Early Developmental Signs in Children with Autism Spectrum Disorder: Results from the Japan Environment and Children’s Study
Abstract
:1. Introduction
2. Materials and Methods
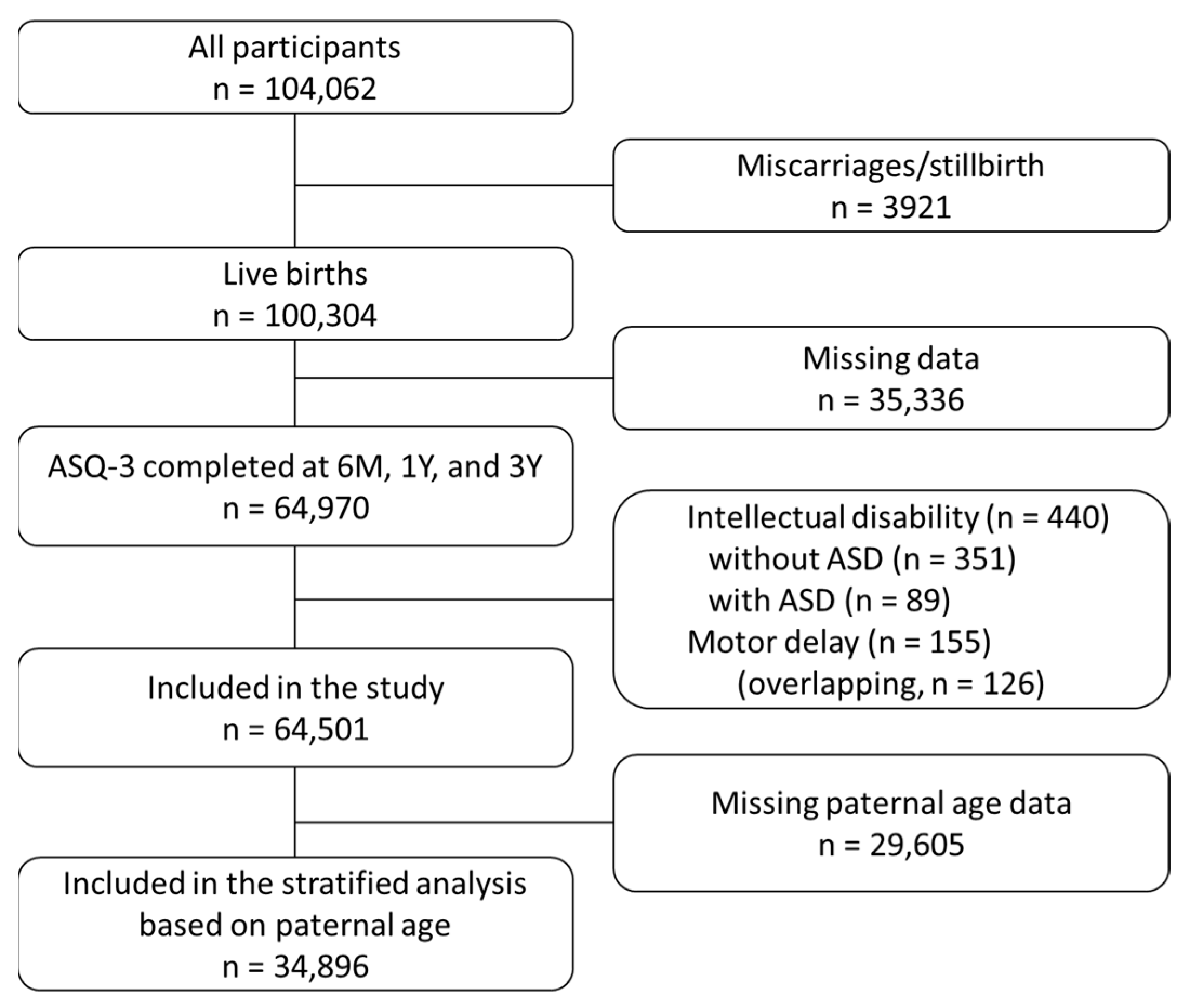
2.1. Participants
2.2. Data Selection of the ASQ-3
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arlington, V.A.; American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-V), 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Chiarotti, F.; Venerosi, A. Epidemiology of Autism Spectrum Disorders: A Review of Worldwide Prevalence Estimates Since 2014. Brain Sci. 2020, 10, 274. [Google Scholar] [CrossRef]
- Christensen, D.L.; Maenner, M.J.; Bilder, D.; Constantino, J.N.; Daniels, J.; Durkin, M.S.; Fitzgerald, R.T.; Kurzius-Spencer, M.; Pettygrove, S.D.; Robinson, C.; et al. Prevalence and Characteristics of Autism Spectrum Disorder among Children Aged 4 Years—Early Autism and Developmental Disabilities Monitoring Network, Seven Sites, United States, 2010, 2012, and 2014. MMWR Surveill. Summ. 2019, 68, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, H.; Shimizu, Y.; Misumi, K.; Niimi, M.; Ohashi, Y. Cumulative incidence and prevalence of childhood autism in children in Japan. Br. J. Psychiatry 1996, 169, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Ashizawa, F.; Inagaki, M. Prevalence estimates of neurodevelopmental disorders in Japan: A community sample questionnaire study. Psychiatry Clin. Neurosci. 2020, 74, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, A.J.O.; Varcin, K.J.; Alvares, G.A.; Barbaro, J.; Bent, C.; Boutrus, M.; Chetcuti, L.; Cooper, M.N.; Clark, A.; Davidson, E.; et al. Pre-emptive intervention versus treatment as usual for infants showing early behavioural risk signs of autism spectrum disorder: A single-blind, randomised controlled trial. Lancet Child Adolesc. Health 2019, 3, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, S.; Brian, J.; Zwaigenbaum, L.; Roberts, W.; Szatmari, P.; Smith, I.; Bryson, S. Early language and communication development of infants later diagnosed with autism spectrum disorder. J. Dev. Behav. Pediatr. 2006, 27, S69–S78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, A.; Tobe, R.; Dias, E.C.; Ardekani, B.A.; Veenstra-VanderWeele, J.; Patel, G.; Breland, M.; Lieval, A.; Silipo, G.; Javitt, D.C. Differential Patterns of Visual Sensory Alteration Underlying Face Emotion Recognition Impairment and Motion Perception Deficits in Schizophrenia and Autism Spectrum Disorder. Biol. Psychiatry 2019, 86, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Fournier, K.A.; Hass, C.J.; Naik, S.K.; Lodha, N.; Cauraugh, J.H. Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. J. Autism Dev. Disord. 2010, 40, 1227–1240. [Google Scholar] [CrossRef]
