Postoperative Epileptic Seizures in Children
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of the Two Groups
3.2. Postoperative Seizures and Epilepsy
3.3. Risk of Postoperative Seizures
4. Discussion
4.1. Epidemiology
4.2. Onset and Type of Seizures
4.3. Risk Factors
4.4. Pathogenesis
4.5. Perioperative ASMs Prophylaxis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- De Santis, A.; Villani, R.; Sinisi, M.; Stocchetti, N.; Perucca, E. Add-on Phenytoin Fails to Prevent Early Seizures after Surgery for Supratentorial Brain Tumors: A Randomized Controlled Study. Epilepsia 2002, 43, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Foy, P.M.; Copeland, G.P.; Shaw, M.D. The Incidence of Postoperative Seizures. Acta Neurochir. 1981, 55, 253–264. [Google Scholar] [CrossRef]
- Grobelny, B.T.; Ducruet, A.F.; Zacharia, B.E.; Hickman, Z.L.; Andersen, K.N.; Sussman, E.; Carpenter, A.; Connolly, E.S. Preoperative Antiepileptic Drug Administration and the Incidence of Postoperative Seizures Following Bur Hole-Treated Chronic Subdural Hematoma. J. Neurosurg. 2009, 111, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Kern, K.; Schebesch, K.M.; Schlaier, J.; Hansen, E.; Feigl, G.C.; Brawanski, A.T.; Lange, M. Levetiracetam Compared to Phenytoin for the Prevention of Postoperative Seizures after Craniotomy for Intracranial Tumours in Patients without Epilepsy. J. Clin. Neurosci. 2012, 19, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Kombogiorgas, D.; Jatavallabhula, N.S.; Sgouros, S.; Josan, V.; Walsh, A.R.; Hockley, A.D. Risk Factors for Developing Epilepsy after Craniotomy in Children. Child’s Nerv. Syst. 2006, 22, 1441–1445. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.D.; Nistal, D.; McGrath, M.; Barros, G.; Shenoy, V.S.; Sekhar, L.N.; Levitt, M.R.; Kim, L.J. De Novo Epilepsy after Microsurgical Resection of Brain Arteriovenous Malformations. Neurosurg. Focus 2022, 53, E6. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Rutkowski, M.J.; Chang, E.F.; Shangari, G.; Kane, A.J.; McDermott, M.W.; Berger, M.S.; Parsa, A.T. Postoperative Seizures Following the Resection of Convexity Meningiomas: Are Prophylactic Anticonvulsants Indicated? Clinical Article. J. Neurosurg. 