Risk, Course, and Effect of SARS-CoV-2 Infection in Children and Adults with Chronic Inflammatory Bowel Diseases
Abstract
:1. Introduction
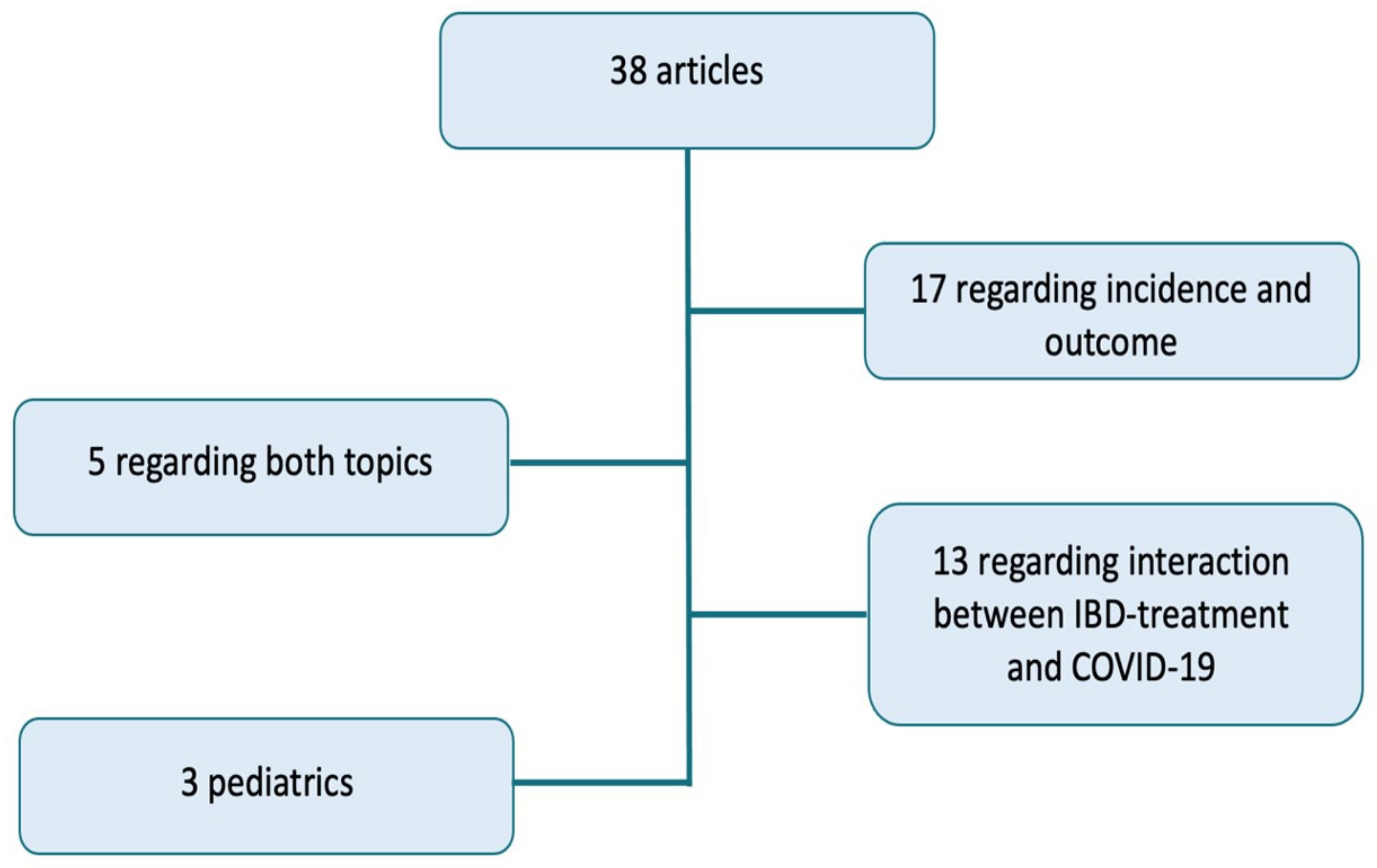
Methods
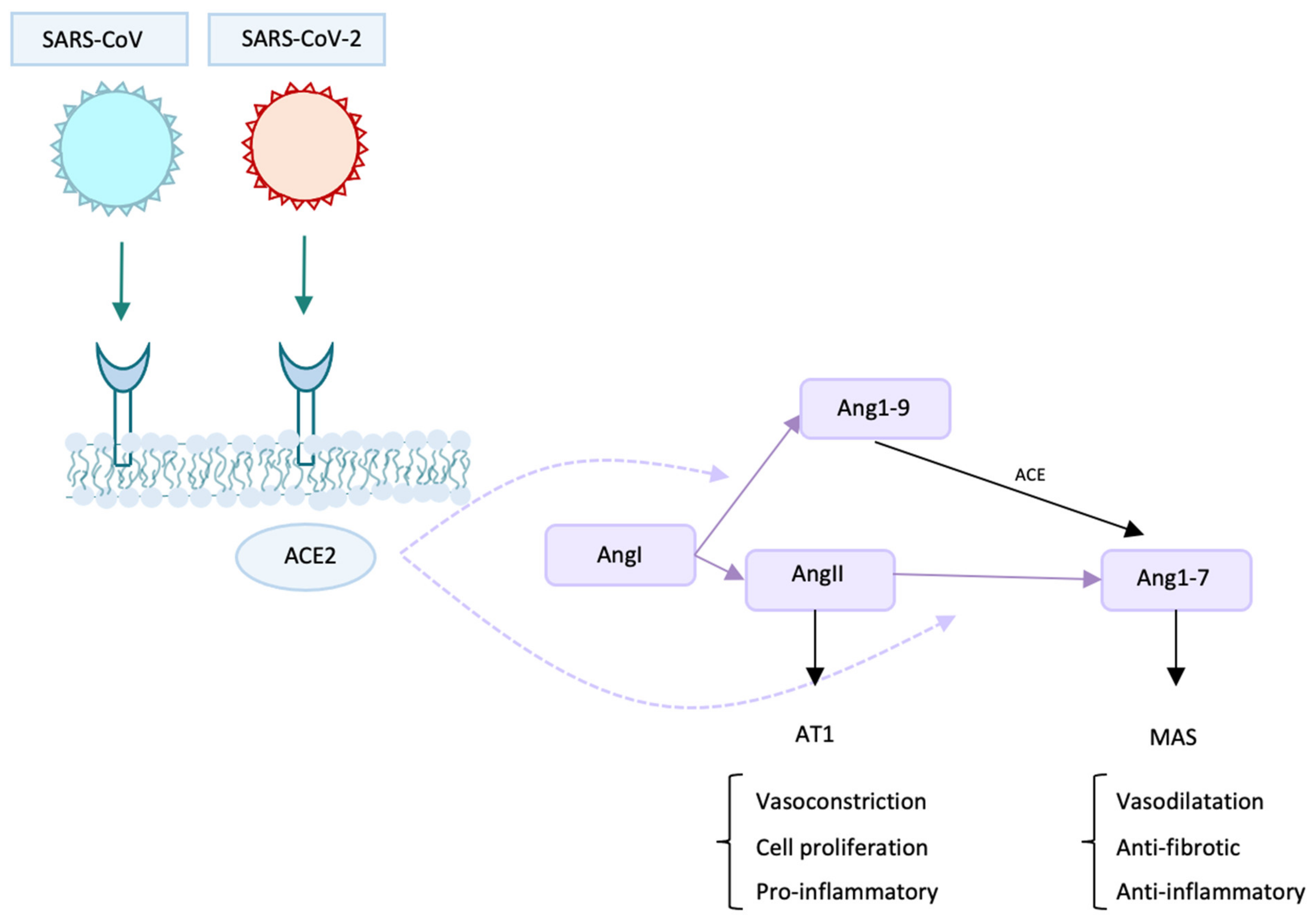
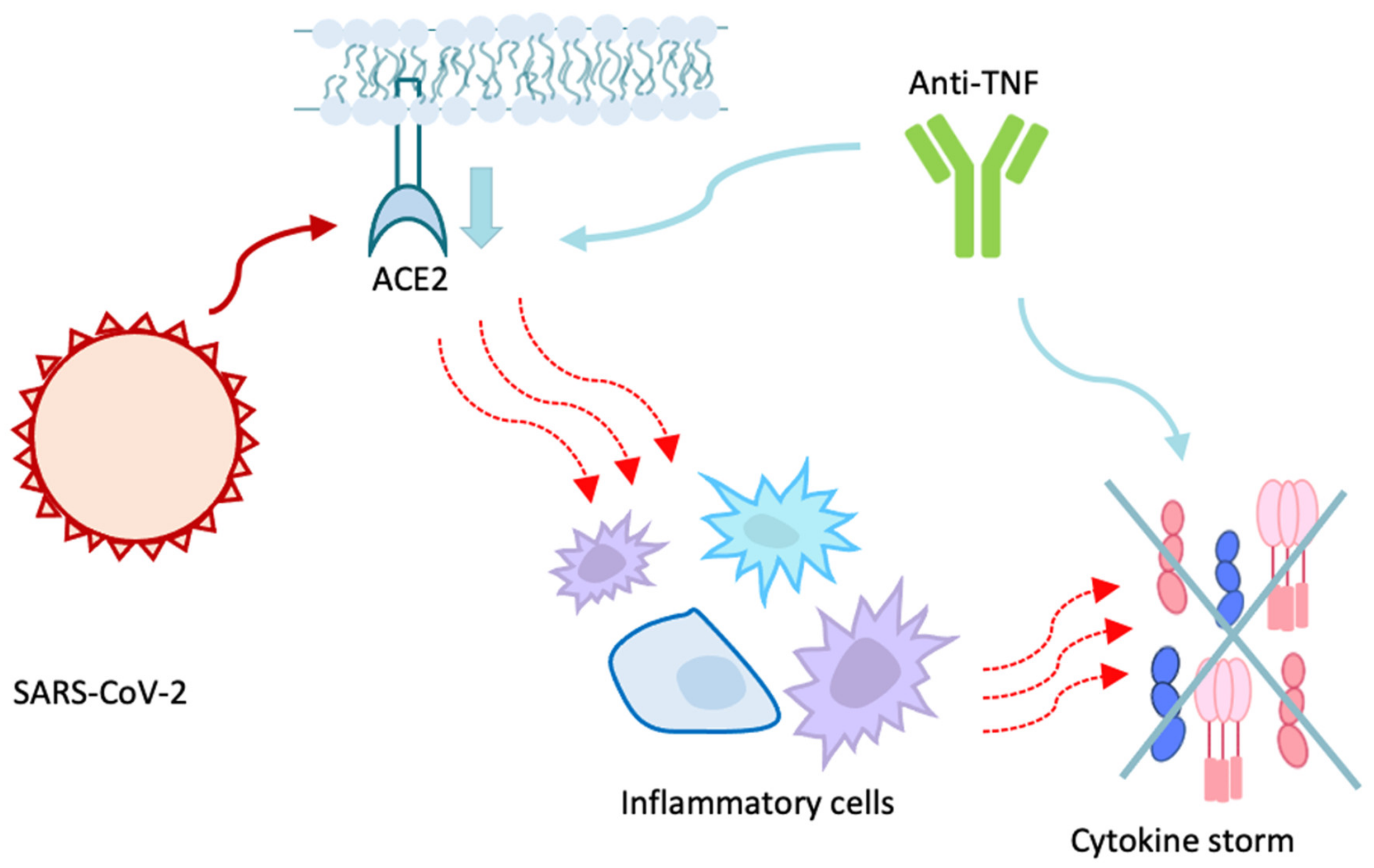
2. Protagonists and Co-Protagonists of The Infection: The Role of ACE2
3. Is SARS-CoV-2 Infection Incidence Increased in Inflammatory Bowel Diseases Affected Patients?
4. Outcome: Which Risks for Inflammatory Bowel Diseases Affected Patients?
Gastrointestinal Symptoms
5. Inflammatory Bowel Diseases, COVID-19 and Children
6. Inflammatory Bowel Diseases and ACE2
7. Drugs and Inflammatory Bowel Diseases: What Have We Learned?
7.1. Corticosteroids: Instruction for Use
7.2. Thiopurines: Should More Attention Be Paid in Elderly Patients?
7.3. Mesalamines: A Summary of Available Literature So Far
7.4. Biologic Drugs Protective Role
7.5. New Drugs
8. What to Do in Case of SARS-CoV-2 Infection: What British Society of Gastroenterology Suggests
9. Vaccines against COVID-19 and Inflammatory Bowel Diseases: A New Challenge
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [Green Version]
- WHO. Corona Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 18 May 2021).
