoffmann, F.; Johannesdottir, G.B.; et al. Paediatric Life Support. Notf. Rettungsmed. 2021, 24, 650–719. [Google Scholar] [CrossRef]
- Matava, C.T.; Kovatsis, P.G.; Lee, J.K.; Castro, P.; Denning, S.; Yu, J.; Park, R.; Lockman, J.L.; Von Ungern-Sternberg, B.; Sabato, S.; et al. Pediatric Airway Management in COVID-19 Patients: Consensus Guidelines from the Society for Pediatric Anesthesia’s Pediatric Difficult Intubation Collaborative and the Canadian Pediatric Anesthesia Society. Anesth. Analg. 2020, 131, 61–73. [Google Scholar] [CrossRef]
- Kneyber, M.C.J.; de Luca, D.; Calderini, E.; Jarreau, P.-H.; Javouhey, E.; Lopez-Herce, J.; Hammer, J.; Macrae, D.; Markhorst, D.G.; Medina, A.; et al. Recommendations for Mechanical Ventilation of Critically Ill Children from the Paediatric Mechanical Ventilation Consensus Conference (PEMVECC). Intensive Care Med. 2017, 43, 1764–1780. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.R. Time to Stop Using Uncuffed Tracheal Tubes in Children? Anaesthesia 2018, 73, 147–150. [Google Scholar] [CrossRef]
- Weiss, M.; Dullenkopf, A.; Gerber, A.C. Microcuff pediatric tracheal tube. A new tracheal tube with a high volume-low pressure cuff for children. Anaesthesist 2004, 53, 73–79. [Google Scholar] [CrossRef]
- Weiss, M.; Gerber, A.C.; Dullenkopf, A. Appropriate Placement of Intubation Depth Marks in a New Cuffed Paediatric Tracheal Tube. Br. J. Anaesth. 2005, 94, 80–87. [Google Scholar] [CrossRef]
- Talekar, C.R.; Udy, A.A.; Boots, R.J.; Lipman, J.; Cook, D. Tracheal Cuff Pressure Monitoring in the ICU: A Literature Review and Survey of Current Practice in Queensland. Anaesth. Intensive Care 2014, 42, 761–770. [Google Scholar] [CrossRef]
- Efrati, S.; Bolotin, G.; Levi, L.; Zaaroor, M.; Guralnik, L.; Weksler, N.; Levinger, U.; Soroksky, A.; Denman, W.T.; Gurman, G.M. Optimization of Endotracheal Tube Cuff Pressure by Monitoring CO2 Levels in the Subglottic Space in Mechanically Ventilated Patients: A Randomized Controlled Trial. Anesth. Analg. 2017, 125, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Cole, F. Pediatric Formulas for the Anesthesiologist. AMA Am. J. Dis. Child. 1957, 94, 672–673. [Google Scholar] [CrossRef] [PubMed]
- Motoyama: Smith’s Anesthesia for Infants and Children. Available online: https://scholar.google.com/scholar_lookup?title=Smith%E2%80%99s+Anesthesia+for+Infants+and+Children&author=EK+Motoyama&publication_year=2006&#d=gs_cit&t=1655893791867&u=%2Fscholar%3Fq%3Dinfo%3AEaqV7urwcwkJ%3Ascholar.google.com%2F%26output%3Dcite%26scirp%3D0%26hl%3Dcs (accessed on 22 June 2022).