- Geschwind, D.H.; State, M.W. Gene hunting in autism spectrum disorder: On the path to precision medicine. Lancet Neurol. 2015, 14, 1109–1120. [Google Scholar] [CrossRef] [Green Version]
- Chaste, P.; Leboyer, M. Autism risk factors: Genes, environment, and gene-environment interactions. Dialogues Clin. Neurosci. 2012, 14, 281–292. [Google Scholar]
- Wu, S.; Wu, F.; Ding, Y.; Hou, J.; Bi, J.; Zhang, Z. Advanced parental age and autism risk in children: A systematic review and meta-analysis. Acta Psychiatr. Scand. 2017, 135, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, A.; Frigge, M.L.; Masson, G.; Besenbacher, S.; Sulem, P.; Magnusson, G.; Gudjonsson, S.A.; Sigurdsson, A.; Jonasdottir, A.; Jonasdottir, A.; et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012, 488, 471–475. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.T. Meiosis in oocytes: Predisposition to aneuploidy and its increased incidence with age. Hum. Reprod. Update 2008, 14, 143–158. [Google Scholar] [CrossRef]
- Barnhill, J.; Bedford, J.; Crowley, J.; Soda, T. A search for the common ground between Tic; Obsessive-compulsive and Autism Spectrum Disorders: Part I, Tic disorders. AIMS Genet. 2017, 4, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowski, J.A.; Smith, T.F.; Shrewsbury, A.M.; Thomas, L.R.; Knopik, V.S.; Acosta, M.T. Neurofibromatosis Type 1 Implicates Ras Pathways in the Genetic Architecture of Neurodevelopmental Disorders. Behav. Genet. 2020, 50, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Di Filippo, T.; Glorioso, P.; La Grutta, S.; Epifanio, M.S.; Roccella, M. Autism spectrum disorders in children affected by Duchenne muscular dystrophy. Minerva Pediatr. 2018, 70, 233–239. [Google Scholar] [CrossRef]
- Eyoh, E.E.; Failla, M.D.; Williams, Z.J.; Schwartz, K.L.; Cutting, L.E.; Landman, B.A.; Cascio, C.J. Brief Report: The Characterization of Medical Comorbidity Prior to Autism Diagnosis in Children Before Age Two. J. Autism Dev. Disord. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Squires, J.; Bricker, D. Ages & Stages Questionnaires®, Third Edition (ASQ-3™). A Parent-Completed Child-Monitoring System; Paul H. Brookes Publishing Co.: Baltimore, MD, USA, 2009. [Google Scholar]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef] [Green Version]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline Profile of Participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Glascoe, F.P. Screening for developmental and behavioral problems. Ment. Retard. Dev. Disabil. Res. Rev. 2005, 11, 173–179. [Google Scholar] [CrossRef]
- Mezawa, H.; Aoki, S.; Nakayama, S.F.; Nitta, H.; Ikeda, N.; Kato, K.; Tamai, S.; Takekoh, M.; Sanefuji, M.; Ohga, S.; et al. Psychometric profile of the Ages and Stages Questionnaires, Japanese translation. Pediatr. Int. 2019, 61, 1086–1095. [Google Scholar] [CrossRef]
