Analysis of the Influence of Pre-Pregnancy BMI and Weight Gain during Pregnancy on the Weight of Healthy Children during the First 2 Years of Life: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
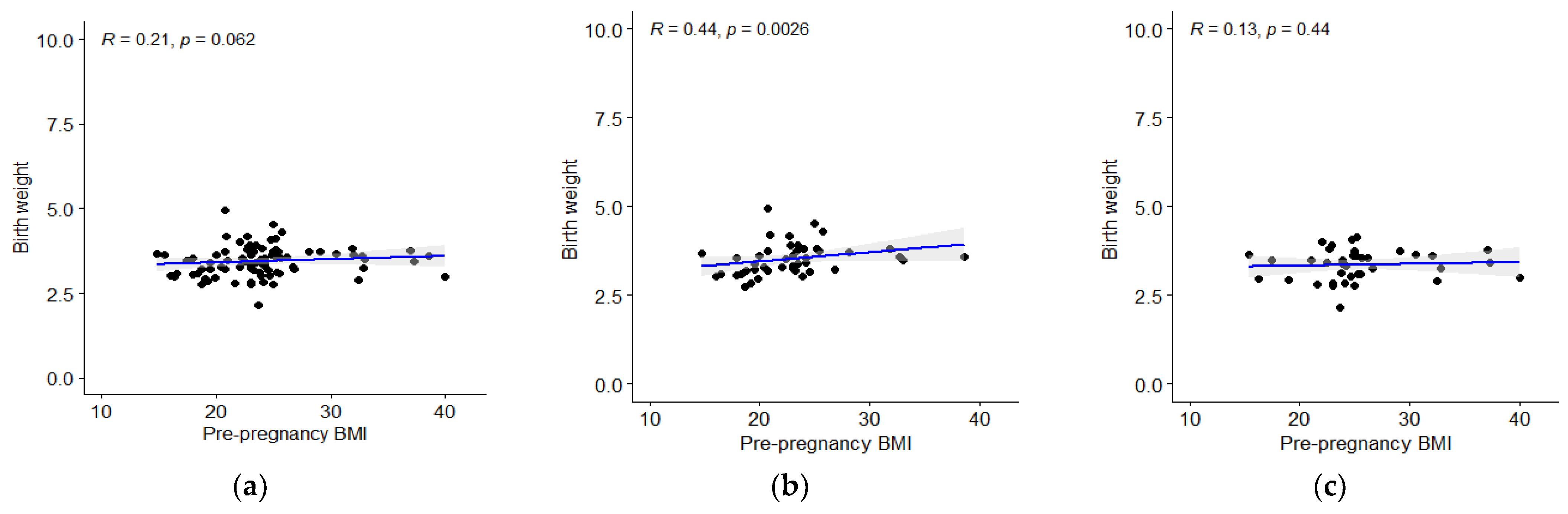
3. Results
3.1. Group Characteristics
3.2. Analysis of Children’s Body Mass
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.Y.; Sharma, A.J.; Sappenfield, W.; Wilson, H.G.; Salihu, H.M. Association of Maternal Body Mass Index, Excessive Weight Gain, and Gestational Diabetes Mellitus With Large-for-Gestational-Age Births. Obstet. Gynecol. 2014, 123, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, L.; Wang, Y.; Zhang, Y.; Du, Y.; Sun, Y.; Wang, Z. Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obes. Rev. 2016, 17, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Voerman, E.; Amiano, P.; Barros, H.; Beilin, L.J.; Bergström, A.; Charles, M.-A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P.; et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: An individual participant data meta-analysis of European, North American and Australian cohorts. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Gul, R.; Iqbal, S.; Anwar, Z.; Ahdi, S.G.; Ali, S.H.; Pirzada, S. Pre-pregnancy maternal BMI as predictor of neonatal birth weight. PLoS ONE 2020, 15, e0240748. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, H.; Feng, Y.; Zhan, Y.; Wu, S.; Cai, S.; Shi, Y.; Chen, Y.; Ma, L.; Jiang, Y. Association among pre-pregnancy body mass index, gestational weight gain and neonatal birth weight: A prospective cohort study in China. BMC Pregnancy Childbirth 2020, 20, 690. [Google Scholar] [CrossRef] [PubMed]
- Voerman, E.; Santos, S.; Patro Golab, B.; Amiano, P.; Ballester, F.; Barros, H.; Bergström, A.; Charles, M.-A.; Chatzi, L.; Chevrier, C.; et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, Y.; Dai, Y.; Xiao, L.; Zhao, P.; Ben, X. Prepregnancy body mass index and gestational weight gain affect the offspring neurobehavioral development at one year of age. J. Matern. Neonatal Med. 2021, 1–10. [Google Scholar] [CrossRef]
- Oteng-Ntim, E.; Varma, R.; Croker, H.; Poston, L.; Doyle, P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: Systematic review and meta-analysis. BMC Med. 2012, 10, 47. [Google Scholar] [CrossRef]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. 2018, 19, 464–484. [Google Scholar] [CrossRef]
- Whiteman, V.E.; Salemi, J.L.; Mejia De Grubb, M.C.; Ashley Cain, M.; Mogos, M.F.; Zoorob, R.J.; Salihu, H.M. Additive effects of Pre-pregnancy body mass index and gestational diabetes on health outcomes and costs. Obesity 2015, 23, 2299–2308. [Google Scholar] [CrossRef]