- Bae, J.-Y.; Byon, H.-J.; Han, S.-S.; Kim, H.-S.; Kim, J.-T. Usefulness of Ultrasound for Selecting a Correctly Sized Uncuffed Tracheal Tube for Paediatric Patients. Anaesthesia 2011, 66, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, M.; Nakajima, Y.; Ishii, S.; Shimizu, F.; Shime, N.; Sessler, D.I. Prediction of Pediatric Endotracheal Tube Size by Ultrasonography. Anesthesiology 2010, 113, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Schramm, C.; Knop, J.; Jensen, K.; Plaschke, K. Role of Ultrasound Compared to Age-Related Formulas for Uncuffed Endotracheal Intubation in a Pediatric Population. Pediatr. Anesth. 2012, 22, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Ekor, O.E.; Olatosi, J.O.; Rotimi, M.K.; Dada, O.I.O.; Awodesu, T.; Menkiti, D.I.; Olowoyeye, O.A. Airway Ultrasound Predicts Endotracheal Tube Size More Accurately than Cole’s Age-Based Formula in Paediatric Patients. S. Afr. J. Anaesth. Analg. 2022, 28, 99–103. [Google Scholar] [CrossRef]
- Raphael, P.O.; Thasim, E.; Simon, B.P.; Rajagopal, P. Comparative Study on Prediction of Paediatric Endotracheal Tube Size by Ultrasonography and by Age Based Formulas. Int. J. Res. Med. Sci. 2017, 4, 2528–2532. [Google Scholar] [CrossRef]
- Adler, A.C.; Siddiqui, A.; Chandrakantan, A.; Matava, C.T. Lung and Airway Ultrasound in Pediatric Anesthesia. Paediatr. Anaesth. 2022, 32, 202–208. [Google Scholar] [CrossRef]
- Tessaro, M.O.; Salant, E.P.; Arroyo, A.C.; Haines, L.E.; Dickman, E. Tracheal Rapid Ultrasound Saline Test (T.R.U.S.T.) for Confirming Correct Endotracheal Tube Depth in Children. Resuscitation 2015, 89, 8–12. [Google Scholar] [CrossRef]
- Litman, R.S.; Maxwell, L.G. Cuffed versus Uncuffed Endotracheal Tubes in Pediatric Anesthesia: The Debate Should Finally End. Anesthesiology 2013, 118, 500–501. [Google Scholar] [CrossRef]
- Sathyamoorthy, M.; Lerman, J.; Okhomina, V.I.; Penman, A.D. Use of Cuffed Tracheal Tubes in Neonates, Infants and Children: A Practice Survey of Members of the Society of Pediatric Anesthesia. J. Clin. Anesth. 2016, 33, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Calder, A.; Hegarty, M.; Erb, T.O.; von Ungern-Sternberg, B.S. Predictors of Postoperative Sore Throat in Intubated Children. Paediatr. Anaesth. 2012, 22, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Xiao, Y.; Xiong, W.; Zhou, Q.; Huang, X. Cuffed versus Uncuffed Endotracheal Tubes in Children: A Meta-Analysis. J. Anesth. 2016, 30, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Foley, L.J.; Urdaneta, F.; Berkow, L.; Aziz, M.F.; Baker, P.A.; Jagannathan, N.; Rosenblatt, W.; Straker, T.M.; Wong, D.T.; Hagberg, C.A. Difficult Airway Management in Adult Coronavirus Disease 2019 Patients: Statement by the Society of Airway Management. Anesth. Analg. 2021, 133, 876–890. [Google Scholar] [CrossRef]
- Zander, D.; Grass, B.; Weiss, M.; Buehler, P.K.; Schmitz, A. Cuffed Endotracheal Tubes in Neonates and Infants of Less than 3 Kg Body Weight—A Retrospective Audit. Pediatr. Anesth. 2021, 31, 604–610. [Google Scholar] [CrossRef]
