Parent Mental Health and Family Coping over Two Years after the Birth of a Child with Acute Neonatal Seizures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. Statistical Analysis
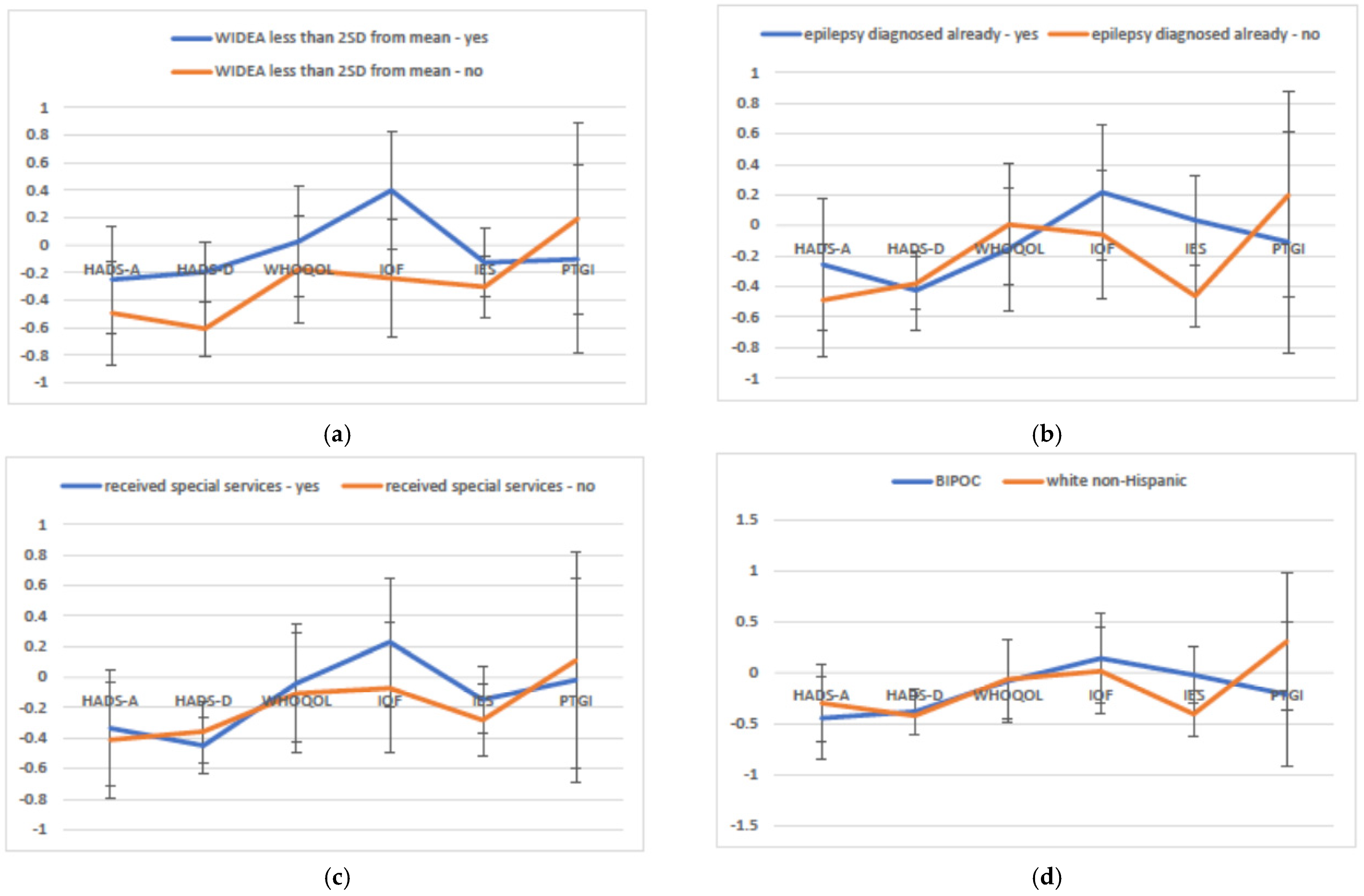
3. Results
3.1. Sample Characteristics
3.2. Linear Modeling of Variables Associated with Each Well-Being Outcome
3.2.1. Anxiety and Depression
3.2.2. Post-Traumatic Stress
3.2.3. Impact on Families
3.2.4. Quality of Life
3.2.5. Post-Traumatic Growth
3.3. Multivariate Modeling of Variables Associated with Parent and Family Well-Being over Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glass, H.C.; Grinspan, Z.M.; Shellhaas, R.A. Outcomes after acute symptomatic seizures in neonates. Semin. Fetal Neonatal Med. 2018, 23, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Glass, H.C.; Numis, A.L.; Gano, D.; Bali, V.; Rogers, E.E. Outcomes after acute symptomatic seizures in children admitted to a neonatal neurocritical care service. Pediatr. Neurol. 2018, 84, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Franck, L.S.; Ye, X.Y.; Hutchinson, S.A.; Lee, S.K.; O’Brien, K. Evaluating the effect of Family Integrated Care on maternal stress and anxiety in neonatal intensive care units. J. Reprod. Infant Psychol. 2021, 39, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Gateau, K.; Song, A.; Vanderbilt, D.L.; Gong, C.; Friedlich, P.; Kipke, M.; Lakshmanan, A. Maternal post-traumatic stress and depression symptoms and outcomes after NICU discharge in a low-income sample: A cross-sectional study. BMC Pregnancy Childbirth 2021, 21, 48. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, A.; Agni, M.; Lieu, T.; Fleegler, E.; Kipke, M.; Friedlich, P.S.; McCormick, M.C.; Belfort, M.B. The impact of preterm birth <37 weeks on parents and families: A cross-sectional study in the 2 years after discharge from the neonatal intensive care unit. Health Qual. Life Outcomes 2017, 15, 38. [Google Scholar] [CrossRef] [Green Version]
- Franck, L.S.; Shellhaas, R.A.; Lemmon, M.; Sturza, J.; Soul, J.S.; Chang, T.; Wusthoff, C.J.; Chu, C.J.; Massey, S.L.; Abend, N.S.; et al. Associations between infant and parent characteristics and measures of family well-being in neonates with seizures: A cohort study. J. Pediatr. 2020, 221, 64–71. [Google Scholar] [CrossRef]