- Martin, H.; Thevenet-Morrison, K.; Dozier, A. Maternal pre-pregnancy body mass index, gestational weight gain and breastfeeding outcomes: A cross-sectional analysis. BMC Pregnancy Childbirth 2020, 20, 471. [Google Scholar] [CrossRef]
- Nommsen-Rivers, L.A. Does Insulin Explain the Relation between Maternal Obesity and Poor Lactation Outcomes? An Overview of the Literature. Adv. Nutr. 2016, 7, 407–414. [Google Scholar] [CrossRef]
- Peaceman, A.M.; Clifton, R.G.; Phelan, S.; Gallagher, D.; Evans, M.; Redman, L.M.; Knowler, W.C.; Joshipura, K.; Haire-Joshu, D.; Yanovski, S.Z.; et al. Lifestyle Interventions Limit Gestational Weight Gain in Women with Overweight or Obesity: LIFE-Moms Prospective Meta-Analysis. Obes. Silver Spring 2018, 26, 1396–1404. [Google Scholar] [CrossRef]
- Guo, L.; Liu, J.; Ye, R.; Liu, J.; Zhuang, Z.; Ren, A. Gestational Weight Gain and Overweight in Children Aged 3–6 Years. J. Epidemiol. 2015, 25, 536–543. [Google Scholar] [CrossRef]
- Hochner, H.; Friedlander, Y.; Calderon-Margalit, R.; Meiner, V.; Sagy, Y.; Avgil-Tsadok, M.; Burger, A.; Savitsky, B.; Siscovick, D.S.; Manor, O. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: The Jerusalem Perinatal Family Follow-up Study. Circulation 2012, 125, 1381–1389. [Google Scholar] [CrossRef]
- Whitaker, R.C.; Dietz, W.H. Role of the prenatal environment in the development of obesity. J. Pediatr. 1998, 132, 768–776. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, M.; Pérez-Muñoz, C.; Carretero-Bravo, J.; Ruíz-Ruíz, C.; Serrano-Santamaría, M.; Ferriz-Mas, B.C. Early Risk Factors for Obesity in the First 1000 Days—Relationship with Body Fat and BMI at 2 Years. Int. J. Environ. Res. Public. Health 2021, 18, 8179. [Google Scholar] [CrossRef]
- Dieterich, C.M.; Felice, J.P.; O’Sullivan, E.; Rasmussen, K.M. Breastfeeding and health outcomes for the mother-infant dyad. Pediatr. Clin. N. Am. 2013, 60, 31–48. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Nowak, M.; Kalwa, M.; Oleksy, P.; Marszalek, K.; Radon-Pokracka, M.; Huras, H. The relationship between pre-pregnancy BMI, gestational weight gain and neonatal birth weight: A retrospective cohort study. Ginekol. Pol. 2019, 90, 50–54. [Google Scholar] [CrossRef]
- GUS Odsetek Osób w Wieku Powyżej 15 lat Według Indeksu Masy Ciała (BMI). stat.gov.pl n.d. Available online: https://stat.gov.pl/obszary-tematyczne/zdrowie/zdrowie/odsetek-osob-w-wieku-powyzej-15-lat-wedlug-indeksu-masy-ciala-bmi,23,1.html (accessed on 8 September 2022).