- Ozonoff, S.; Macari, S.; Young, G.S.; Goldring, S.; Thompson, M.; Rogers, S.J. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism 2008, 12, 457–472. [Google Scholar] [PubMed] [Green Version]
- Gima, H.; Kihara, H.; Watanabe, H.; Nakano, H.; Nakano, J.; Konishi, Y.; Nakamura, T.; Taga, G. Early motor signs of autism spectrum disorder in spontaneous position and movement of the head. Exp. Brain Res. 2018, 236, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Zwaigenbaum, L.; Bauman, M.L.; Stone, W.L.; Yirmiya, N.; Estes, A.; Hansen, R.L.; McPartland, J.C.; Natowicz, M.R.; Choueiri, R.; Fein, D.; et al. Early Identification of Autism Spectrum Disorder: Recommendations for Practice and Research. Pediatrics 2015, 136 (Suppl. S1), S10–S40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bejarano-Martín, Á.; Canal-Bedia, R.; Magán-Maganto, M.; Fernández-Álvarez, C.; Cilleros-Martín, M.V.; Sánchez-Gómez, M.C.; García-Primo, P.; Rose-Sweeney, M.; Boilson, A.; Linertová, R.; et al. Early Detection, Diagnosis and Intervention Services for Young Children with Autism Spectrum Disorder in the European Union (ASDEU): Family and Professional Perspectives. J. Autism Dev. Disord. 2020, 50, 3380–3394. [Google Scholar] [PubMed]
- Levy, S.E.; Wolfe, A.; Coury, D.; Duby, J.; Farmer, J.; Schor, E.; Van Cleave, J.; Warren, Z. Screening Tools for Autism Spectrum Disorder in Primary Care: A Systematic Evidence Review. Pediatrics 2020, 145, S47–S59. [Google Scholar] [CrossRef] [Green Version]
- Dawson, G.; Campbell, K.; Hashemi, J.; Lippmann, S.J.; Smith, V.; Carpenter, K.; Egger, H.; Espinosa, S.; Vermeer, S.; Baker, J.; et al. Atypical postural control can be detected via computer vision analysis in toddlers with autism spectrum disorder. Sci. Rep. 2018, 8, 17008. [Google Scholar]
- Vanvuchelen, M.; Van Schuerbeeck, L.; Braeken, M.A. Screening accuracy of the parent-completed Ages and Stages Questionnaires—Second edition as a broadband screener for motor problems in preschoolers with autism spectrum disorders. Autism 2017, 21, 29–36. [Google Scholar] [CrossRef]
- Hardy, S.; Haisley, L.; Manning, C.; Fein, D. Can Screening with the Ages and Stages Questionnaire Detect Autism? J. Dev. Behav. Pediatr. 2015, 36, 536–543. [Google Scholar]
- Warrier, V.; Baron-Cohen, S. Childhood trauma, life-time self-harm, and suicidal behaviour and ideation are associated with polygenic scores for autism. Mol. Psychiatry 2021, 26, 1670–1684. [Google Scholar] [CrossRef] [Green Version]
- Rutter, M.; Andersen-Wood, L.; Beckett, C.; Bredenkamp, D.; Castle, J.; Groothues, C.; Kreppner, J.; Keaveney, L.; Lord, C.; O’Connor, T.G. Quasi-autistic patterns following severe early global privation. English and Romanian Adoptees (ERA) Study Team. J. Child Psychol. Psychiatry 1999, 40, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Kerns, C.M.; Newschaffer, C.J.; Berkowitz, S.J. Traumatic Childhood Events and Autism Spectrum Disorder. J. Autism Dev. Disord. 2015, 45, 3475–3486. [Google Scholar] [CrossRef] [PubMed]