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Guan, W.-J.; Liang, W.-H.; He, J.-X.; Zhong, N.-S. Cardiovascular comorbidity and its impact on patients with COVID-19. Eur. Respir. J. 2020, 55, 2001227. [Google Scholar] [CrossRef] [PubMed]
- DiPasquale, V.; Cucchiara, S.; Martinelli, M.; Miele, E.; Aloi, M.; Romano, C. Challenges in paediatric inflammatory bowel diseases in the COVID-19 time. Dig. Liver Dis. 2020, 52, 593–594. [Google Scholar] [CrossRef]
- Neurath, M.F. COVID-19 and immunomodulation in IBD. Gut 2020, 69, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Jablaoui, A.; Kriaa, A.; Mkaouar, H.; Akermi, N.; Soussou, S.; Wysocka, M.; Wołoszyn, D.; Amouri, A.; Gargouri, A.; Maguin, E.; et al. Fecal Serine Protease Profiling in Inflammatory Bowel Diseases. Front. Cell. Infect. Microbiol. 2020, 10, 21. [Google Scholar] [CrossRef]
- Garg, M.; Burrell, L.M.; Velkoska, E.; Griggs, K.; Angus, P.W.; Gibson, P.R.; Lubel, J.S. Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: A pilot study. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 559–569. [Google Scholar] [CrossRef]
- Kim, K.O.; Jang, B.I. Management of inflammatory bowel disease in the COVID-19 era. Intest. Res. 2021, 3. [Google Scholar] [CrossRef]
- Carparelli, S.; Pastore, M.R.; Valvano, M.R.; Marseglia, A.; Latiano, A.; Palmieri, O.; Guerra, M.; Martino, G.; Perri, F.; Bossa, F. Worse impact of second wave COVID-19 pandemic in adults but not in children with inflammatory bowel disease: An Italian single tertiary center experience. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2744–2747. [Google Scholar] [PubMed]
- Derikx, L.A.A.P.; Lantinga, M.A.; de Jong, D.J.; van Dop, W.A.; Creemers, R.H.; Römkens, T.E.H.; Jansen, J.M.; Mahmmod, N.; West, R.L.; Tan, A.C.I.T.L.; et al. Clinical Outcomes of Covid-19 in Patients with Inflammatory Bowel Disease: A Nationwide Cohort Study. J. Crohn’s Colitis 2021, 15, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, F.; Calabrese, C.; Salice, M.; Calandrini, L.; Privitera, H.; Melotti, L.; Peruzzi, G.; Dussias, N.; Belluzzi, A.; Scaioli, E.; et al. COVID-19 in IBD: The experience of a single tertiary IBD center. Dig. Liver Dis. 2021, 53, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Axelrad, J.; Halfvarson, J.; Khalili, H.; Larsson, E.; Lochhead, P.; Roelstraete, B.; Simon, T.G.; Söderling, J.; Olén, O. Inflammatory bowel disease and risk of severe COVID-19: A nationwide population-based cohort study in Sweden. United Eur. Gastroenterol. J. 2021, 9, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Guerra, I.; Algaba, A.; Jiménez, L.; Mar Aller, M.; Garza, D.; Bonillo, D.; Molina Esteban, L.M.; Bermejo, F. Incidence, Clinical Characteristics, and Evolution of SARS-CoV-2 Infection in Patients with Inflammatory Bowel Disease: A Single-Center Study in Madrid, Spain. Inflamm. Bowel Dis. 2021, 27, 25–33. [Google Scholar] [CrossRef]
- Marafini, I.; Salvatori, S.; Sena, G.; Calabrese, E.; Biancone, L.; Monteleone, G. Low frequency of COVID-19 in inflammatory bowel diseases. Dig. Liver Dis. 2020, 52, 1234–1235. [Google Scholar] [CrossRef] [PubMed]
- Łodyga, M.; Maciejewska, K.; Eder, P.; Waszak, K.; Stawczyk-Eder, K.; Michalak, M.; Dobrowolska, A.; Wiśniewska-Jarosińska, M.; Gąsiorowska, A.; Cicha, M.; et al. Inflammatory bowel disease is associated with higher seroprevalence rates of antibodies against SARS-CoV-2. Pol. Arch. Intern. Med. 2021, 131, 226–232. [Google Scholar]
- Taxonera, C.; Sagastagoitia, I.; Alba, C.; Mañas, N.; Olivares, D.; Rey, E. 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2020, 52, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.W.Y.; Weng, M.; Wei, S.C.; Ng, S.C. Zero COVID-19 infection in inflammatory bowel disease patients: Findings from population-based inflammatory bowel disease registries in Hong Kong and Taiwan. J. Gastroenterol. Hepatol. 2021, 36, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Maconi, G.; Bosetti, C.; De Monti, A.; Boyapati, R.K.; Shelton, E.; Piazza, N.; Gabrielli, A.M.C.; Lenti, M.V.; Bezzio, C.; Ricci, C.; et al. Risk of COVID 19 in patients with inflammatory bowel diseases compared to a control population. Dig. Liver Dis. 2021, 53, 263–270. [Google Scholar] [CrossRef]
- Allocca, M.; Chaparro, M.; Gonzalez, H.; Bosca-Watts, M.; Palmela, C.; D’Amico, F.; Zacharopoulou, E.; Kopylov, U.; Ellul, P.; Bamias, G.; et al. Patients with Inflammatory Bowel Disease Are Not at Increased Risk of COVID-19: A Large Multinational Cohort Study. J. Clin. Med. 2020, 9, 3533. [Google Scholar] [CrossRef]
- Norsa, L.; Indriolo, A.; Sansotta, N.; Cosimo, P.; Greco, S.; D’Antiga, L. Uneventful Course in Patients with Inflammatory Bowel Disease during the Severe Acute Respiratory Syndrome Coronavirus 2 Outbreak in Northern Italy. Gastroenterology 2020, 159, 371–372. [Google Scholar] [CrossRef]
- Quera, R.; Pizarro, G.; Simian, D.; Ibáñez, P.; Lubascher, J.; Kronberg, U.; Flores, L.; Figueroa, C. Impact of COVID-19 on a cohort of patients with inflammatory bowel disease at a specialised centre in Chile. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Viganò, C.; Massironi, S.; Pirola, L.; Cristoferi, L.; Fichera, M.; Bravo, M.; Mauri, M.; Redaelli, A.E.; Dinelli, M.E.; Invernizzi, P. COVID-19 in Patients With Inflammatory Bowel Disease: A Single-center Observational Study in Northern Italy. Inflamm. Bowel Dis. 2020, 26, e138–e139. [Google Scholar] [CrossRef]
- Lukin, D.J.; Kumar, A.; Hajifathalian, K.; Sharaiha, R.Z.; Scherl, E.J.; Longman, R.S.; Roberts, J.; Center Study Group Study Group; Weill Cornell Medicine-Gastrointestinal Study Group. Baseline Disease Activity and Steroid Therapy Stratify Risk of COVID-19 in Patients with Inflammatory Bowel Disease. Gastroenterology 2020, 159, 1541–1544. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Pugliese, D.; Privitera, G.; Onali, S.; Lopetuso, L.R.; Rizzatti, G.; Settanni, C.R.; Pizzoferrato, M.; Schiavoni, E.; Turchini, L.; et al. Impact of COVID-19 pandemic on the daily management of biotechnological therapy in inflammatory bowel disease patients: Reorganisational response in a high-volume Italian inflammatory bowel disease centre. United Eur. Gastroenterol. J. 2020, 8, 775–781. [Google Scholar] [CrossRef]