- Kaczmarczyk, M.; Löber, U.; Adamek, K.; Węgrzyn, D.; Skonieczna-Żydecka, K.; Malinowski, D.; Łoniewski, I.; Markó, L.; Ulas, T.; Forslund, S.K.; et al. The gut microbiota is associated with the small intestinal paracellular permeability and the development of the immune system in healthy children during the first two years of life. J. Transl. Med. 2021, 19, 177. [Google Scholar] [CrossRef]
- Łoniewska, B.; Adamek, K.; Węgrzyn, D.; Kaczmarczyk, M.; Skonieczna-Żydecka, K.; Clark, J.; Adler, G.; Tousty, J.; Uzar, I.; Tousty, P.; et al. Analysis of Faecal Zonulin and Calprotectin Concentrations in Healthy Children During the First Two Years of Life. An Observational Prospective Cohort Study. J. Clin. Med. 2020, 9, 777. [Google Scholar] [CrossRef]
- CDC. Defining Adult Overweight and Obesity. Centers for Disease Control and Prevention 2022. Available online: https://www.cdc.gov/obesity/basics/adult-defining.html (accessed on 8 September 2022).
- Santos, S.; Eekhout, I.; Voerman, E.; Gaillard, R.; Barros, H.; Charles, M.-A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P.; Corpeleijn, E.; et al. Gestational weight gain charts for different body mass index groups for women in Europe, North America, and Oceania. BMC Med. 2018, 16, 201. [Google Scholar] [CrossRef]
- The WHO Child Growth Standards. Available online: https://www.who.int/tools/child-growth-standards/standards (accessed on 23 February 2022).
- Schnurr, T.M.; Ängquist, L.; Nøhr, E.A.; Hansen, T.; Sørensen, T.I.A.; Morgen, C.S. Smoking during pregnancy is associated with child overweight independent of maternal pre-pregnancy BMI and genetic predisposition to adiposity. Sci. Rep. 2022, 12, 3135. [Google Scholar] [CrossRef]
- Ralphs, E.; Pembrey, L.; West, J.; Santorelli, G. Association between mode of delivery and body mass index at 4-5 years in White British and Pakistani children: The Born in Bradford birth cohort. BMC Public Health 2021, 21, 987. [Google Scholar] [CrossRef]
- Scott, J.A.; Ng, S.Y.; Cobiac, L. The relationship between breastfeeding and weight status in a national sample of Australian children and adolescents. BMC Public Health 2012, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Tombeau Cost, K.; Fuller, A.; Birken, C.S.; Anderson, L.N. Sex and gender differences in childhood obesity: Contributing to the research agenda. BMJ Nutr. Prev. Health 2020, 3, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Stuebe, A.M.; Forman, M.R.; Michels, K.B. Maternal-recalled gestational weight gain, pre-pregnancy body mass index, and obesity in the daughter. Int. J. Obes. 2009, 33, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tylavsky, F.A.; Han, J.C.; Kocak, M.; Fowke, J.H.; Davis, R.L.; Lewinn, K.; Bush, N.R.; Zhao, Q. Maternal Metabolic Factors during Pregnancy Predict Early Childhood Growth Trajectories and Obesity Risk: The CANDLE Study. Int. J. Obes. 2019, 43, 1914–1922. [Google Scholar] [CrossRef]
- Bouyer, K.; Simerly, R.B. Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice. J. Neurosci. 2013, 33, 840–851. [Google Scholar] [CrossRef]