2011, 114, 705–709. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, G.; Yang, H.-F.; Wang, D.; Yu, J.-L.; Huang, H.-Y. Assessment of Risk Factors for Early Seizures Following Surgery for Meningiomas Using Logistic Regression Analysis. J. Int. Med. Res. 2011, 39, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, F.; Lubrano, V.; Ribeiro-Filho, T.; Pradel, V.; Roche, P.-H. Incidence and clinical impact of seizures after surgery for chronic subdural haematoma. Neurochirurgie 2012, 58, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Goertz, L.; Speier, J.; Schulte, A.P.; Stavrinou, P.; Krischek, B.; Goldbrunner, R.; Timmer, M. Independent Risk Factors for Postoperative Seizures in Chronic Subdural Hematoma Identified by Multiple Logistic Regression Analysis. World Neurosurg. 2019, 132, e716–e721. [Google Scholar] [CrossRef] [PubMed]
- Hardesty, D.A.; Sanborn, M.R.; Parker, W.E.; Storm, P.B. Perioperative Seizure Incidence and Risk Factors in 223 Pediatric Brain Tumor Patients without Prior Seizures. J. Neurosurg. Pediatrics 2011, 7, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Komotar, R.J.; Raper, D.M.S.; Starke, R.M.; Iorgulescu, J.B.; Gutin, P.H. Prophylactic Antiepileptic Drug Therapy in Patients Undergoing Supratentorial Meningioma Resection: A Systematic Analysis of Efficacy. J. Neurosurg. 2011, 115, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Manaka, S.; Ishijima, B.; Mayanagi, Y. Postoperative Seizures: Epidemiology, Pathology, and Prophylaxis. Neurol. Med.-Chir. 2003, 43, 589–600; discussion 600. [Google Scholar] [CrossRef]
- Raper, D.M.S.; Kokabi, N.; McGee-Collett, M. The Efficacy of Antiepileptic Drug Prophylaxis in the Prevention of Early and Late Seizures Following Repair of Intracranial Aneurysms. J. Clin. Neurosci. 2011, 18, 1174–1179. [Google Scholar] [CrossRef]
- Visudthibhan, A.; Visudhiphan, P.; Chiemchanya, S.; Srirattanajaree, C. Seizures after Intracranial Surgery in Pediatric Patients. J. Med. Assoc. Thail. Chotmaihet Thangphaet 1999, 82 (Suppl. S1), S111–S116. [Google Scholar]