- Allocca, M.; Fiorino, G.; Zallot, C.; Furfaro, F.; Gilardi, D.; Radice, S.; Danese, S.; Peyrin-Biroulet, L. Incidence and Patterns of COVID-19 Among Inflammatory Bowel Disease Patients from the Nancy and Milan Cohorts. Clin. Gastroenterol. Hepatol. 2020, 18, 2134–2135. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khan, A.; Chowdhry, M.; Bilal, M.; Kochhar, G.S.; Clarke, K. Risk of Severe Coronavirus Disease 2019 in Patients with Inflammatory Bowel Disease in the United States: A Multicenter Research Network Study. Gastroenterology 2020, 159, 1575–1578.e4. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Levitte, S.; Balabanis, T.; Patel, A.; Sharma, A.; Habtezion, A. SARS-CoV-2 Testing, Prevalence, and Predictors of COVID-19 in Patients with Inflammatory Bowel Disease in Northern California. Gastroenterology 2020, 159, 1141–1144.e2. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, J.; Nielsen, J.; Ellingsen, T.; Knudsen, T.; Nielsen, R.G.; Larsen, M.D.; Lund, K.; Nørgård, B.M. Outcome of COVID-19 in hospitalized patients with chronic inflammatory diseases. A population based national register study in Denmark. J. Autoimmun. 2021, 120, 102632. [Google Scholar] [CrossRef]
- Mao, R.; Liang, J.; Shen, J.; Ghosh, S.; Zhu, L.-R.; Yang, H.; Wu, K.-C.; Chen, M.-H. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol. Hepatol. 2020, 5, 425–427. [Google Scholar] [CrossRef]
- Attauabi, M.; Poulsen, A.; Theede, K.; Pedersen, N.; Larsen, L.; Jess, T.; Hansen, M.R.; Verner-Andersen, M.K.; Haderslev, K.V.; Lødrup, A.B.; et al. Prevalence and Outcomes of COVID-19 Among Patients with Inflammatory Bowel Disease—A Danish Prospective Population-based Cohort Study. J. Crohn’s Colitis 2021, 15, 540–550. [Google Scholar] [CrossRef]
- Brenner, E.J.; Pigneur, B.; Focht, G.; Zhang, X.; Ungaro, R.C.; Colombel, J.-F.; Turner, D.; Kappelman, M.D.; Ruemmele, F.M. Benign Evolution of SARS-Cov2 Infections in Children with Inflammatory Bowel Disease: Results from Two International Databases. Clin. Gastroenterol. Hepatol. 2021, 19, 394–396.e5. [Google Scholar] [CrossRef]
- Turner, D.; Huang, Y.; Martín-De-Carpi, J.; Aloi, M.; Focht, G.; Kang, B.; Zhou, Y.; Sanchez, C.; Kappelman, M.D.; Uhlig, H.H.; et al. Corona Virus Disease 2019 and Paediatric Inflammatory Bowel Diseases: Global Experience and Provisional Guidance (March 2020) from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 727–733. [Google Scholar] [CrossRef]
- Sansotta, N.; Norsa, L.; Zuin, G.; Panceri, R.; Dilillo, D.; Pozzi, E.; De Giacomo, C.; Moretti, C.; Celano, R.; Nuti, F.; et al. Children with Inflammatory Bowel Disease in the COVID-19 Main Endemic Focus: The Lombardy Experience. Front. Pediatr. 2021, 9, 607285. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.C.; Brenner, E.J.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.-F.; Reinisch, W.; Steinwurz, F.; et al. Effect of IBD medications on COVID-19 outcomes: Results from an international registry. Gut 2021, 70, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Bezzio, C.; Pellegrini, L.; Manes, G.; Arena, I.; Picascia, D.; Della Corte, C.; Devani, M.; Schettino, M.; Saibeni, S. Biologic Therapies May Reduce the Risk of COVID-19 in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, e107–e109. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Brunton, A.E.; Beekmann, S.; Polgreen, P.; Baddley, J.; Saag, K.G.; Calabrese, C.; Calabrese, L.; Robinson, P.C.; Wallace, Z.S.; et al. SARS CoV-2 infection among patients using immunomodulatory therapies. Ann. Rheum. Dis. 2021, 80, 269–271. [Google Scholar] [CrossRef]
- Burke, K.E.; Kochar, B.; Allegretti, J.R.; Winter, R.W.; Lochhead, P.; Khalili, H.; Colizzo, F.P.; Hamilton, M.J.; Chan, W.W.; Ananthakrishnan, A.N. Immunosuppressive Therapy and Risk of COVID-19 Infection in Patients with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2021, 27, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.A.; Goodhand, J.R.; Bewshea, C.; Nice, R.; Chee, D.; Lin, S.; Chanchlani, N.; Butterworth, J.; Cooney, R.; Croft, N.M.; et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut 2021, 70, 865–875. [Google Scholar] [CrossRef]
- Bossa, F.; Carparelli, S.; Latiano, A.; Palmieri, O.; Tavano, F.; Panza, A.; Pastore, M.; Marseglia, A.; D’Altilia, M.; Latiano, T.; et al. Impact of the COVID-19 outbreak and the serum prevalence of SARS-CoV-2 antibodies in patients with inflammatory bowel disease treated with biologic drugs. Dig. Liver. Dis. 2021, 53, 277–282. [Google Scholar] [CrossRef]
- Khan, N.; Mahmud, N.; Trivedi, C.; Reinisch, W.; Lewis, J.D. Risk factors for SARS-CoV-2 infection and course of COVID19 disease in patients with IBD in the Veterans Affair Healthcare System. Gut 2021, 22. [Google Scholar] [CrossRef]
- Berte’, R.; Mazza, S.; Stefanucci, M.R.; Noviello, D.; Costa, S.; Ciafardini, C.; Mileti, E.; Mapelli, M.; Pasqualato, S.; Pinto, S.; et al. Seroprevalence of SARS-CoV2 in IBD Patients Treated with Biologic Therapy. J. Crohn’s Colitis 2021, 15, 864–868. [Google Scholar] [CrossRef]
- Agrawal, M.; Zhang, X.; Brenner, E.J.; Ungaro, R.C.; Kappelman, M.D.; Colombel, J.-F. The impact of vedolizumab on COVID-19 outcomes among adult IBD patients in the SECURE-IBD registry. J. Crohn’s Colitis 2021, 22, jjab071. [Google Scholar] [CrossRef]
- Brenner, E.J.; Ungaro, R.C.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.-F.; Reinisch, W.; Ruemmele, F.M.; et al. Corticosteroids, But Not TNF Antagonists, Are Associated with Adverse COVID-19 Outcomes in Patients with Inflammatory Bowel Diseases: Results from an International Registry. Gastroenterology 2020, 159, 481–491.e3. [Google Scholar] [CrossRef] [PubMed]
- Hormati, A.; Ghadir, M.R.; Zamani, F.; Khodadadi, J.; Khodadust, F.; Afifian, M.; Aminnejad, R.; Ahmadpour, S. Are there any association between COVID-19 severity and immunosuppressive therapy? Immunol. Lett. 2020, 224, 12–13. [Google Scholar] [CrossRef]
- Khan, N.; Patel, D.; Xie, D.; Lewis, J.; Trivedi, C.; Yang, Y.-X. Impact of Anti-Tumor Necrosis Factor and Thiopurine Medications on the Development of COVID-19 in Patients with Inflammatory Bowel Disease: A Nationwide Veterans Administration Cohort Study. Gastroenterology 2020, 159, 1545–1546.e1. [Google Scholar] [CrossRef]
- Allocca, M.; Guidelli, G.M.; Borroni, R.G.; Selmi, C.; Narcisi, A.; Danese, S.; Fiorino, G. Clinical course of COVID-19 in 41 patients with immune-mediated inflammatory diseases: Experience from humanitas center, Milan. Pharmacol. Res. 2020, 160, 105061. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xiao, S.-Y. COVID-19 and inflammatory bowel disease: A pathophysiological assessment. Biomed. Pharmacother. 2021, 135, 111233. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Yang, C.; Li, Y.; Xiao, S.-Y. Differential expression of ACE2 in the respiratory tracts and its relationship to COVID-19 pathogenesis. EBioMedicine 2020, 60, 103004. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Cai, G.; Chen, F.; Christiani, D.C.; Zhang, Z.; Wang, M. Multiomics Evaluation of Gastrointestinal and Other Clinical Characteristics of COVID-19. Gastroenterology 2020, 158, 2298–2301.e7. [Google Scholar] [CrossRef]