- Abreu, L.R.S.; Shirley, M.K.; Castro, N.P.; Euclydes, V.V.; Bergamaschi, D.P.; Luzia, L.A.; Cruz, A.M.; Rondó, P.H.C. Gestational diabetes mellitus, pre-pregnancy body mass index, and gestational weight gain as risk factors for increased fat mass in Brazilian newborns. PLoS ONE 2019, 14, e0221971. [Google Scholar] [CrossRef]
- Schellong, K.; Schulz, S.; Harder, T.; Plagemann, A. Birth weight and long-term overweight risk: Systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS ONE 2012, 7, e47776. [Google Scholar] [CrossRef]
- Belbasis, L.; Savvidou, M.D.; Kanu, C.; Evangelou, E.; Tzoulaki, I. Birth weight in relation to health and disease in later life: An umbrella review of systematic reviews and meta-analyses. BMC Med. 2016, 14, 147. [Google Scholar] [CrossRef]
- Karachaliou, M.; Georgiou, V.; Roumeliotaki, T.; Chalkiadaki, G.; Daraki, V.; Koinaki, S.; Dermitzaki, E.; Sarri, K.; Vassilaki, M.; Kogevinas, M.; et al. Association of trimester-specific gestational weight gain with fetal growth, offspring obesity, and cardiometabolic traits in early childhood. Am. J. Obstet. Gynecol. 2015, 212, 502.e1–501.e14. [Google Scholar] [CrossRef]
- Li, N.; Liu, E.; Guo, J.; Pan, L.; Li, B.; Wang, P.; Liu, J.; Wang, Y.; Liu, G.; Baccarelli, A.A.; et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS ONE 2013, 8, e82310. [Google Scholar] [CrossRef]
- Olson, C.M.; Demment, M.M.; Carling, S.J.; Strawderman, M.S. Associations Between Mothers’ and Their Children’s Weights at 4 Years of Age. Child. Obes. 2010, 6, 201–207. [Google Scholar] [CrossRef]
- von Kries, R.; Ensenauer, R.; Beyerlein, A.; Amann-Gassner, U.; Hauner, H.; Rosario, A.S. Gestational weight gain and overweight in children: Results from the cross-sectional German KiGGS study. Int. J. Pediatr. Obes. 2011, 6, 45–52. [Google Scholar] [CrossRef]
- Wrotniak, B.H.; Shults, J.; Butts, S.; Stettler, N. Gestational weight gain and risk of overweight in the offspring at age 7 y in a multicenter, multiethnic cohort study. Am. J. Clin. Nutr. 2008, 87, 1818–1824. [Google Scholar] [CrossRef]
- Drake, A.J.; Reynolds, R.M. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reprod. Camb. Engl. 2010, 140, 387–398. [Google Scholar] [CrossRef]
- Chen, C.-N.; Yu, H.-C.; Chou, A.-K. Association between Maternal Pre-pregnancy Body Mass Index and Breastfeeding Duration in Taiwan: A Population-Based Cohort Study. Nutrients 2020, 12, 2361. [Google Scholar] [CrossRef]
- Boudet-Berquier, J.; Salanave, B.; Desenclos, J.-C.; Castetbon, K. Association between maternal prepregnancy obesity and breastfeeding duration: Data from a nationwide prospective birth cohort. Matern. Child. Nutr. 2018, 14, e12507. [Google Scholar] [CrossRef]
- Nomura, K.; Kido, M.; Tanabe, A.; Ando, K. Prepregnancy obesity as a risk factor for exclusive breastfeeding initiation in Japanese women. Nutr. Burbank Los Angel. Cty. Calif. 2019, 62, 93–99. [Google Scholar] [CrossRef]
- Briere, C.-E.; McGrath, J.; Cong, X.; Cusson, R. An integrative review of factors that influence breastfeeding duration for premature infants after NICU hospitalization. J. Obstet. Gynecol. Neonatal Nurs. 2014, 43, 272–281. [Google Scholar] [CrossRef]