- Fischer, M.; Grass, B.; Kemper, M.; Weiss, M.; Dave, M.H. Cuffed Pediatric Endotracheal Tubes—Outer Cuff Diameters Compared to Age-related Airway Dimensions. Pediatr. Anesth. 2020, 30, 424–434. [Google Scholar] [CrossRef]
- Sun, X.-L.; Li, J.; Wang, Z.-Y.; Li, M.-Y.; Duan, W.; Wu, N.; Yang, O. Reinforced Laryngeal Mask in Pediatric Laparoscopic Surgery. J. Coll. Physicians Surg. 2019, 29, 915–918. [Google Scholar] [CrossRef]
- Greif, R.; Theiler, L. The Use of Supraglottic Airway Devices in Pediatric Laparoscopic Surgery. Minerva Anestesiol. 2010, 76, 575–576. [Google Scholar]
- Thomas-Kattappurathu, G.; Kasisomayajula, A.; Short, J. Best Position and Depth of Anaesthesia for Laryngeal Mask Airway Removal in Children: A Randomised Controlled Trial. Eur. J. Anaesthesiol. 2015, 32, 624–630. [Google Scholar] [CrossRef]
- Sharma, B.; Sood, J.; Sehgal, R.; Sahai, C.; Gera, A. ProSeal Laryngeal Mask AirwayTM Insertion in the Prone Position: Optimal Utilization of Operation Theatre Personnel and Time? J. Anaesthesiol. Clin. Pharmacol. 2014, 30, 177–182. [Google Scholar] [CrossRef]
- Klučka, J.; Štourač, P.; Štoudek, R.; Ťoukálková, M.; Harazim, H.; Kosinová, M. Controversies in Pediatric Perioperative Airways. BioMed Res. Int. 2015, 2015, 368761. [Google Scholar] [CrossRef] [PubMed]
- Patki, A. Laryngeal Mask Airway vs. the Endotracheal Tube in Paediatric Airway Management: A Meta-Analysis of Prospective Randomised Controlled Trials. Indian J. Anaesth. 2011, 55, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Klučka, J.; Šenkyřík, J.; Skotáková, J.; Štoudek, R.; Ťoukalková, M.; Křikava, I.; Mareček, L.; Pavlík, T.; Štouračová, A.; Štourač, P. Laryngeal Mask Airway UniqueTM Position in Paediatric Patients Undergoing Magnetic Resonance Imaging (MRI): Prospective Observational Study. BMC Anesthesiol. 2018, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.L.; Baratt-Due, A.; Englund, P.N.; Harju, J.A.; Sigurðsson, T.S.; Liberg, J.-P. Paediatric Ventilation Treatment of Acute Lung Injury in Nordic Intensive Care Units. Acta Anaesthesiol. Scand. 2015, 59, 568–575. [Google Scholar] [CrossRef]
- Kollef, M.H. What Is Ventilator-Associated Pneumonia and Why Is It Important? Respir. Care 2005, 50, 714–721; discussion 721–724. [Google Scholar]
- Zolfaghari, P.S.; Wyncoll, D.L.A. The Tracheal Tube: Gateway to Ventilator-Associated Pneumonia. Crit. Care 2011, 15, 310. [Google Scholar] [CrossRef]
- Muscedere, J.G.; Martin, C.M.; Heyland, D.K. The Impact of Ventilator-Associated Pneumonia on the Canadian Health Care System. J. Crit. Care 2008, 23, 5–10. [Google Scholar] [CrossRef]
- Warren, D.K.; Shukla, S.J.; Olsen, M.A.; Kollef, M.H.; Hollenbeak, C.S.; Cox, M.J.; Cohen, M.M.; Fraser, V.J. Outcome and Attributable Cost of Ventilator-Associated Pneumonia among Intensive Care Unit Patients in a Suburban Medical Center. Crit. Care Med. 2003, 31, 1312–1317. [Google Scholar] [CrossRef]