- Kariyawasam, J.C.; Jayarajah, U.; Riza, R.; Abeysuriya, V.; Seneviratne, S.L. Gastrointestinal manifestations in COVID-19. Trans. R. Soc. Trop. Med. Hyg. 2021, 16, trab042. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Luo, W.; Huang, L.; Xiao, J.; Li, F.; Qin, S.; Song, X.; Wu, Y.; Zeng, Q.; et al. A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int. J. Med. Sci. 2020, 17, 1522–1531. [Google Scholar] [CrossRef]
- Cholankeril, G.; Podboy, A.; Aivaliotis, V.I.; Tarlow, B.; Pham, E.A.; Spencer, S.P.; Kim, D.; Hsing, A.; Ahmed, A. High prevalence of concurrent gastrointestinal manifestations in patients with severe acute respiratory syndrome Coronavirus 2: Early experience from California. Gastroenterology 2020, 159, 775–777. [Google Scholar] [CrossRef]
- Werion, A.; Belkhir, L.; Perrot, M.; Schmit, G.; Aydin, S.; Chen, Z.; Penaloza, A.; De Greef, J.; Yildiz, H.; Pothen, L.; et al. Cliniques universitaires Saint-Luc (CUSL) COVID-19 Research Group SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020, 98, 1296–1307. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J. Trilogy of ACE2: A peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010, 128, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Simões, E.; Silva, A.C.; Teixeira, M.M. ACE inhibition, ACE2 and angiotensin-(1–7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol. Res. 2016, 107, 154–162. [Google Scholar] [CrossRef]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef]
- Matthai, J.; Shanmugam, N.; Sobhan, P.; Indian Society of Pediatric Gastroenterology, Hepatology and Nutrition. Pediatric Gastroenterology Chapter of Indian Academy of Pediatrics. Coronavirus Disease (COVID-19) and the Gastrointestinal System in Children. Indian Pediatr. 2020, 57, 533–535. [Google Scholar] [CrossRef]
- Xiao, F.; Sun, J.; Xu, Y.; Li, F.; Huang, X.; Li, H.; Zhao, J.; Huang, J.; Zhao, J. Infectious SARS-CoV-2 in Feces of Patient with Severe COVID-19. Emerg. Infect. Dis. 2020, 26, 1920–1922. [Google Scholar] [CrossRef]
- Gu, J.; Han, B.; Wang, J. COVID-19: Gastrointestinal Manifestations and Potential Fecal–Oral Transmission. Gastroenterology 2020, 158, 1518–1519. [Google Scholar] [CrossRef]
- Patankar, J.V.; Chiriac, M.T.; Lehmann, M.; Kühl, A.A.; Atreya, R.; Becker, C.; Gonzalez-Acera, M.; Schmitt, H.; Gamez-Belmonte, R.; Mahapatro, M.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Attachment Receptor Angiotensin-Converting Enzyme 2 Is Decreased in Crohn’s Disease and Regulated by Microbial and Inflammatory Signaling. Gastroenterology 2021, 160, 925–928.e4. [Google Scholar] [CrossRef]
- Suárez-Fariñas, M.; Tokuyama, M.; Wei, G.; Huang, R.; Livanos, A.; Jha, D.; Levescot, A.; Irizar, H.; Kosoy, R.; Cording, S.; et al. Intestinal Inflammation Modulates the Expression of ACE2 and TMPRSS2 and Potentially Overlaps with the Pathogenesis of SARS-CoV-2-related Disease. Gastroenterology 2021, 160, 287–301.e20. [Google Scholar] [CrossRef] [PubMed]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Zoufaly, A.; Poglitsch, M.; Aberle, J.H.; Hoepler, W.; Seitz, T.; Traugott, M.; Grieb, A.; Pawelka, E.; Laferl, H.; Wenisch, C.; et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020, 8, 1154–1158. [Google Scholar] [CrossRef]
- Senchenkova, E.Y.; Russell, J.; Almeida-Paula, L.D.; Harding, J.W.; Granger, D.N. Angiotensin II–Mediated Microvascular Thrombosis. Hypertension 2010, 56, 1089–1095. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Runge, M.S.; Brasier, A.R. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kappa B transcription factors. Circ. Res. 1999, 84, 695–703. [Google Scholar] [CrossRef]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Arrigo, S.; Alvisi, P.; Banzato, C.; Bramuzzo, M.; Civitelli, F.; Corsello, A.; D’Arcangelo, G.; Di Lillo, A.; Dipasquale, V.; Felici, E.; et al. Management of paediatric IBD after the peak of COVID-19 pandemic in Italy: A position paper on behalf of the SIGENP IBD working group. Dig. Liver Dis. 2021, 53, 183–189. [Google Scholar] [CrossRef]
- Prentice, R.E.; Al-Ani, A.; Christensen, B. Managing COVID -19 in patients with inflammatory bowel disease: Navigating unprecedented challenges. Intern. Med. J. 2021, 51, 284–287. [Google Scholar] [CrossRef]
- Miller, D.C.; Patel, J.; Gill, J.; Mattie, R.; Saffarian, M.; Schneider, B.J.; Popescu, A.; Babaria, V.; McCormick, Z.L. Corticosteroid Injections and COVID-19 Infection Risk. Pain Med. 2020, 21, 1703–1706. [Google Scholar] [CrossRef] [PubMed]
- Stockman, L.J.; Bellamy, R.; Garner, P. SARS: Systematic Review of Treatment Effects. PLoS Med. 2006, 3, e343. [Google Scholar] [CrossRef] [Green Version]
- Arabi, Y.M.; Mandourah, Y.; Al-Hameed, F.; Sindi, A.A.; Almekhlafi, G.; Hussein, M.A.; Jose, J.; Pinto, R.; Al-Omari, A.; Kharaba, A.; et al. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 2018, 197, 757–767. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Jones, G.-R.; Lamb, C.A.; Appleby, R.; Arnott, I.; Beattie, R.M.; Bloom, S.; Brooks, A.J.; Cooney, R.; Dart, R.J.; et al. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut 2020, 69, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Ruemmele, F.M.; Veres, G.; Kolho, K.L.; Griffiths, A.; Levine, A.; Escher, J.C.; Amil Dias, J.; Barabino, A.; Braegger, C.P.; Bronsky, J.; et al. European Society of Pediatric Gastroenterology, Hepatology and Nutrition Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J. Crohn’s Colitis 2014, 8, 1179–1207. [Google Scholar] [CrossRef] [Green Version]
- Kirchgesner, J.; Lemaitre, M.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Risk of Serious and Opportunistic Infections Associated with Treatment of Inflammatory Bowel Diseases. Gastroenterology 2018, 155, 337–346.e10. [Google Scholar] [CrossRef] [Green Version]
- Wisniewski, A.; Kirchgesner, J.; Seksik, P.; Landman, C.; Bourrier, A.; Nion-Larmurier, I.; Marteau, P.; Cosnes, J.; Sokol, H.; Beaugerie, L.; et al. Increased incidence of systemic serious viral infections in patients with inflammatory bowel disease associates with active disease and use of thiopurines. United Eur. Gastroenterol. J. 2020, 8, 303–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lees, C.W.; Irving, P.M.; Beaugerie, L. COVID-19 and IBD drugs: Should we change anything at the moment? Gut 2021, 70, 632–634. [Google Scholar] [CrossRef]
- Al-Ani, A.H.; Prentice, R.E.; Rentsch, C.A.; Johnson, D.; Ardalan, Z.; Heerasing, N.; Garg, M.; Campbell, S.; Sasadeusz, J.; Macrae, F.A.; et al. Review article: Prevention, diagnosis and management of COVID-19 in the IBD patient. Aliment. Pharmacol. Ther. 2020, 52, 54–72. [Google Scholar] [CrossRef]
- Abdullah, A.; Neurath, M.F.; Atreya, R. Mild COVID-19 Symptoms in an Infliximab-Treated Ulcerative Colitis Patient: Can Ongoing Anti-TNF Therapy Protect against the Viral Hyperinflammatory Response and Avoid Aggravated Outcomes? Visc. Med. 2020, 36, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, Z.; Li, J.W.; Zhao, H.; Wang, G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents 2020, 29, 105954. [Google Scholar] [CrossRef]