- Earls, M.F.; Yogman, M.W.; Mattson, G.; Rafferty, J. Committee on Psychosocial Aspects of Child and Family Health. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics 2019, 143, e20183259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, A.; Obst, S.; Teague, S.J.; Rossen, L.; Spry, E.A.; Macdonald, J.A.; Sunderland, M.; Olsson, C.A.; Youssef, G.; Hutchinson, D. Association between maternal perinatal depression and anxiety and child and adolescent development: A meta-analysis. JAMA Pediatr. 2020, 174, 1082–1092. [Google Scholar] [CrossRef]
- Glass, H.C.; Soul, J.S.; Chang, T.; Wusthoff, C.J.; Chu, C.J.; Massey, S.L.; Abend, N.S.; Lemmon, M.; Thomas, C.; Numis, A.L.; et al. Safety of Early Discontinuation of Antiseizure Medication After Acute Symptomatic Neonatal Seizures. JAMA Neurol. 2021, 78, 817–825. [Google Scholar] [CrossRef]
- Glass, H.C.; Shellhaas, R.A.; Wusthoff, C.J.; Chang, T.; Abend, N.S.; Chu, C.J.; Cilio, M.R.; Glidden, D.V.; Bonifacio, S.L.; Massey, S.; et al. Contemporary profile of seizures in neonates: A prospective cohort study. J. Pediatr. 2016, 174, 98–103.e1. [Google Scholar] [CrossRef] [Green Version]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, D.S.; Marmar, C.R. The Impact of Event Scale-Revised. In Assessing Psychological Trauma and PTSD: A Practitioner’s Handbook; Wilson, J.P., Keane, T.M., Eds.; Guilford Press: New York, NY, USA, 1997; pp. 399–411. [Google Scholar]
- Williams, A.R.; Piamjariyakul, U.; Williams, P.D.; Bruggeman, S.K.; Cabanela, R.L. Validity of the revised Impact on Family (IOF) scale. J. Pediatr. 2006, 149, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Skevington, S.M.; Lotfy, M.; O’Connell, K.A. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A Report from the WHOQOL Group. Qual. Life Res. 2004, 13, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R.G.; Calhoun, L.G. The Posttraumatic Growth Inventory: Measuring the positive legacy of trauma. J. Trauma. Stress 1996, 9, 455–472. [Google Scholar] [CrossRef]
- Peyton, C.; Msall, M.E.; Wroblewski, K.; Rogers, E.E.; Kohn, M.; Glass, H.C. Concurrent validity of the Warner Initial Developmental Evaluation of Adaptive and Functional Skills and the Bayley Scales of Infant and Toddler Development, Third Edition. Dev. Med. Child Neurol. 2021, 63, 349–354. [Google Scholar] [CrossRef]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Agajany, N.; Gigi, M.; Ross, J.; Roth, J.; Eshel, R.; Constantini, S.; Bassan, H. The impact of neonatal posthemorrhagic hydrocephalus of prematurity on family function at preschool age. Early Hum. Dev. 2019, 137, 104827. [Google Scholar] [CrossRef]
- Pace, C.C.; Anderson, P.J.; Lee, K.J.; Spittle, A.J.; Treyvaud, K. Posttraumatic stress symptoms in mothers and fathers of very preterm infants over the first 2 years. J. Dev. Behav. Pediatr. 2020, 41, 612–618. [Google Scholar] [CrossRef]