- Saadeh, F.S.; Melamed, E.F.; Rea, N.D.; Krieger, M.D. Seizure Outcomes of Supratentorial Brain Tumor Resection in Pediatric Patients. Neuro-Oncology 2018, 20, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE Classification of the Epilepsies: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Hwang, K.; Joo, J.-D.; Kim, Y.-H.; Han, J.H.; Oh, C.W.; Yun, C.-H.; Park, S.-H.; Kim, C.-Y. Risk Factors for Preoperative and Late Postoperative Seizures in Primary Supratentorial Meningiomas. Clin. Neurol. Neurosurg. 2019, 180, 34–39. [Google Scholar] [CrossRef]
- Ranger, A.; Diosy, D. Seizures in Children with Dysembryoplastic Neuroepithelial Tumors of the Brain--A Review of Surgical Outcomes across Several Studies. Child’s Nerv. Syst. 2015, 31, 847–855. [Google Scholar] [CrossRef]
- Matthew, E.; Sherwin, A.L.; Welner, S.A.; Odusote, K.; Stratford, J.G. Seizures Following Intracranial Surgery: Incidence in the First Post-Operative Week. Can. J. Neurol. Sci. 1980, 7, 285–290. [Google Scholar] [CrossRef]
- North, J.B.; Penhall, R.K.; Hanieh, A.; Hann, C.S.; Challen, R.G.; Frewin, D.B. Postoperative Epilepsy: A Double-Blind Trial of Phenytoin after Craniotomy. Lancet 1980, 1, 384–386. [Google Scholar] [CrossRef]
- Oushy, S.; Sillau, S.H.; Ney, D.E.; Damek, D.M.; Youssef, A.S.; Lillehei, K.O.; Ormond, D.R. New-Onset Seizure during and after Brain Tumor Excision: A Risk Assessment Analysis. J. Neurosurg. 2018, 128, 1713–1718. [Google Scholar] [CrossRef] [PubMed]
- Suri, A.; Mahapatra, A.K.; Bithal, P. Seizures Following Posterior Fossa Surgery. Br. J. Neurosurg. 1998, 12, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wu, Y.; Wang, S.; Huang, T.; Tian, Q.; Wang, J.; Qin, H.; Feng, D. Preoperative Antiepileptic Drug Prophylaxis for Early Postoperative Seizures in Supratentorial Meningioma: A Single-Center Experience. J. Neurooncol. 2022, 158, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Elbadry Ahmed, R.; Tang, H.; Asemota, A.; Huang, L.; Boling, W.; Bannout, F. Meningioma Related Epilepsy- Pathophysiology, Pre/Postoperative Seizures Predicators and Treatment. Front. Oncol. 2022, 12, 905976. [Google Scholar] [CrossRef] [PubMed]
- Strand, P.S.; Sagberg, L.M.; Gulati, S.; Solheim, O. Brain Infarction Following Meningioma Surgery-Incidence, Risk Factors, and Impact on Function, Seizure Risk, and Patient-Reported Quality of Life. Neurosurg. Rev. 2022, 45, 3237–3244. [Google Scholar] [CrossRef]