- Leal, R.F.; Planell, N.; Kajekar, R.; Lozano, J.J.; Ordás, I.; Dotti, I.; Esteller, M.; Masamunt, M.C.; Parmar, H.; Ricart, E.; et al. Identification of inflammtaory mediators in patients with Crohn’s disease unresponsive to anti-TNFα therapy. Gut 2015, 64, 233–242. [Google Scholar] [CrossRef]
- Garg, M.; Royce, S.G.; Tikellis, C.; Shallue, C.; Batu, D.; Velkoska, E.; Burrell, L.M.; Patel, S.K.; Beswick, L.; Jackson, A.; et al. Imbalance of the renin–angiotensin system may contribute to inflammation and fibrosis in IBD: A novel therapeutic target? Gut 2020, 69, 841–851. [Google Scholar] [CrossRef]
- Li, X.-Z.; Qiu, Y.; Jeffery, L.; Liu, F.; Feng, R.; He, J.-S.; Tan, J.-Y.; Ye, Z.-Y.; Lin, S.-N.; Ghosh, S.; et al. Down-Regulation of Colonic ACE2 Expression in Patients with Inflammatory Bowel Disease Responding to Anti-TNF Therapy: Implications for COVID-19. Front. Med. 2021, 7, 613475. [Google Scholar] [CrossRef]
- Pasparakis, M.; Alexopoulou, L.; Episkopou, V.; Kollias, G. Immune and inflammatory responses in TNF alpha-deficient mice: A critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 1996, 184, 1397–1411. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Feldmann, M.; Maini, R.N.; Woody, J.N.; Holgate, S.T.; Winter, G.; Rowland, M.; Richards, D.; Hussell, T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet 2020, 395, 1407–1409. [Google Scholar] [CrossRef]
- Dolinger, M.T.; Person, H.; Smith, R.; Jarchin, L.; Pittman, N.; Dubinsky, M.C.; Lai, J. Pediatric Crohn Disease and Multisystem Inflammatory Syndrome in Children (MIS-C) and COVID-19 Treated with Infliximab. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 153–155. [Google Scholar] [CrossRef]
- Ling, K.L.; Hilmi, I.; Ali, R.A.R.; Leong, R.W.L.; Leung, W.K.; Ng, S.C.; Wu, K.C.; Chen, M.H.; Ran, Z.H.; Hisamatsu, T.; et al. Asian Pacific Association of Gastroenterology (APAGE) Inflammatory Bowel Disease (IBD) Working Party guidelines on IBD management during the COVID-19 pandemic. JGH Open 2020, 4, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Anikhindi, S.A.; Kumar, A.; Arora, A. COVID-19 in patients with inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1187–1193. [Google Scholar] [CrossRef]
- Kontzias, A.; Kotlyar, A.; Laurence, A.; Changelian, P.; O’Shea, J.J. Jakinibs: A new class of kinase inhibitors in cancer and autoimmune disease. Curr. Opin. Pharmacol. 2012, 12, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Jefremow, A.; Neurath, M.F. SARS-CoV-2 Virus Manifestations in the Gastrointestinal Tract: Therapeutic Implications. Visc. Med. 2021, 37, 63–69. [Google Scholar] [CrossRef]
- Cantini, F.; Niccoli, L.; Nannini, C.; Matarrese, D.; Di Natale, M.E.; Lotti, P.; Aquilini, D.; Landini, G.; Cimolato, B.; Di Pietro, M.A.; et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Melmed, G.Y.; Vermeire, S.; Long, M.D.; Chan, G.; Pedersen, R.D.; Lawendy, N.; Thorpe, A.J.; Nduaka, C.; Su, C. Herpes Zoster Infection in Patients with Ulcerative Colitis Receiving Tofacitinib. Inflamm. Bowel Dis. 2018, 24, 2258–2265. [Google Scholar] [CrossRef] [Green Version]
- Scribano, M.L. Why Do Immunosuppressed Patients with Inflammatory Bowel Disease Not Seem to be at a Higher Risk of COVID-19? Dig. Dis. Sci. 2021, 66, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef] [PubMed]
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef]
- Gupta, S.; Wang, W.; Hayek, S.S.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Association between Early Treatment with Tocilizumab and Mortality Among Critically Ill Patients with COVID-19. JAMA Intern. Med. 2021, 181, 41–51. [Google Scholar] [CrossRef]
- Stone, J.H.; Frigault, M.J.; Serling-Boyd, N.J.; Fernandes, A.D.; Harvey, L.; Foulkes, A.S.; Horick, N.K.; Healy, B.C.; Shah, R.; Bensaci, A.M.; et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N. Engl. J. Med. 2020, 383, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T.; Feuerstein, J.D.; Wang, A.Y.; Cohen, R.D. AGA Clinical Practice Update on Management of Inflammatory Bowel Disease during the COVID-19 Pandemic: Expert Commentary. Gastroenterology 2020, 159, 350–357. [Google Scholar] [CrossRef]
- Siegel, C.A.; Christensen, B.; Kornbluth, A.; Rosh, J.R.; Kappelman, M.D.; Ungaro, R.C.; Johnson, D.F.; Chapman, S.; Wohl, D.A.; Mantzaris, G.J. Guidance for Restarting Inflammatory Bowel Disease Therapy in Patients Who Withheld Immunosuppressant Medications during COVID-19. J. Crohn’s Colitis 2020, 14, S769–S773. [Google Scholar] [CrossRef]
- D’Amico, F.; Rabaud, C.; Peyrin-Biroulet, L.; Danese, S. SARS-CoV-2 vaccination in IBD: More pros than cons. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 211–213. [Google Scholar] [CrossRef]
- Mamula, P.; Markowitz, J.E.; Piccoli, D.A.; Klimov, A.; Cohen, L.; Baldassano, R.N. Immune Response to Influenza Vaccine in Pediatric Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2007, 5, 851–856. [Google Scholar] [CrossRef]
- Fiorino, G.; Peyrin-Biroulet, L.; Naccarato, P.; Szabò, H.; Sociale, O.R.; Vetrano, S.; Fries, W.; Montanelli, A.; Repici, A.; Malesci, A.; et al. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: A prospective study. Inflamm. Bowel Dis. 2012, 18, 1042–1047. [Google Scholar] [CrossRef]
- Alexander, J.L.; Powell, N. British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group. SARS-CoV-2 vaccination in immunosuppressed patients with inflammatory bowel disease: Should our approach change? Lancet. Gastroenterol. Hepatol. 2021, 6, 528–529. [Google Scholar] [CrossRef]
- About Our COVID-19 Volunteer Trials. Available online: https://www.bsg.org.uk/covid-19-advice/british-society-of-gastroenterology-inflammatory-bowel-disease-section-andibd-clinical-research-group-position-statement-on-sars-cov2-vaccination (accessed on 20 May 2021).
- Squire, J.D.; Gonzalez-Estrada, A.; Caldera, F.; Farraye, F.A. COVID-19 Vaccination in Patients with Inflammatory Bowel Disease and History of Reaction to Injectable Therapies. Inflamm. Bowel Dis. 2021, 27, 1358–1360. [Google Scholar] [CrossRef]
- Lichtenstein, L.; Ron, Y.; Kivity, S.; Ben-Horin, S.; Israeli, E.; Fraser, G.M.; Dotan, I.; Chowers, Y.; Confino-Cohen, R.; Weiss, B. Infliximab-Related Infusion Reactions: Systematic Review. J. Crohn’s Colitis 2015, 9, 806–815. [Google Scholar] [CrossRef] [Green Version]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pr. 2021, 9, 1423–1437. [Google Scholar] [CrossRef]
- United States Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States; United States Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/clinicalconsiderations.html (accessed on 18 May 2021).