- Safdar, N.; Dezfulian, C.; Collard, H.R.; Saint, S. Clinical and Economic Consequences of Ventilator-Associated Pneumonia: A Systematic Review. Crit. Care Med. 2005, 33, 2184–2193. [Google Scholar] [CrossRef]
- Niederman, M.S. The Clinical Diagnosis of Ventilator-Associated Pneumonia. Respir. Care 2005, 50, 788–796; discussion 807–812. [Google Scholar]
- Rello, J.; Soñora, R.; Jubert, P.; Artigas, A.; Rué, M.; Vallés, J. Pneumonia in Intubated Patients: Role of Respiratory Airway Care. Am. J. Respir. Crit. Care Med. 1996, 154, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Charles, M.P.; Kali, A.; Easow, J.M.; Joseph, N.M.; Ravishankar, M.; Srinivasan, S.; Kumar, S.; Umadevi, S. Ventilator-Associated Pneumonia. Australas. Med. J. 2014, 7, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Pitts, R.; Fisher, D.; Sulemanji, D.; Kratohvil, J.; Jiang, Y.; Kacmarek, R. Variables Affecting Leakage Past Endotracheal Tube Cuffs: A Bench Study. Intensive Care Med. 2010, 36, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- Young, P.J.; Pakeerathan, S.; Blunt, M.C.; Subramanya, S. A Low-Volume, Low-Pressure Tracheal Tube Cuff Reduces Pulmonary Aspiration. Crit. Care Med. 2006, 34, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Dullenkopf, A.; Gerber, A.; Weiss, M. Fluid Leakage Past Tracheal Tube Cuffs: Evaluation of the New Microcuff Endotracheal Tube. Intensive Care Med. 2003, 29, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Greaney, D.; Russell, J.; Dawkins, I.; Healy, M. A Retrospective Observational Study of Acquired Subglottic Stenosis Using Low-Pressure, High-Volume Cuffed Endotracheal Tubes. Paediatr. Anaesth. 2018, 28, 1136–1141. [Google Scholar] [CrossRef]
- Higgins, K.M.; Punthakee, X. Meta-Analysis Comparison of Open versus Percutaneous Tracheostomy. Laryngoscope 2007, 117, 447–454. [Google Scholar] [CrossRef]
- Schneider, J.; Mulale, U.; Yamout, S.; Pollard, S.; Silver, P. Impact of Monitoring Endotracheal Tube Cuff Leak Pressure on Postextubation Stridor in Children. J. Crit. Care 2016, 36, 173–177. [Google Scholar] [CrossRef]
- Tellez, D.W.; Galvis, A.G.; Storgion, S.A.; Amer, H.N.; Hoseyni, M.; Deakers, T.W. Dexamethasone in the Prevention of Postextubation Stridor in Children. J. Pediatr. 1991, 118, 289–294. [Google Scholar] [CrossRef]
- Deakers, T.W.; Reynolds, G.; Stretton, M.; Newth, C.J. Cuffed Endotracheal Tubes in Pediatric Intensive Care. J. Pediatr. 1994, 125, 57–62. [Google Scholar] [CrossRef]
- Newth, C.J.L.; Rachman, B.; Patel, N.; Hammer, J. The Use of Cuffed versus Uncuffed Endotracheal Tubes in Pediatric Intensive Care. J. Pediatr. 2004, 144, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Harless, J.; Ramaiah, R.; Bhananker, S.M. Pediatric Airway Management. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.J.; Parwani, V.; Hahn, I.-H. Experienced Emergency Medicine Physicians Cannot Safely Inflate or Estimate Endotracheal Tube Cuff Pressure Using Standard Techniques. Am. J. Emerg. Med. 2006, 24, 139–143. [Google Scholar] [CrossRef]