- Chandra, V.; Rock, A.K.; Opalak, C.; Stary, J.M.; Sima, A.P.; Carr, M.; Vega, R.A.; Broaddus, W.C. A Systematic Review of Perioperative Seizure Prophylaxis during Brain Tumor Resection: The Case for a Multicenter Randomized Clinical Trial. Neurosurg. Focus 2017, 43, E18. [Google Scholar] [CrossRef]
- Greenhalgh, J.; Weston, J.; Dundar, Y.; Nevitt, S.J.; Marson, A.G. Antiepileptic Drugs as Prophylaxis for Postcraniotomy Seizures. Cochrane Database Syst. Rev. 2020, 4, CD007286. [Google Scholar] [CrossRef]
- Walbert, T.; Harrison, R.A.; Schiff, D.; Avila, E.K.; Chen, M.; Kandula, P.; Lee, J.W.; Le Rhun, E.; Stevens, G.H.J.; Vogelbaum, M.A.; et al. SNO and EANO Practice Guideline Update: Anticonvulsant Prophylaxis in Patients with Newly Diagnosed Brain Tumors. Neuro-Oncology 2021, 23, 1835–1844. [Google Scholar] [CrossRef]
- Hwang, S.L.; Lieu, A.S.; Kuo, T.H.; Lin, C.L.; Chang, C.Z.; Huang, T.Y.; Howng, S.L. Preoperative and Postoperative Seizures in Patients with Astrocytic Tumours: Analysis of Incidence and Influencing Factors. J. Clin. Neurosci. 2001, 8, 426–429. [Google Scholar] [CrossRef]
- Wilne, S.; Collier, J.; Kennedy, C.; Koller, K.; Grundy, R.; Walker, D. Presentation of Childhood CNS Tumours: A Systematic Review and Meta-Analysis. Lancet Oncol. 2007, 8, 685–695. [Google Scholar] [CrossRef]
- Massimi, L.; Battaglia, D.; Bianchi, F.; Peraio, S.; Peppucci, E.; Di Rocco, C. Postoperative Epileptic Seizures in Children: Is the Brain Incision a Risk Factor? Neurosurgery 2018, 82, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Jennett, W.B. Early Traumatic Epilepsy. Definition and Identity. Lancet 1969, 1, 1023–1025. [Google Scholar] [CrossRef]
- Mauro, A.M.; Bomprezzi, C.; Morresi, S.; Provinciali, L.; Formica, F.; Iacoangeli, M.; Scerrati, M. Prevention of Early Postoperative Seizures in Patients with Primary Brain Tumors: Preliminary Experience with Oxcarbazepine. J. Neurooncol. 2007, 81, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, E.; Lenzi, B.; Vannozzi, R.; Parenti, G.F.; Iudice, A. Incidence and Management of Late Postsurgical Seizures in Clinical Practice. Turk. Neurosurg. 