- Regan, J.; Thompson, A.; DeFranco, E. The influence of mode of delivery on breastfeeding initiation in women with a prior cesarean delivery: A population-based study. Breastfeed. Med. 2013, 8, 181–186. [Google Scholar] [CrossRef]
- Kitsantas, P.; Gaffney, K.F.; Kornides, M.L. Prepregnancy body mass index, socioeconomic status, race/ethnicity and breastfeeding practices. J. Perinat. Med. 2011, 40, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Huang, Y.; Ouyang, Y.-Q.; Redding, S.R. Maternal Prepregnancy Body Mass Index, Gestational Weight Gain, and Cessation of Breastfeeding: A Systematic Review and Meta-Analysis. Breastfeed. Med. 2019, 14, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Hu, J.; Aris, I.M.; Oken, E.; Ma, Y.; Ding, N.; Gao, M.; Wei, X.; Wen, D. Association of Total and Trimester-Specific Gestational Weight Gain Rate with Early Infancy Weight Status: A Prospective Birth Cohort Study in China. Nutrients 2019, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Broskey, N.T.; Wang, P.; Li, N.; Leng, J.; Li, W.; Wang, L.; Gilmore, L.A.; Hu, G.; Redman, L.M. Early Pregnancy Weight Gain Exerts the Strongest Effect on Birth Weight, Posing a Critical Time to Prevent Childhood Obesity. Obesity 2017, 25, 1569–1576. [Google Scholar] [CrossRef]


| Child’s Weight Status | Centile Charts | 12 Month | 24 Month | ||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| Underweight | Z-score (kg): <−2 | <7.7 | <7.0 | <9.7 | <9.0 |
| Centile charts (kg): <2.3 centile (abnormal growth) | <7.8 | <7.1 | <9.8 | <9.2 | |
| BMI (kg/m2): <3 percentile | <14.5 | <13.9 | <13.7 | <13.2 | |
| Overweight | Z-score (kg): 2–3 | 12.0–13.3 | 11.5–13.1 | 15.3–17.2 | 14.8–17.0 |
| Obesity | Z-score (kg): >3 | >13.3 | >13.1 | >17.2 | >17.0 |
| Centile charts (kg): >97.7 centile (abnormal growth+) | >11.8 | >11.3 | >15.1 | >14.6 | |
| BMI (kg/m2): >97 percentile | >19.6 | >19.4 | >18.3 | >18.2 | |
| pBMI (kg/m2) | p Adjusted | ||||
|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25–29.9 | ≥30 | ||
| Number of subjects; n (%) | 9 (11) | 46 (55) | 17 (20) | 11 (13) | |
| Maternal characteristics | |||||
| pBMI(kg/m2) | 16.73 (1.23) | 22.34 (1.81) | 25.94 (1.17) | 34.38 (3.21) | NA |
| Mass gain(kg) | 16.13 (4.94) | 17.13 (6.04) | 16.3 (5.51) | 7.12 (7.7) | 0.0004 |
| Breastfeeding (weeks) | 17.75 (17.35) | 26.05 (26.01) | 28.03 (22.75) | 24.91 (24.55) | 0.799 |
| Breastfeeding at 6th month; n (%) | 2 (25) | 19 (41.3) | 9 (52.9) | 5 (45.4) | 0.875 |
| Smoking+; n (%) | 2 (22) | 13 (28.2) | 1 (6) | 2 (18) | 0.3973 |
| ATB+; n (%) | 2 (22) | 12 (26) | 7 (41) | 5 (45) | 0.441 |
| GBs+; n (%) | 1 (12.5) | 10 (29.4) | 5 (38.5) | 4 (40) | 0.614 |
| Child characteristics | |||||
| Delivery C, n (%) | 5 (55.6) | 30 (65.2) | 11 (64.7) | 6 (54.5) | 0.875 |
| Gender male; n (%) | 6 (66.7) | 28 (60.8) | 6 (35.3) | 4 (36.4) | 0.315 |
| Weight [kg] | |||||
| Birth | 3.29 (0.29) | 3.41 (0.49) | 3.61 (0.45) | 3.47 (0.3) | 0.010 |
| 1 month | 4.17 (0.34) | 4.47 (0.65) | 4.59 (0.39) | 4.81 (0.71) | 0.010 |
| 6 months | 6.96 (0.54) | 8.06 (1.18) | 7.83 (0.96) | 8.19 (0.9) | 0.315 |