- Felten, M.-L.; Schmautz, E.; Delaporte-Cerceau, S.; Orliaguet, G.A.; Carli, P.A. Endotracheal Tube Cuff Pressure Is Unpredictable in Children. Anesth. Analg. 2003, 97, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Khemani, R.G.; Randolph, A.; Markovitz, B. Corticosteroids for the Prevention and Treatment of Post-Extubation Stridor in Neonates, Children and Adults. Cochrane Database Syst. Rev. 2009, 3, CD001000. [Google Scholar] [CrossRef] [PubMed]
- Venkategowda, P.M.; Mahendrakar, K.; Rao, S.M.; Mutkule, D.P.; Shirodkar, C.G.; Yogesh, H. Laryngeal Air Column Width Ratio in Predicting Post Extubation Stridor. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2015, 19, 170–173. [Google Scholar] [CrossRef]
- Walner, D.L.; Loewen, M.S.; Kimura, R.E. Neonatal Subglottic Stenosis—Incidence and Trends. Laryngoscope 2001, 111, 48–51. [Google Scholar] [CrossRef]
- Dankle, S.K.; Schuller, D.E.; McClead, R.E. Risk Factors for Neonatal Acquired Subglottic Stenosis. Ann. Otol. Rhinol. Laryngol. 1986, 95, 626–630. [Google Scholar] [CrossRef]
- Kruse, K.E.; Purohit, P.J.; Cadman, C.R.; Su, F.; Aghaeepour, N.; Hammer, G.B. Subglottic Stenosis Following Cardiac Surgery with Cardiopulmonary Bypass in Infants and Children. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2017, 18, 429–433. [Google Scholar] [CrossRef]
- Manica, D.; Schweiger, C.; Maróstica, P.J.C.; Kuhl, G.; Carvalho, P.R.A. Association between Length of Intubation and Subglottic Stenosis in Children. Laryngoscope 2013, 123, 1049–1054. [Google Scholar] [CrossRef]
- Schweiger, C.; Marostica, P.J.C.; Smith, M.M.; Manica, D.; Carvalho, P.R.A.; Kuhl, G. Incidence of Post-Intubation Subglottic Stenosis in Children: Prospective Study. J. Laryngol. Otol. 2013, 127, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E.; Rao, S.C.; Minutillo, C.; Vijayasekaran, S.; Nathan, E.A. Severe Acquired Subglottic Stenosis in Neonatal Intensive Care Graduates: A Case-Control Study. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F349–F354. [Google Scholar] [CrossRef] [PubMed]
- Abid, E.S.; Miller, K.A.; Monuteaux, M.C.; Nagler, J. Association between the Number of Endotracheal Intubation Attempts and Rates of Adverse Events in a Paediatric Emergency Department. Emerg. Med. J. 2022, 39, 601–607. [Google Scholar] [CrossRef]
- Rost, F.; Donaubauer, B.; Kirsten, H.; Schwarz, T.; Zimmermann, P.; Siekmeyer, M.; Gräfe, D.; Ebel, S.; Kleber, C.; Lacher, M.; et al. Tracheal Tube Misplacement after Emergency Intubation in Pediatric Trauma Patients: A Retrospective, Exploratory Study. Children 2022, 9, 289. [Google Scholar] [CrossRef]
- Meakin, G.H. Role of Muscle Relaxants in Pediatric Anesthesia. Curr. Opin. Anaesthesiol. 2007, 20, 227–231. [Google Scholar] [CrossRef]
- Klučka, J.; Ťoukalková, M.; Křikava, I.; Štoudek, R.; Klabusayová, E.; Moravská, M.; Štourač, P. Neuromuscular Blockade in Clinical Practice in Paediatric Anaesthesia: Retrospective Cohort Trial in a Tertiary Paediatric Anaesthesia Centre. Available online: https://www.signavitae.com/articles/10.22514/SV141.042018.10 (accessed on 25 August 2022).