2012, 22, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Maehara, T.; Ichimura, K.; Suzuki, R.; Hirakawa, K.; Monma, S. Low Incidence of Seizures in Patients with Chronic Subdural Haematoma. J. Neurol. Neurosurg. Psychiatry 1993, 56, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.N.; Belzer, J.S.; Riva-Cambrin, J.; Presson, A.P.; Bratton, S.L. The Incidence of Postoperative Hyponatremia and Associated Neurological Sequelae in Children with Intracranial Neoplasms. J. Neurosurg. Pediatrics 2014, 13, 283–290. [Google Scholar] [CrossRef]
- Moussazadeh, N.; Souweidane, M. Brain Tumors in the First Two Years of Life. In Principles and Practice of Pediatric Neurosurgery; Thieme: New York, NY, USA, 2015; pp. 423–444. [Google Scholar]
- Brokinkel, B.; Hinrichs, F.L.; Schipmann, S.; Grauer, O.; Sporns, P.B.; Adeli, A.; Brokinkel, C.; Hess, K.; Paulus, W.; Stummer, W.; et al. Predicting Postoperative Seizure Development in Meningiomas—Analyses of Clinical, Histological and Radiological Risk Factors. Clin. Neurol. Neurosurg. 2021, 200, 106315. [Google Scholar] [CrossRef]
- Lee, S.T.; Lui, T.N.; Chang, C.N.; Cheng, W.C. Early Postoperative Seizures after Posterior Fossa Surgery. J. Neurosurg. 1990, 73, 541–544. [Google Scholar] [CrossRef]
- Temkin, N.R. Antiepileptogenesis and Seizure Prevention Trials with Antiepileptic Drugs: Meta-Analysis of Controlled Trials. Epilepsia 2001, 42, 515–524. [Google Scholar] [CrossRef]
- Durmus, N.; Gültürk, S.; Kaya, T.; Demir, T.; Parlak, M.; Altun, A. Evaluation of Effects of T and N Type Calcium Channel Blockers on the Electroencephalogram Recordings in Wistar Albino Glaxo/Rij Rats, an Absence Epilepsy Model. Indian J. Pharmacol. 2015, 47, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Losi, G.; Marcon, I.; Mariotti, L.; Sessolo, M.; Chiavegato, A.; Carmignoto, G. A Brain Slice Experimental Model to Study the Generation and the Propagation of Focally-Induced Epileptiform Activity. J. Neurosci. Methods 2016, 260, 125–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marino Gammazza, A.; Colangeli, R.; Orban, G.; Pierucci, M.; Di Gennaro, G.; Lo Bello, M.; D’Aniello, A.; Bucchieri, F.; Pomara, C.; Valentino, M.; et al. Hsp60 Response in Experimental and Human Temporal Lobe Epilepsy. Sci. Rep. 2015, 5, 9434. [Google Scholar] [CrossRef]