- Caron, B.; Neuville, E.; Peyrin-Biroulet, L. Inflammatory Bowel Disease and COVID-19 Vaccination: A Patients’ Survey. Dig. Dis. Sci. 2021, 12, 1–7. [Google Scholar] [CrossRef]
- Martinelli, M.; Strisciuglio, C.; Fedele, F.; Miele, E.; Staiano, A. Clinical and Psychological Issues in Children with Inflammatory Bowel Disease during COVID-19 Pandemic. Inflamm. Bowel Dis. 2020, 26, e95–e96. [Google Scholar] [CrossRef]

| Author | Patients | Diagnostic Method | Geographic Area | Time/Duration of the Study | Results |
|---|---|---|---|---|---|
| Carparelli et al., 2021 [10] | IBD: 600 COVID-19: 25 | Molecular swab (PCR) or serological test | Foggia (Italy) | Until January 2021 | COVID-19 incidence in IBD patients (4.1%) > incidence in general population (2.8%) Hospitalization in IBD patients (12%) > hospitalization in Italian population (4.8%) |
| Derikx et al., 2021 [11] | IBD: 34,763 COVID-19: 100 (0.29%) | PCR 96/100 TC 3/100 Serological test and symptoms 1/100 | Netherlands | From March to June 2020 | COVID-19 incidence in IBD patients (287.6/100,000) comparable to general population (333/100,000), p = 0.15 Mortality in IBD patients (37.3/100,000) comparable to general population (44.9/100,000), 0 = 0.51 Among 100 infections, 59 hospitalizations and 13 deaths Hospitalization risk in IBD patients (177.2/100,000) > general population (84.5/100,000), p < 0.01 |
| Rizzello et al., 2020 [12] | IBD: 1158 COVID-19: 26 (2.2%) | Molecular swab (PCR) | Italy | 10 March 2020–10 June 2020 | COVID-19 incidence in IBD patients (22.4/1000) > incidence in Italy (3.91/1000, respectively 9.01, 6.27, and 7.10/1000 in Lombardy, Emilia Romagna, and Piedmont) |
| Ludvigsson et al., 2021 [13] | IBD: 67,292 (of which 6569 < 18aa) COVID-19: 811 (1.21%) Controls: 297,910 (of which 30,891 < 18aa) COVID-19: 2890 (0.97%) | Laboratory diagnosis | Sweden | 01 February 2020–31 July 2020 | COVID-19 incidence in IBD patients (5.4/1000) > controls (3.4/1000) 1/185 IBD patients that required hospitalization < 1/295 in controls (179 vs. 500): the risk of hospitalization is increased 43% in IBD patients (0.27% vs. 0.17% in controls) No increased risk of severe forms (ICU or death) |
| Guerra et al., 2020 [14] | IBD: 805 COVID-19: 82 | PCR 28 patients Clinic 54 patients (highly suspected) | Madrid (Spain) | Until 27 May 2020 | COVID-19 incidence: 10.2% 79.3% mild symptoms, 12.2% moderate symptoms, 8.5% severe symptoms, 1 death |
| Marafini et al., 2020 [15] | IBD: 672 COVID-19: 3 | Molecular swab (PCR) | Tor Vergata, Rome (Italy) | Until 30 April 2020 | COVID-19 incidence in IBD patients (4.46/1000) > Italian population (3.41/1000) p = 0.5 |
| Lodyga et al., 2021 [16] | IBD: 432 Controls: 432 | Serological test | Warsaw, Lodz and Poznan (Poland) | 01 May 2020–15 June 2020 | IgG: 4.6% of IBD patients and 1.6% of controls, p < 0.05 IgA + IgM: 6% of IBD patients and 1.1% of controls, p < 0.05 |
| Author | Patients | Diagnostic Method | Geographic Area | Time/Duration of the Study | Results |
|---|---|---|---|---|---|
| Taxonera et al., 2020 [17] | IBD: 1912 COVID-19: 12 | Molecular swab (PCR) | Madrid (Spain) | Until 08 April 2020 | COVID-19 incidence in IBD patients (4.9/1000) < general population (6.6/1000), OR:0.74, p < 0.001 Mortality in IBD patients (0.82/1000) < general population (0.9/1000) but not statistically significative, p = 0.36 |
| Mak et al., 2021 [18] | Hong Kong IBD: 2954 Taiwan IBD: 2554 | Molecular swab (PCR) | Hong Kong and Taiwan (China) | 21 January 2020–15 April 2020 | 0 COVID-19 cases among IBD patients General population: 1017 cases in Hong Kong, 429 cases in Taiwan |
| Maconi et al., 2020 [19] | IBD: 941 COVID-19: 2 Controls: 869 COVID-19: 10 | Molecular swab (PCR) (certain cases) Clinic (highly suspected cases) | Lombardy (Italy) | Until 25 April 2020 | Certain diagnosis of COVID-19: 2 IBD patients and 10 controls, p = 0.018 Highly suspected COVID-19: 3.8% of IBD patients < 6.3% of controls, p = 0.006 |
| Allocca et al., 2020 [20] | IBD: 23,879 COVID-19: 97 | Molecular swab (PCR): 64 patients Clinic + contact or radiology: 33 patients (highly suspected) | Italy, United Kingdom, France, Spain, Portugal, Malta, Kastoria, Attica, Greece, Russia, Israel | 21 February 2020–30 June 2020 | COVID-19 incidence in IBD patients (0.406%) comparable to general population (0.402%) Lethality in IBD patients (1%) < general population (9%) |
| Norsa et al., 2020 [21] | IBD: 522, of which 59 < 18 aa Controls with COVID-19: 479 | Molecular swab (PCR) | Hospital “Papa Giovanni XXIII”, Bergamo (Italy) | 19 February 2020–23 March 2020 | 0 cases of COVID-19 in IBD patients 479 COVID-19 patients accessed the hospital during the same period |
| Quera et al., 2020 [22] | IBD: 1432 COVID-19: 32 | Molecular swab (PCR) | Chile | 01 March 2020–31 August 2020 | Hospitalization in 4 patients. No death. IBD patients do not have an increased risk of severe symptoms |
| Viganò et al., 2020 [23] | IBD: 704 COVID-19: 53 | Laboratory diagnosis (9 patients, 1.2%) or highly suspected clinic based on WHO criteria (+ contact or flu vaccine) | Lombardy | Until April 2020 | COVID-19 incidence in IBD patients (1.2%) comparable to general population (0.81%) Association between IBD severity and COVID-19 (OR:12.6, p = 0.01) |
| Lukin et al., 2020 [24] | IBD e COVID-19: 80 COVID-19 non IBD: 160 | Molecular swab (PCR) or highly suspected clinic | New York (USA) | 01 February 2020–30 April 2020 | Risk of ICU admission, intubation and death resulted minor in IBD patients compared to controls (24% vs. 35%) but the result is not statistically significative (p = 0.352) |
| Scaldaferri et al., 2020 [25] | IBD: 1451 COVID-19: 5 | Molecular swab (PCR) | Rome (Italy) | 04 March 2020–15 April 2020 | Only mild symptoms in positive patients |
| Allocca, Fiorino et al., 2020 [26] | IBD: 6000 patients COVID-19: 15 | Molecular swab (PCR) | Nancy (France) and Milan (Italy) | Since the beginning of pandemic (publication date: 30 April 2020) | COVID-19 incidence in IBD patients (0.0025) comparable to general population (0.0017) Mortality and need for hospitalization higher in general population (13% vs. 5%), 5 hospitalizations, 0 ICU admission 0 deaths |