- Gupta, A.; Kaur, R.; Malhotra, R.; Kale, S. Comparative Evaluation of Different Doses of Propofol Preceded by Fentanyl on Intubating Conditions and Pressor Response during Tracheal Intubation without Muscle Relaxants. Paediatr. Anaesth. 2006, 16, 399–405. [Google Scholar] [CrossRef]
- Hanci, V.; Erdoğan, G.; Okyay, R.D.; Yurtlu, B.S.; Ayoğlu, H.; Baydilek, Y.; Turan, I.O. Effects of Fentanyl-Lidocaine-Propofol and Dexmedetomidine-Lidocaine-Propofol on Tracheal Intubation without Use of Muscle Relaxants. Kaohsiung J. Med. Sci. 2010, 26, 244–250. [Google Scholar] [CrossRef]
- Siddik-Sayyid, S.M.; Taha, S.K.; Kanazi, G.E.; Chehade, J.-M.A.; Zbeidy, R.A.; Al Alami, A.A.; Zahreddine, B.W.; Khatib, M.F.; Baraka, A.S.; Aouad, M.T. Excellent Intubating Conditions with Remifentanil-Propofol and Either Low-Dose Rocuronium or Succinylcholine. Can. J. Anaesth. J. Can. Anesth. 2009, 56, 483–488. [Google Scholar] [CrossRef][Green Version]
- Julien-Marsollier, F.; Michelet, D.; Bellon, M.; Horlin, A.-L.; Devys, J.-M.; Dahmani, S. Muscle Relaxation for Tracheal Intubation during Paediatric Anaesthesia: A Meta-Analysis and Trial Sequential Analysis. Eur. J. Anaesthesiol. 2017, 34, 550–561. [Google Scholar] [CrossRef]
- Nauheimer, D.; Fink, H.; Fuchs-Buder, T.; Geldner, G.; Hofmockel, R.; Ulm, K.; Wallek, B.; Blobner, M. Muscle Relaxant Use for Tracheal Intubation in Pediatric Anaesthesia: A Survey of Clinical Practice in Germany. Paediatr. Anaesth. 2009, 19, 225–231. [Google Scholar] [CrossRef]
- Tokmaji, G.; Vermeulen, H.; Müller, M.C.; Kwakman, P.H.; Schultz, M.J.; Zaat, S.A. Silver-coated Endotracheal Tubes for Prevention of Ventilator-associated Pneumonia in Critically Ill Patients. Cochrane Database Syst. Rev. 2015, 2015, CD009201. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ling, X.; Liu, G.; Xiao, J. Antimicrobial Coating: Tracheal Tube Application. Int. J. Nanomed. 2022, 17, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- De Orange, F.A.; Andrade, R.G.; Lemos, A.; Borges, P.S.; Figueiroa, J.N.; Kovatsis, P.G. Cuffed versus Uncuffed Endotracheal Tubes for General Anaesthesia in Children Aged Eight Years and Under. Cochrane Database Syst. Rev. 2017, 2017, CD011954. [Google Scholar] [CrossRef] [PubMed]