| Domains | Total | Non-ASD | ASD | RR | 95% CI | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| 6 months old | |||||||||
| Communication | Monitoring | 4171 | 4148 | 23 | 1.90 | 1.29 | 2.78 | 0.0041 | |
| Cut-off | 415 | 412 | 3 | 2.49 | 0.81 | 7.69 | 0.1218 | ||
| Gross motor | Monitoring | 19,813 | 19,735 | 78 | 1.35 | 1.14 | 1.60 | 0.0019 | |
| Cut-off | 6914 | 6878 | 36 | 1.79 | 1.33 | 2.40 | 0.0005 | ||
| Fine motor | Monitoring | 19,228 | 19,144 | 84 | 1.50 | 1.28 | 1.76 | <0.0001 | |
| Cut-off | 3444 | 3422 | 22 | 2.20 | 1.48 | 3.26 | 0.0005 | ||
| Problem solving | Monitoring | 16,935 | 16,862 | 73 | 1.48 | 1.24 | 1.77 | 0.0002 | |
| Cut-off | 7259 | 7224 | 35 | 1.66 | 1.23 | 2.24 | 0.0025 | ||
| Personal−social | Monitoring | 16,347 | 16,277 | 70 | 1.47 | 1.22 | 1.77 | 0.0003 | |
| Cut-off | 2470 | 2456 | 14 | 1.95 | 1.18 | 3.23 | 0.0194 | ||
| 1 year old | |||||||||
| Communication | Monitoring | 4582 | 4530 | 52 | 3.93 | 3.11 | 4.96 | <0.0001 | |
| Cut-off | 77 | 75 | 2 | 9.12 | 2.26 | 36.88 | 0.0214 | ||
| Gross motor | Monitoring | 12,250 | 12,187 | 63 | 1.77 | 1.44 | 2.16 | <0.0001 | |
| Cut-off | 3660 | 3634 | 26 | 2.45 | 1.71 | 3.50 | <0.0001 | ||
| Fine motor | Monitoring | 10,130 | 10,076 | 54 | 1.83 | 1.46 | 2.30 | <0.0001 | |
| Cut-off | 3732 | 3707 | 25 | 2.31 | 1.60 | 3.33 | 0.0001 | ||
| Problem solving | Monitoring | 10,134 | 10,065 | 69 | 2.35 | 1.94 | 2.83 | <0.0001 | |
| Cut-off | 3340 | 3308 | 32 | 3.31 | 2.41 | 4.55 | <0.0001 | ||
| Personal−social | Monitoring | 11,139 | 11,056 | 83 | 2.57 | 2.18 | 3.02 | <0.0001 | |
| Cut-off | 783 | 770 | 13 | 5.78 | 3.40 | 9.80 | <0.0001 | ||
| 3 years old | |||||||||
| Communication | Monitoring | 7254 | 7118 | 136 | 6.54 | 5.97 | 7.16 | <0.0001 | |
| Cut-off | 2279 | 2180 | 99 | 15.54 | 13.48 | 17.90 | <0.0001 | ||
| Gross motor | Monitoring | 7486 | 7391 | 95 | 4.40 | 3.81 | 5.07 | <0.0001 | |
| Cut-off | 2671 | 2614 | 57 | 7.46 | 5.99 | 9.29 | <0.0001 | ||
| Fine motor | Monitoring | 10,102 | 9993 | 109 | 3.73 | 3.30 | 4.22 | <0.0001 | |
| Cut-off | 4634 | 4556 | 78 | 5.86 | 4.93 | 6.96 | <0.0001 | ||
| Problem solving | Monitoring | 10,311 | 10,177 | 134 | 4.50 | 4.11 | 4.94 | <0.0001 | |
| Cut-off | 4496 | 4391 | 105 | 8.18 | 7.18 | 9.32 | <0.0001 | ||
| Personal−social | Monitoring | 11,523 | 11,374 | 149 | 4.48 | 4.16 | 4.83 | <0.0001 | |
| Cut-off | 1849 | 1760 | 89 | 17.30 | 14.78 | 20.25 | <0.0001 | ||
| Domains | Non-ASD | ASD | RR | 95% CI | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Under 35 years old, n = 20,830 | ||||||||