- Werner, H.; Latal, B.; Buechel, E.V.; Beck, I.; Landolt, M.A. The impact of an infant’s severe congenital heart disease on the family: A prospective cohort study. Congenit. Heart Dis. 2014, 9, 203–210. [Google Scholar] [CrossRef]
- Bemister, T.B.; Brooks, B.L.; Dyck, R.H.; Kirton, A. Predictors of caregiver depression and family functioning after perinatal stroke. BMC Pediatr. 2015, 15, 75. [Google Scholar] [CrossRef] [Green Version]
- Peterson, R.K.; Williams, T.; Dlamini, N.; Westmacott, R. Parent experiences and developmental outcomes following neonatal stroke. Clin. Neuropsychol. 2021, 35, 973–987. [Google Scholar] [CrossRef]
- Berg, A.T.; Kaiser, K.; Dixon-Salazar, T.; Elliot, A.; McNamara, N.; Meskis, M.A.; Golbeck, E.; Tatachar, P.; Laux, L.; Raia, C.; et al. Seizure burden in severe early-life epilepsy: Perspectives from parents. Epilepsia Open 2019, 4, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Spindler, U.P.; Hotopp, L.C.; Bach, V.A.; Hornemann, F.; Syrbe, S.; Andreas, A.; Merkenschlager, A.; Kiess, W.; Bernhard, M.K.; Bertsche, T.; et al. Seizure disorders and developmental disorders: Impact on life of affected families-a structured interview. Eur. J. Pediatr. 2017, 176, 1121–1129. [Google Scholar] [CrossRef]
- Phillips, N.L.; Widjaja, E.; Smith, M.L. Family resources moderate the relationship between seizure control and health-related quality of life in children with drug-resistant epilepsy. Epilepsia 2020, 61, 1638–1648. [Google Scholar] [CrossRef]
- Danguecan, A.; El Shahed, A.I.; Somerset, E.; Fan, C.S.; Ly, L.G.; Williams, T. Towards a biopsychosocial understanding of neurodevelopmental outcomes in children with hypoxic-ischemic encephalopathy: A mixed-methods study. Clin. Neuropsychol. 2021, 35, 925–947. [Google Scholar] [CrossRef]
- McLaughlin, R.M.; Schraegle, W.A.; Nussbaum, N.L.; Titus, J.B. Parental coping and its role in predicting health-related quality of life in pediatric epilepsy. Epilepsy Behav. 2018, 87, 1–6. [Google Scholar] [CrossRef]
- Picoraro, J.A.; Womer, J.W.; Kazak, A.E.; Feudtner, C. Posttraumatic growth in parents and pediatric patients. J. Palliat. Med. 2014, 17, 209–218. [Google Scholar] [CrossRef]
- Carmassi, C.; Dell’Oste, V.; Foghi, C.; Bertelloni, C.A.; Conti, E.; Calderoni, S.; Battini, R.; Dell’Osso, L. Post-traumatic stress reactions in caregivers of children and adolescents/young adults with severe diseases: A systematic review of risk and protective factors. Int. J. Environ. Res. Public Health 2020, 18, 189. [Google Scholar] [CrossRef] [PubMed]