| 12 months | 9.91 (0.91) | 10.17 (1.18) | 9.91 (1.04) | 10.31 (1.02) | 0.491 |
| 24 months | 12.63 (2.05) | 12.91 (1.59) | 12.51 (1.21) | 13.7 (2.23) | 0.251 |
| Classification by Z-Score, 12 Months | |||||
| Child’s Weight Category (n) | pBMI Category | p Value | |||
| Underweight | Normal | Overweight | Obesity | ||
| Underweight | 0 | 0 | 0 | 0 | 0.63 |
| Normal | 7 | 16 | 15 | 9 | |
| Overweight | 0 | 6 | 1 | 1 | |
| Obesity | 0 | 0 | 0 | 0 | |
| Classification by Z-score, 24 months | |||||
| Child’s Weight Category (n) | pBMI category | p value | |||
| Underweight | Normal | Overweight | Obesity | ||
| Underweight | 0 | 0 | 0 | 0 | 0.15 |
| Normal | 5 | 35 | 15 | 7 | |
| Overweight | 1 | 4 | 0 | 2 | |
| Obesity | 0 | 0 | 0 | 1 | |
| Classification by centile charts: weight-for-age, 12 months | |||||
| Child’s weight status (n) | pBMI category | p value | |||
| Underweight | Normal | Overweight | Obesity | ||
| abnormal growth − | 0 | 0 | 0 | 0 | 0.527 |
| Normal | 5 | 21 | 5 | 2 | |
| abnormal growth + | 0 | 4 | 0 | 1 | |
| Classification by centile charts: weight-for-age, 24 months | |||||
| Child’s weight status (n) | pBMI category | p value | |||
| Underweight | Normal | Overweight | Obesity | ||
| abnormal growth − | 0 | 0 | 0 | 0 | 0.252 |
| Normal | 5 | 21 | 5 | 2 | |
| abnormal growth + | 1 | 1 | 0 | 1 | |
| Classification by centile charts: BMI-for-age, 12 months | |||||
| Child’s BMI category (n) | pBMI category | p value | |||
| Underweight | Normal | Overweight | Obesity | ||
| Underweight | 1 | 3 | 0 | 2 | 0.582 |
| Other groups † | 7 | 41 | 10 | 6 | |
| Other groups * | 8 | 36 | 10 | 7 | 0.241 |
| Obesity | 0 | 8 | 0 | 1 | |
| Classification by centile charts: BMI-for-age, 24 months | |||||
| Child’s BMI category (n) | pBMI category | p value | |||
| Underweight | Normal | Overweight | Obesity | ||
| Underweight | 1 | 2 | 1 | 0 | 0.745 |
| Other groups † | 3 | 28 | 12 | 9 | |
| Other groups * | 4 | 24 | 13 | 4 | 0.04 |
| Obesity | 0 | 6 | 0 | 5 | |
| GWG Category | p Adjusted | |||
|---|---|---|---|---|
| Inadequate | Adequate | Excessive | ||
| Number of subjects; n (%) | 18(22) | 16(19.5) | 48(58.5) | |
| Maternal characteristics | ||||
| BMI gain (kg/m2) | 2.28(1.68) | 4.83(0.93) | 7.14(1.64) | NA |
| pBMI (kg/m2) | 25.17(7.74) | 22.58(3.72) | 24.20(4.24) | 1 |
| Natural breastfeeding; n (%) | 11(61.11) | 11(68.75) | 34(70.83) | 1 |
| Breastfeeding (weeks) | 30.00(30.76) | 20.09(24.62) | 26.16(21.6) | 1 |
| Breastfeeding at 6th month | 7(41.2) | 5(31.2) | 23(47.9) | 1 |
| Smoking+; n (%) | 4(22.22) | 3(18.75) | 10(20.83) | 1 |
| Child characteristics | ||||
| Delivery C, n (%) | 8(50) | 8(44.44) | 35(72.91) | 0.165 |
| Gender male; n (%) | 8(44.44) | 11(68.75) | 25(52) | 0.507 |
| Weight (kg) | ||||
| Birth | 3.19(0.41) | 3.38(0.3) | 3.57(0.45) | 0.018 |
| 1 month | 4.28(0.75) | 4.57(0.44) | 4.59(0.56) | 0.081 |
| 6 months | 7.45(1.21) | 8.01(0.62) | 8.07(1.14) | 0.375 |
| 12 months | 9.69(1.07) | 10.44(1.01) | 10.17(1.1) | 0.307 |
| 24 months | 12.20(1.63) | 12.54(1.26) | 13.27(1.69) | 0.288 |
| Classification by Z-Score, 12 Months | ||||
| Child’s Weight Category (n) | Maternal GWG Category | p Value | ||