| 6 months old | ||||||||
| Communication | Monitoring | 1111 | 6 | 2.61 | 1.24 | 5.50 | 0.026 | |
| Cut-off | 99 | 1 | 4.88 | 0.70 | 34.22 | 0.187 | ||
| Gross motor | Monitoring | 6173 | 16 | 1.25 | 0.85 | 1.85 | 0.316 | |
| Cut-off | 2072 | 7 | 1.63 | 0.83 | 3.22 | 0.195 | ||
| Fine motor | Monitoring | 5736 | 17 | 1.43 | 0.99 | 2.07 | 0.088 | |
| Cut-off | 915 | 6 | 3.17 | 1.50 | 6.68 | 0.011 | ||
| Problem solving | Monitoring | 4876 | 10 | 0.99 | 0.58 | 1.71 | 1.000 | |
| Cut-off | 1979 | 4 | 0.98 | 0.38 | 2.49 | 1.000 | ||
| Personal−social | Monitoring | 4780 | 15 | 1.52 | 1.01 | 2.28 | 0.071 | |
| Cut-off | 664 | 3 | 2.18 | 0.73 | 6.52 | 0.158 | ||
| 1 year old | ||||||||
| Communication | Monitoring | 1291 | 13 | 4.87 | 3.08 | 7.69 | <0.0001 | |
| Cut-off | 18 | 1 | 26.86 | 3.67 | 196.73 | 0.039 | ||
| Gross motor | Monitoring | 3593 | 15 | 2.02 | 1.34 | 3.04 | 0.007 | |
| Cut-off | 1010 | 3 | 1.44 | 0.48 | 4.28 | 0.466 | ||
| Fine motor | Monitoring | 3038 | 15 | 2.39 | 1.58 | 3.60 | 0.001 | |
| Cut-off | 1035 | 7 | 3.27 | 1.66 | 6.46 | 0.005 | ||
| Problem solving | Monitoring | 2971 | 14 | 2.28 | 1.48 | 3.51 | 0.003 | |
| Cut-off | 870 | 8 | 2.28 | 1.48 | 3.50 | 0.003 | ||
| Personal−social | Monitoring | 3160 | 16 | 2.45 | 1.66 | 3.61 | 0.0004 | |
| Cut-off | 195 | 4 | 9.92 | 3.86 | 25.48 | 0.001 | ||
| 3 years old | ||||||||
| Communication | Monitoring | 2029 | 35 | 8.34 | 7.2 | 9.7 | <0.0001 | |
| Cut-off | 592 | 22 | 17.96 | 13.3 | 24.3 | <0.0001 | ||
| Gross motor | Monitoring | 2208 | 20 | 4.38 | 3.2 | 6.0 | <0.0001 | |
| Cut-off | 750 | 12 | 7.73 | 4.8 | 12.6 | <0.0001 | ||
| Fine motor | Monitoring | 3065 | 27 | 4.26 | 3.4 | 5.4 | <0.0001 | |
| Cut-off | 1361 | 19 | 6.75 | 4.8 | 9.5 | <0.0001 | ||
| Problem solving | Monitoring | 3017 | 31 | 4.97 | 4.1 | 6.0 | <0.0001 | |
| Cut-off | 1241 | 21 | 8.18 | 6.0 | 11.2 | <0.0001 | ||
| Personal−social | Monitoring | 3464 | 35 | 4.88 | 4.2 | 5.7 | <0.0001 | |
| Cut-off | 498 | 19 | 18.44 | 13.0 | 26.1 | <0.0001 | ||
| 35 years old and over, n = 14,065 | ||||||||
| 6 months old | ||||||||
| Communication | Monitoring | 1095 | 8 | 1.90 | 1.00 | 3.60 | 0.070 | |
| Cut-off | 115 | 1 | 2.26 | 0.32 | 15.86 | 0.361 | ||
| Gross motor | Monitoring | 4625 | 26 | 1.46 | 1.10 | 1.93 | 0.021 | |
| Cut-off | 1711 | 13 | 1.97 | 1.23 | 3.17 | 0.019 | ||
| Fine motor | Monitoring | 4612 | 28 | 1.58 | 1.22 | 2.04 | 0.005 | |
| Cut-off | 902 | 7 | 2.01 | 1.01 | 4.03 | 0.085 | ||
| Problem solving | Monitoring | 4177 | 25 | 1.55 | 1.16 | 2.07 | 0.011 | |
| Cut-off | 1831 | 16 | 2.27 | 1.50 | 3.43 | 0.002 | ||
| Personal−social | Monitoring | 3902 | 22 | 1.46 | 1.06 | 2.02 | 0.047 | |
| Cut-off | 659 | 3 | 1.18 | 0.39 | 3.56 | 0.741 | ||
| 1 year old | ||||||||