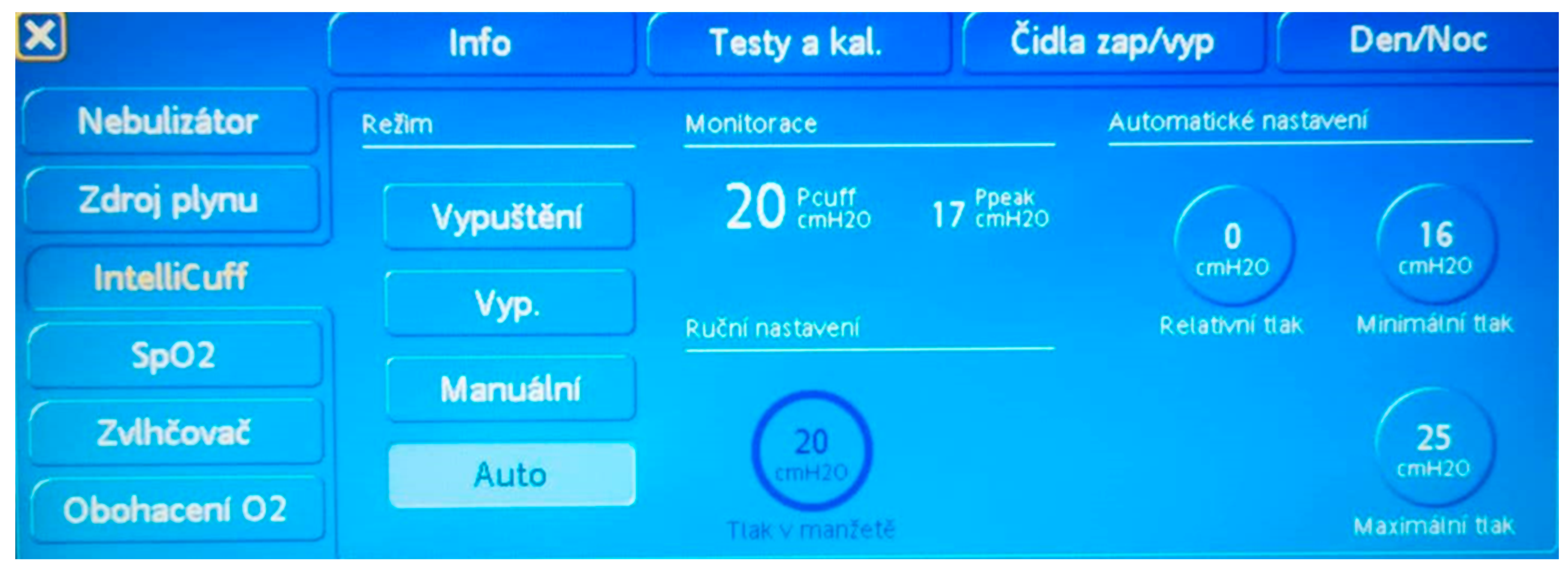
| Age | Uncuffed Tracheal Tube | Cuffed Tracheal Tube | Age | Microcuff® Tracheal Tube |
|---|---|---|---|---|
| Modified Cole’s Formula | Motoyama Formula | |||
| ID = (Age/4) + 4 | ID = (Age/4) + 3.5 | |||
| term-1 year | 3.5 mm | 3 mm | term to < 8 months | 3 mm |
| <2 years | 4 mm | 3.5 mm | 8 months to < 2 years | 3.5 mm |
| <4 years | 4.5 mm | 4 mm | <4 years | 4 mm |
| <6 years | 5 mm | 4.5 mm | <6 years | 4.5 mm |
| <8 years | 5.5 mm | 5 mm | <8 years | 5 mm |
| <10 years | 6 mm | 5.5 mm | <10 years | 5.5 mm |
| <12 years | 6.5 mm | 6 mm | <12 years | 6 mm |
| <14 years | 7 mm | 6.5 mm | <14 years | 6.5 mm |
| <16 years | 7.5 mm | 7 mm | <16 years | 7 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klabusayová, E.; Klučka, J.; Kratochvíl, M.; Musilová, T.; Vafek, V.; Skříšovská, T.; Djakow, J.; Kosinová, M.; Havránková, P.; Štourač, P. Airway Management in Pediatric Patients: Cuff-Solved Problem? Children 2022, 9, 1490. https://doi.org/10.3390/children9101490
Klabusayová E, Klučka J, Kratochvíl M, Musilová T, Vafek V, Skříšovská T, Djakow J, Kosinová M, Havránková P, Štourač P. Airway Management in Pediatric Patients: Cuff-Solved Problem? Children. 2022; 9(10):1490. https://doi.org/10.3390/children9101490
Chicago/Turabian StyleKlabusayová, Eva, Jozef Klučka, Milan Kratochvíl, Tereza Musilová, Václav Vafek, Tamara Skříšovská, Jana Djakow, Martina Kosinová, Pavla Havránková, and Petr Štourač. 2022. "Airway Management in Pediatric Patients: Cuff-Solved Problem?" Children 9, no. 10: 1490. https://doi.org/10.3390/children9101490
APA StyleKlabusayová, E., Klučka, J., Kratochvíl, M., Musilová, T., Vafek, V., Skříšovská, T., Djakow, J., Kosinová, M., Havránková, P., & Štourač, P. (2022). Airway Management in Pediatric Patients: Cuff-Solved Problem? Children, 9(10), 1490. https://doi.org/10.3390/children9101490