| Inadequate | Adequate | Excessive | ||
| Underweight | 0 | 0 | 0 | 0.87 |
| Normal | 16 | 12 | 39 | |
| Overweight | 1 | 2 | 5 | |
| Obesity | 0 | 0 | 0 | |
| Classification by Z-score, 24 months | ||||
| Child’s weight category (n) | Maternal GWG category | p value | ||
| Inadequate | Adequate | Excessive | ||
| Underweight | 0 | 0 | 0 | 0.08 |
| Normal | 15 | 11 | 36 | |
| Overweight | 0 | 0 | 7 | |
| Obesity | 1 | 0 | 0 | |
| Classification by centile charts: weight-for-age, 12 months | ||||
| Child’s weight status (n) | Maternal GWG category | p value | ||
| Inadequate | Adequate | Excessive | ||
| abnormal growth − | 0 | 0 | 0 | 1 |
| Normal | 6 | 8 | 19 | |
| abnormal growth+ | 1 | 1 | 3 | |
| Classification by centile charts: weight-for-age, 24 months | ||||
| Child’s weight status (n) | Maternal GWG category | p value | ||
| Inadequate | Adequate | Excessive | ||
| abnormal growth − | 0 | 0 | 0 | 0.756 |
| Normal | 6 | 7 | 17 | |
| abnormal growth + | 0 | 0 | 3 | |
| Classification by centile charts: BMI-for-age, 12 months | ||||
| Child’s BMI category (n) | Maternal GWG category | p value | ||
| Inadequate | Adequate | Excessive | ||
| Underweight | 4 | 0 | 4 | 0.76 |
| Other groups * | 10 | 12 | 40 | |
| Other groups † | 13 | 11 | 38 | 0.159 |
| Obesity | 1 | 1 | 6 | |
| Classification by centile charts: BMI-for-age, 24 months | ||||
| Child’s weight status (n) | Maternal GWG category | p value | ||
| Inadequate | Adequate | Excessive | ||
| Underweight | 1 | 1 | 2 | 0.76 |
| Other groups * | 11 | 8 | 33 | |
| Other groups † | 9 | 8 | 28 | 1 |
| Obesity | 3 | 1 | 7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łoniewska, B.; Michalczyk, K.; Podsiadło, K.; Adamek, K.; Michalczyk, B.; Tousty, P.; Kaczmarczyk, M.; Łoniewski, I. Analysis of the Influence of Pre-Pregnancy BMI and Weight Gain during Pregnancy on the Weight of Healthy Children during the First 2 Years of Life: A Prospective Study. Children 2022, 9, 1431. https://doi.org/10.3390/children9101431
Łoniewska B, Michalczyk K, Podsiadło K, Adamek K, Michalczyk B, Tousty P, Kaczmarczyk M, Łoniewski I. Analysis of the Influence of Pre-Pregnancy BMI and Weight Gain during Pregnancy on the Weight of Healthy Children during the First 2 Years of Life: A Prospective Study. Children. 2022; 9(10):1431. https://doi.org/10.3390/children9101431
Chicago/Turabian StyleŁoniewska, Beata, Kaja Michalczyk, Konrad Podsiadło, Karolina Adamek, Barbara Michalczyk, Piotr Tousty, Mariusz Kaczmarczyk, and Igor Łoniewski. 2022. "Analysis of the Influence of Pre-Pregnancy BMI and Weight Gain during Pregnancy on the Weight of Healthy Children during the First 2 Years of Life: A Prospective Study" Children 9, no. 10: 1431. https://doi.org/10.3390/children9101431
APA StyleŁoniewska, B., Michalczyk, K., Podsiadło, K., Adamek, K., Michalczyk, B., Tousty, P., Kaczmarczyk, M., & Łoniewski, I. (2022). Analysis of the Influence of Pre-Pregnancy BMI and Weight Gain during Pregnancy on the Weight of Healthy Children during the First 2 Years of Life: A Prospective Study. Children, 9(10), 1431. https://doi.org/10.3390/children9101431