- Lai, L.; Morgan, M.K.; Trooboff, S.; Harvey, R.J. A Systematic Review of Published Evidence on Expanded Endoscopic Endonasal Skull Base Surgery and the Risk of Postoperative Seizure. J. Clin. Neurosci. 2013, 20, 197–203. [Google Scholar] [CrossRef]
- Forcelli, P.A.; Kalikhman, D.; Gale, K. Delayed Effect of Craniotomy on Experimental Seizures in Rats. PLoS ONE 2013, 8, e81401. [Google Scholar] [CrossRef] [PubMed]
- Tremont-Lukats, I.W.; Ratilal, B.O.; Armstrong, T.; Gilbert, M.R. Antiepileptic Drugs for Preventing Seizures in People with Brain Tumors. Cochrane Database Syst. Rev. 2008, 2, CD004424. [Google Scholar] [CrossRef]
- Wu, A.S.; Trinh, V.T.; Suki, D.; Graham, S.; Forman, A.; Weinberg, J.S.; McCutcheon, I.E.; Prabhu, S.S.; Heimberger, A.B.; Sawaya, R.; et al. A Prospective Randomized Trial of Perioperative Seizure Prophylaxis in Patients with Intraparenchymal Brain Tumors. J. Neurosurg. 2013, 118, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Mekitarian Filho, E.; de Carvalho, W.B.; Cavalheiro, S. Perioperative Patient Management in Pediatric Neurosurgery. Rev. Assoc. Med. Bras. 2012, 58, 388–396. [Google Scholar] [CrossRef]
- Ansari, S.F.; Bohnstedt, B.N.; Perkins, S.M.; Althouse, S.K.; Miller, J.C. Efficacy of Postoperative Seizure Prophylaxis in Intra-Axial Brain Tumor Resections. J. Neurooncol. 2014, 118, 117–122. [Google Scholar] [CrossRef]
- Lockney, D.T.; Vaziri, S.; Walch, F.; Kubilis, P.; Neal, D.; Murad, G.J.A.; Rahman, M. Prophylactic Antiepileptic Drug Use in Patients with Brain Tumors Undergoing Craniotomy. World Neurosurg. 2017, 98, 28–33. [Google Scholar] [CrossRef] [PubMed]
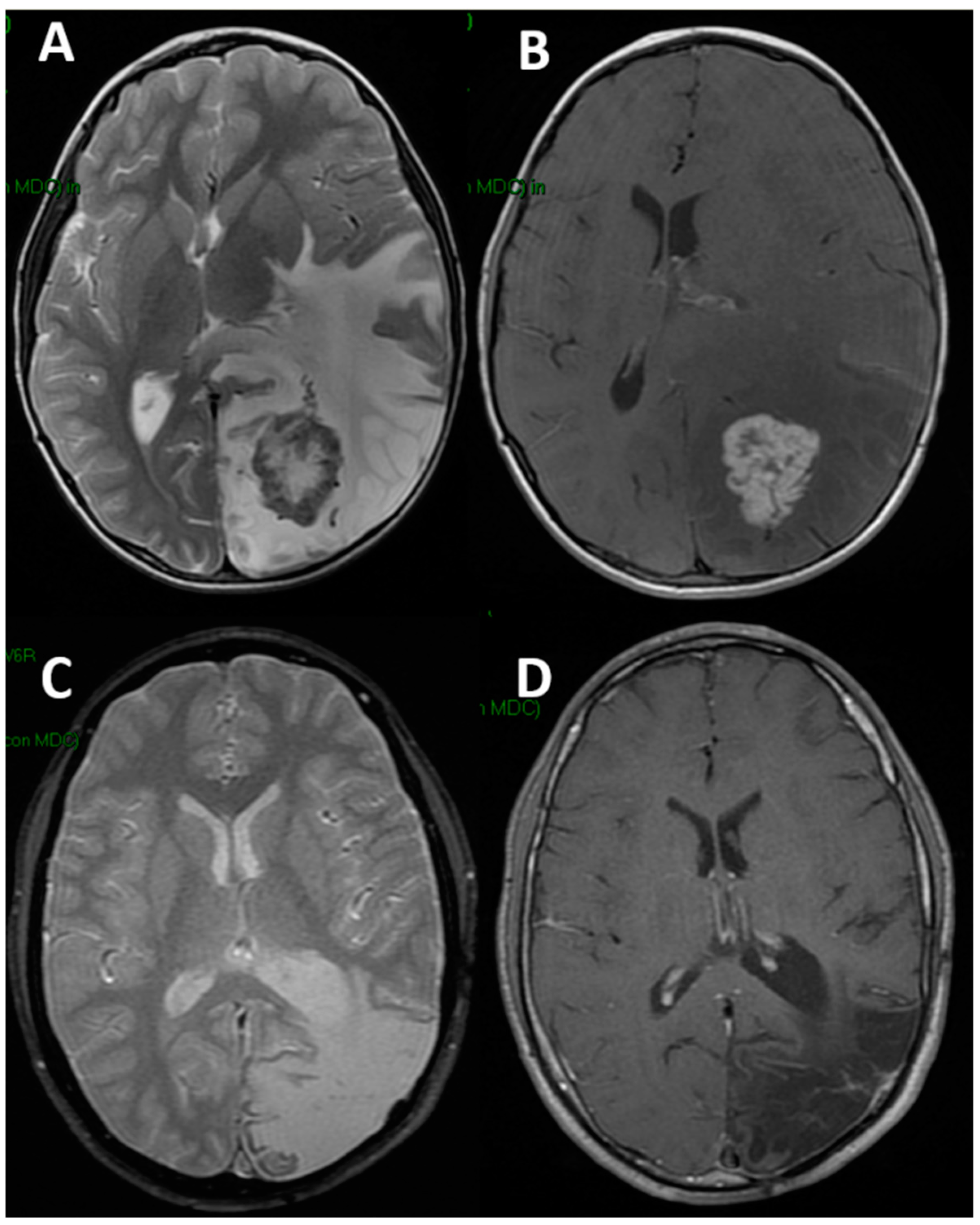
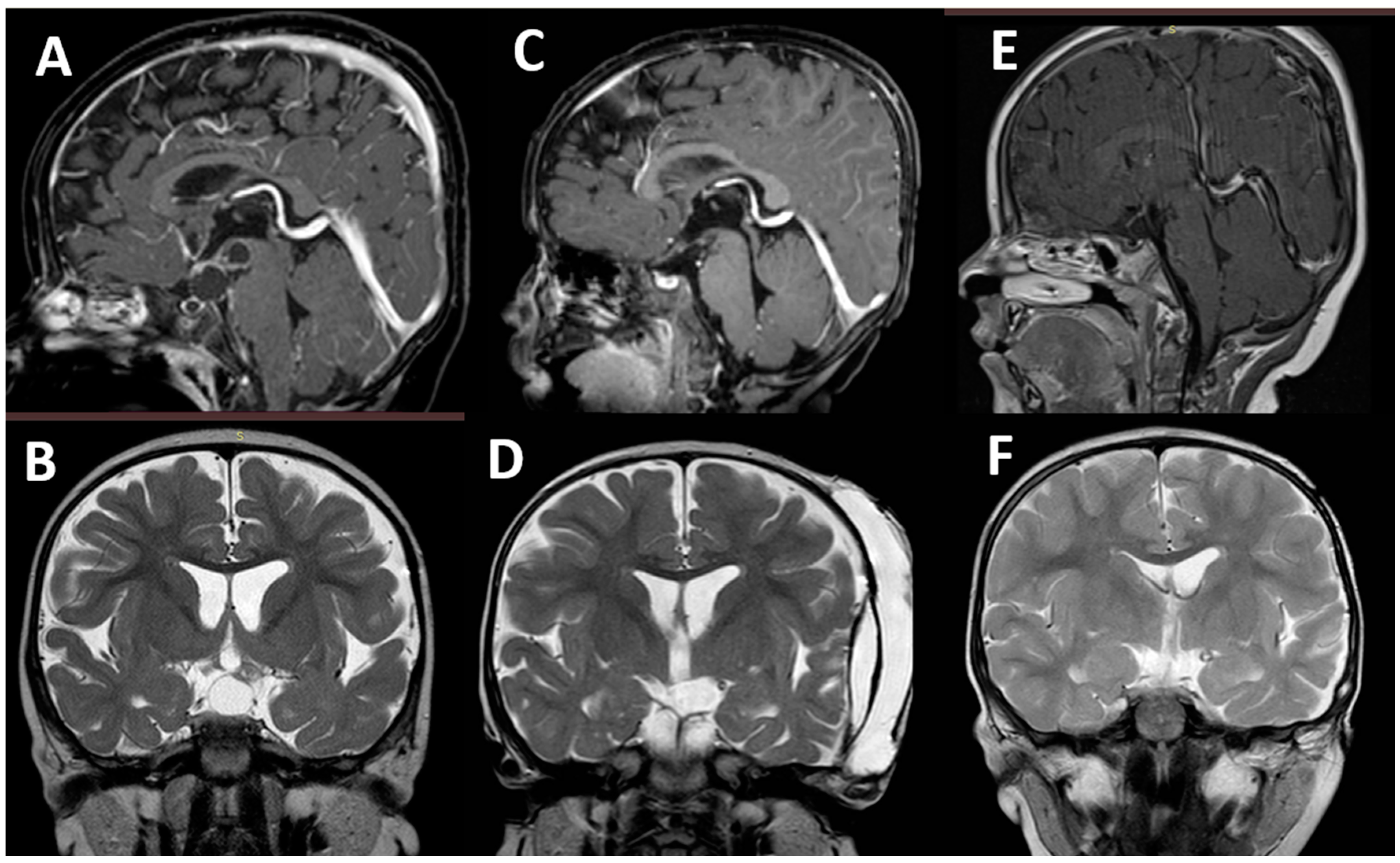
| Group 1 | Group 2 | |
|---|---|---|
| No | 74 | 91 |
| M/F ratio | 1.05 | 1.21 |
| Mean age at surgery | 11.2 years | 10.6 years |
| Children ≤ 2 year-old) | 10 (13.5%) | 11 (12%) |
| Type of lesion | High grade gliomas: 10 (13.5%) | Craniopharyngioma: 38 (42%) |
| Low-grade gliomas: 17 (23%) | Pituitary adenoma: 2 (2%) | |
| DNET: 7 (9.5%) | Optic glioma: 16 (17%) | |
| Choroid plexus tumors: 14 (19%) | Pineal tumors: 14 (15%) | |