- Greening, L.; Stoppelbein, L.; Cheek, K. Racial/ethnic disparities in the risk of posttraumatic stress disorder symptoms among mothers of children diagnosed with cancer and Type-1 diabetes mellitus. Psychol. Trauma 2017, 9, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Slopen, N.; Heard-Garris, N. Structural Racism and Pediatric Health-A Call for Research to Confront the Origins of Racial Disparities in Health. JAMA Pediatr. 2021. [Google Scholar] [CrossRef] [PubMed]

| n (%) or Mean (SD) | |
|---|---|
| Male sex | 170 (56.1%) |
| Public insurance | 128 (42.3%) |
| Child race/ethnicity | |
| Hispanic, any race | 47 (15.5%) |
| Non-Hispanic, Native Hawaiian Pacific Islander | 2 (0.7%) |
| Non-Hispanic, American Indian Alaska Native | 2 (0.7%) |
| Non-Hispanic, Asian | 20 (6.6%) |
| Non-Hispanic, Black/African American | 35 (11.6%) |
| Non-Hispanic, More than 1 race | 8 (2.6%) |
| Non-Hispanic, Other race | 6 (2.0%) |
| Non-Hispanic, White | 175 (57.8%) |
| Unknown | 8 (2.6%) |
| Parent role | |
| Mother (all time points) | 227 (74.9%) |
| Father (all time points) | 27 (8.9%) |
| Mother and/or father or legal guardian (varied by time) | 49 (16.2%) |
| Maternal education, high school or less | 68 (23.5%) |
| Discharged on medication | 194 (64.0%) |
| Preterm birth | 50 (16.5%) |
| Complex medical condition, includes preterm | 79 (26.1%) |
| Age in days at discharge from hospital, median (IQR) | 15 (9–31) |
| Child diagnosed with epilepsy at 24 months | 37 (13.1%) |
| Child diagnosed with cerebral palsy at 24 months | 80 (29.3%) |
| WIDEA-FS less than 2 SD from mean | |
| At 12 months | 22/187 (11.8%) |
| At 18 months | 49/220 (22.3%) |
| At 24 months | 91/270 (33.7%) |
| Child receiving developmental support services | |
| At 12 months | 132/187 (70.6%) |
| At 18 months | 140/220 (63.6%) |
| At 24 months | 167/268 (62.3%) |
| Outcome | Timepoint | ||||
|---|---|---|---|---|---|
| Discharge n = 143 | 12 Months n = 171 | 18 Months n = 209 | 24 Months n = 246 | Change over Time (Controlling for Covariates) | |
| HADS—Depression (0–21; higher scores indicate worse symptoms), mean (SD) | 5.6 (4.0) | 3.5 (3.2) | 3.2 (3.2) 2 | 3.3 (3.1) 2 | Discharge > 12 m, 18 m, 24 m |
| 12 m = 18 m = 24 m | |||||
| HADS—Depression categorical | |||||
| Borderline abnormal (8–10) | 28 (19.6%) | 19 (11.1%) | 14 (6.7%) | 21 (8.5%) | |
| Abnormal (>10) | 17 (11.9%) | 5 (2.9%) | 6 (2.9%) | 8 (3.2%) | |
| HADS—Anxiety (0–21; higher scores indicate worse symptoms), mean (SD) | 8.3 (4.3) | 6.1 (4.3) | 5.6 (3.8) | 6.0 (4.1) | Discharge > 12 m, 18 m, 24 m |