| Communication | Monitoring | 1149 | 16 | 3.61 | 2.39 | 5.47 | <0.0001 | |
| Cut-off | 23 | 1 | 11.28 | 1.55 | 82.05 | 0.088 | ||
| Gross motor | Monitoring | 3003 | 18 | 1.56 | 1.07 | 2.27 | 0.045 | |
| Cut-off | 923 | 10 | 2.81 | 1.60 | 4.94 | 0.003 | ||
| Fine motor | Monitoring | 2436 | 13 | 1.38 | 0.86 | 2.23 | 0.207 | |
| Cut-off | 970 | 7 | 1.87 | 0.94 | 3.75 | 0.099 | ||
| Problem solving | Monitoring | 2498 | 16 | 1.66 | 1.10 | 2.51 | 0.032 | |
| Cut-off | 849 | 5 | 1.53 | 0.66 | 3.53 | 0.381 | ||
| Personal−social | Monitoring | 2747 | 24 | 2.27 | 1.68 | 3.06 | <0.0001 | |
| Cut-off | 209 | 1 | 1.24 | 0.18 | 8.69 | 0.557 | ||
| 3 years old | ||||||||
| Communication | Monitoring | 1739 | 32 | 4.77 | 3.8 | 6.0 | <0.0001 | |
| Cut-off | 566 | 22 | 10.09 | 7.2 | 14.1 | <0.0001 | ||
| Gross motor | Monitoring | 1740 | 27 | 4.03 | 3.1 | 5.3 | <0.0001 | |
| Cut-off | 656 | 16 | 6.33 | 4.2 | 9.6 | <0.0001 | ||
| Fine motor | Monitoring | 2252 | 26 | 3.00 | 2.3 | 4.0 | <0.0001 | |
| Cut-off | 1089 | 19 | 4.53 | 3.1 | 6.5 | <0.0001 | ||
| Problem solving | Monitoring | 2040 | 35 | 3.75 | 3.1 | 4.6 | <0.0001 | |
| Cut-off | 1103 | 24 | 5.65 | 4.2 | 7.6 | <0.0001 | ||
| Personal−social | Monitoring | 2777 | 37 | 3.46 | 2.9 | 4.2 | <0.0001 | |
| Cut-off | 468 | 22 | 12.20 | 8.7 | 17.0 | <0.0001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimomura, H.; Hasunuma, H.; Tokunaga, S.; Taniguchi, Y.; Taniguchi, N.; Fujino, T.; Utsunomiya, T.; Tanaka, Y.; Tokuda, N.; Okuda, M.; et al. Early Developmental Signs in Children with Autism Spectrum Disorder: Results from the Japan Environment and Children’s Study. Children 2022, 9, 90. https://doi.org/10.3390/children9010090
Shimomura H, Hasunuma H, Tokunaga S, Taniguchi Y, Taniguchi N, Fujino T, Utsunomiya T, Tanaka Y, Tokuda N, Okuda M, et al. Early Developmental Signs in Children with Autism Spectrum Disorder: Results from the Japan Environment and Children’s Study. Children. 2022; 9(1):90. https://doi.org/10.3390/children9010090
Chicago/Turabian StyleShimomura, Hideki, Hideki Hasunuma, Sachi Tokunaga, Yohei Taniguchi, Naoko Taniguchi, Tetsuro Fujino, Takeshi Utsunomiya, Yasuhiko Tanaka, Narumi Tokuda, Masumi Okuda, and et al. 2022. "Early Developmental Signs in Children with Autism Spectrum Disorder: Results from the Japan Environment and Children’s Study" Children 9, no. 1: 90. https://doi.org/10.3390/children9010090
APA StyleShimomura, H., Hasunuma, H., Tokunaga, S., Taniguchi, Y., Taniguchi, N., Fujino, T., Utsunomiya, T., Tanaka, Y., Tokuda, N., Okuda, M., Shima, M., Takeshima, Y., & The Japan Environment and Children’s Study (JECS) Group. (2022). Early Developmental Signs in Children with Autism Spectrum Disorder: Results from the Japan Environment and Children’s Study. Children, 9(1), 90. https://doi.org/10.3390/children9010090