| Ependymomas: 9 (12%) | Arachnoid cyst: 10 (11%) | |
| Cavernous angioma: 8 (11%) | Chordoma: 4 (5%) | |
| AT/RT: 5 (6.5%) | Dermoid cyst: 4 (5%) | |
| Other: 4 (5.5%) | Other: 3 (3%) | |
| Region | Hemispheric: 36 (48.5%) Frontal: 18 Temporal: 6 Parieto-occipital: 12 Intraventricular: 27 (36.5%) Thalamic: 11 (15%) | Sellar/suprasellar: 60 (66%) Pineal: 14 (15%) Middle fossa: 11 (12%) Interhemispheric: 3 (3.5%) Retro-orbital: 3 (3.5%) |
| Max diameter | ||
| ≤3 cm | 39 (52%) | 34 (37%) |
| >3 cm | 35 (48%) | 57 (63%) |
| Extent of tumor removal | ||
| Gross/near total | 59 (80%) | 46 (57%) * |
| Subtotal/partial | 15 (20%) | 35 (43%) * |
| Postop hyponatremia | 6 (8%) | 39 (43%) |
| Group 1 | Group 2 | Total | |
|---|---|---|---|
| No. | 74 | 91 | 165 |
| Postop seizures | 5 (6.7%) | 10 (11%) | 15 (9%) |
| Timing and type | |||
| Immediate | 2: FGTC + GT | 2: both FGTC | 4: FGTC (3) + GT |
| Early | 2: FM + GTC | 5: FM + FNM + GT + GTC (2) | 7: FM (2) + FNM + GT + GTC (3) |
| Late | 1: FM | 3: A + FGTC + GTC | 4: A + FM + FGTC + GTC |
| Postop epilepsy | 2 (2.7%) | 3 (3.2%) | 5 (3%) |
| Type and time from surgery | FGTC: 2.5 month GTC: 4 months | A: 5 months FGTC: 2 month GTC: 3.5 months | A + FGTC (2) + GTC (2) Mean time from surgery: 2.5 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massimi, L.; Frassanito, P.; Bianchi, F.; Fiorillo, L.; Battaglia, D.I.; Tamburrini, G. Postoperative Epileptic Seizures in Children. Children 2022, 9, 1465. https://doi.org/10.3390/children9101465
Massimi L, Frassanito P, Bianchi F, Fiorillo L, Battaglia DI, Tamburrini G. Postoperative Epileptic Seizures in Children. Children. 2022; 9(10):1465. https://doi.org/10.3390/children9101465
Chicago/Turabian StyleMassimi, Luca, Paolo Frassanito, Federico Bianchi, Luigi Fiorillo, Domenica Immacolata Battaglia, and Gianpiero Tamburrini. 2022. "Postoperative Epileptic Seizures in Children" Children 9, no. 10: 1465. https://doi.org/10.3390/children9101465
APA StyleMassimi, L., Frassanito, P., Bianchi, F., Fiorillo, L., Battaglia, D. I., & Tamburrini, G. (2022). Postoperative Epileptic Seizures in Children. Children, 9(10), 1465. https://doi.org/10.3390/children9101465