| 12 m = 18 m = 24 m | |||||
| HADS—Anxiety categorical | |||||
| Borderline abnormal (8–10) | 34 (23.8%) | 35 (20.5%) | 45 (21.5%) | 53 (21.5%) | |
| Abnormal (>10) | 42 (29.4%) | 27 (15.8%) | 18 (8.6%) | 31 (12.6%) | |
| IES (0–88; higher scores indicate worse symptoms), median (IQR) | n/a | 14 (5, 25) | 11 (4, 23) | 10 (4, 23) | 12 m > 18 m |
| 12 m > 24 m | |||||
| 18 m = 24 m | |||||
| IES categorical | |||||
| Suggestive of PTSD (24–32) | n/a | 23 (13.4%) | 23 (11.0%) | 25 (10.2%) | |
| Probable PTSD (>33) | 24 (14.0%) | 28 (13.4%) | 33 (13.5%) | ||
| IOF (15 to 60; higher scores indicate greater impact), mean (SD) | Discharge > 12 m, 18 m, 24 m | ||||
| Overall | 34.8 (9.7) | 28.7 (10.7) | 28.7 (10.3) | 28.0 (10.4) | 12 m = 18 m |
| Financial | 10.6 (3.0) | 9.6 (3.3) | 9.3 (3.4) | 9.2 (3.4) | 12 m = 24 m |
| Coping | 8.3 (2.5) | 8.6 (2.3) | 8.9 (2.4) | 9.0 (2.8) | 18 m >24 m |
| WHOQOL-BREF (0 to 100 transformed scale; higher scores indicate better QOL) 1, mean (SD) | Discharge = 12 m =18 m = 24 m | ||||
| Overall | 73.4 (20.6) | 74.8 (18.9) | 74.8 (18.8) | 76.6 (18.2) | |
| Physical | 70.2 (16.6) | 74.5 (16.6) | 76.2 (15.3) | 75.9 (15.6) | |
| Psychological | 71.6 (18.3) | 68.1 (17.8) | 69.4 (16.5) | 692 (16.7) | |
| Social | 75.4 (19.1) | 65.7 (21.6) | 66.9 (19.0) | 67.2 (19.5) | |
| Environment | 73.1 (16.2) | 74.4 (14.8) | 74.1 (14.9) | 74.0 (14.5) | |
| PTGI (0–105; higher scores indicate more positive reappraisal), mean (SD) | n/a | 63.3 (24.8) | 61.1 (27.2) | 61.1 (25.5) | 12 m = 18 m = 24 m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franck, L.S.; Shellhaas, R.A.; Lemmon, M.E.; Sturza, J.; Barnes, M.; Brogi, T.; Hill, E.; Moline, K.; Soul, J.S.; Chang, T.; et al. Parent Mental Health and Family Coping over Two Years after the Birth of a Child with Acute Neonatal Seizures. Children 2022, 9, 2. https://doi.org/10.3390/children9010002
Franck LS, Shellhaas RA, Lemmon ME, Sturza J, Barnes M, Brogi T, Hill E, Moline K, Soul JS, Chang T, et al. Parent Mental Health and Family Coping over Two Years after the Birth of a Child with Acute Neonatal Seizures. Children. 2022; 9(1):2. https://doi.org/10.3390/children9010002
Chicago/Turabian StyleFranck, Linda S., Renée A. Shellhaas, Monica E. Lemmon, Julie Sturza, Marty Barnes, Trisha Brogi, Elizabeth Hill, Katrina Moline, Janet S. Soul, Taeun Chang, and et al. 2022. "Parent Mental Health and Family Coping over Two Years after the Birth of a Child with Acute Neonatal Seizures" Children 9, no. 1: 2. https://doi.org/10.3390/children9010002