| Singh et al., 2020 [27] | IBD: 196,403 COVID-19: 232 Controls: 19,776 COVID-19 | Laboratory diagnosis or COVID-19 diagnostic code after hospitalization | USA | 26 January 2020–26 May 2020 | Risk of severe disease (hospitalization and/or death within 30 days) comparable between IBD patients (56/232) and controls (4139/19,776), RR: 0.93, p = 0.66 |
| Gubatan et al., 2020 [28] | IBD: 168 (tested) COVID-19: 5 | Molecular swab (PCR) | Northern California (USA) | 04 March 2020–14 April 2020 | Positivity rate comparable between IBD patients (3%) and general population (2.8%) |
| Kjeldsen et al., 2021 [29] | 132 hospitalized patients for COVID-19 having IBD/RA/AS/psoriasis 2811 controls hospitalized for COVID-19 | Hospitalized patients with COVID-19 diagnostic code (from national database) | Denmark | 01 March 2020–31 October 2020 | No significative differences between the group of patients with underlying diseases and controls in terms of hospital persistence (6.8 vs. 5.5 days), need for mechanical ventilation (7.6% vs. 9.4%), need for CPAP (11.4% vs. 8.8%), in-hospital, within 14 and 30 days mortality (17.4%, 20.5% e 21.2% vs. 15.2%, 18.1% e 19.1%, OR 0.71, 0.70 e 0.68) |
| Mao et al., 2020 [30] | IBD: 20,000 COVID-19: 0 (the three biggest centers in Wuhan have been analyzed) | Laboratory diagnosis | China | December 2019–08 March 2020 | 0 COVID-19 diagnosis |
| Attauabi et al., 2020 [31] | IBD: 2486 COVID-19: 76 COVID-19 general population: 8476 out of 231601 swabs | Molecular swab (PCR) | Denmark | 28 January 2020–02 June 2020 | Prevalence in IBD patients (2.5%) < general population (3.7%), p < 0.01 (with more tests performed in percentage in patients with IBD) Hospitalization in 25% of patients, need for oxygen-therapy in 18.4%, 4 deaths Dyspnea as presenting symptom is a risk factor for access ICU (OR: 19.7) |
| Authors | Patients | Diagnostic Method | Geographic Area | Time/Duration of the Study | Results |
|---|---|---|---|---|---|
| Brenner et al., 2021 (pediatric) [32] | IBD and COVID-19: 209 | Laboratory diagnosis | 23 countries (SECURE-IBD and COVID-19 Pediatric IBD Porto Group) | Until 01 October 2020 | 7% hospitalizations, of which 1% mechanical ventilation (sulfasalazine/mesalazine therapy, they developed multisystemic inflammation and superinfection). 0 deaths Hospitalization rate < IBD adult patients (33–66%) |
| Turner et al., 2020 (pediatric) [33] | PIBD: 102 COVID-19: 8 (6 confirmed) | Laboratory diagnosis in 6 patients Highly suspected clinic in 2 patients | Porto Group-affiliated Pediatric IBD centers in Europe | Until 26 March 2020 | Only mild symptoms (fever, cough, ageusia, myalgia, anosmia, asthenia) |
| Laboratory diagnosis or clinic suspect | China | Until 20 March 2020 | Out of 917 pediatric cases of SARS-CoV-2 infection, none had IBD | ||
| Sansotta et al., 2021 (pediatric) [34] | PIBD: 290 COVID-19: 24 (8%) | Clinic in 22 patients Molecular swab in 2 patients | Lombardy (Italy) | 21 February 2020–04 May 2020 (lockdown period) | Only 8% of children developed COVID-like symptoms, on which the supposed diagnosis was based given the scarce availability of swabs. 42% thiopurine therapy, 30% salicylates, 16% organic. No severe course or need for hospitalization |
| Author | Patients | Diagnostic Method | Geographic Area | Time/Duration of the Study | Clinic Results | Main Purposes of the Study | Therapy’s Effects |
|---|---|---|---|---|---|---|---|
| Ungaro et al., 2020 [35] | IBD and COVID-19: 1439 | Laboratory diagnosis | SECURE-IBD (47 countries) | 13 March 2020–09 June 2020 | 112 patients (7.8%) severe form 82 ICU 66 mechanical ventilations 49 (3.4%) deaths | To evaluate the course of COVID-19 in IBD patients under different therapies | Severe form of disease: patients under anti-TNFs (1.1%) < other patients (4.8%), p < 0.001 Anti-TNF are not associated with COVID-19 severe forms (aOR:0.69). Patients under thiopurine or thiopurine + anti-TNFs (9.2% and 8.8%) > patients under anti-TNFs monotherapy (2.2%), p < 0.001. Risk of COVID-19 severe form is increased in patients under thiopurine treatment (aOR:4.08) or combined therapy (aOR:4.01). Patients under mesalamine (sulfasalazine (13.9%) > other patients (5.2%), p < 0.001 Increased risk of COVID-19 severe forms (aOR: 1.47) |
| Bezzio et al., 2020 [36] | IBD: 243 COVID-19: 11 (1 confirmed and the other made by contact + clinic) | Molecular swab in 1 patient Clinic (min 3 symptoms) + contact in 10 patients | Italy | 10 March 2020–03 May 2020 | 124 patients on biologic therapy (2 COVID-19) 119 patients not on biologic therapy (9 COVID-19) | To assess the incidence of COVID-19 in IBD patients relating to the use or biologics | COVID-19 incidence: patients on biologic therapy 1.6% < patients not on biologic therapy 7.6% |
| Winthrop et al., 2020 [37] | COVID-19: 2500, of which 77 on immunomodulator therapy (diagnosis: RA, UC, sarcoidosis and others) | Molecular swab (PCR) | Canada | Until 22 May 2020 | 63 (81.8%) hospitalizations 27 (35.1%) mechanical ventilations 37 (48.1%) ICU 9 (11.7%) deaths | To report COVID-19 cases among patients assuming immunomodulatory therapies | Hospitalization required in 50% of patients on anti-TNF therapy 73.3% of patients on biologic (non anti-TNFs) therapy 90.9% of patients on DMARDs therapy 100% of patients od DMARDs + corticosteroids or only corticosteroids 66.7% of patients on JAK inhibitors treatment 0 deaths among patients on anti-TNF therapy |
| Rizzello et al., 2020 [12] | IBD: 1158 COVID-19: 26 (2.2%) | Molecular swab (PCR) | Italy | 10 March 2020–10 June 2020 | 521 patients on biologic therapy. Treatment interrupted in 85 patients and delayed in 195. Worsening of symptoms in 200 patients on biologic therapy (189 interrupted it) | To understand the incidence of COVID-19 between IBD patients and to evaluate possible risk factors for the infection | 5 patients on biologics, 16 on mesalazine, 5 on corticosteroids and 1 on thiopurines Hospitalization in 7 patients (none was in biologic therapy) 2 deaths (mesalazine therapy) Anti-TNFs could reduce the infection severity The continuous corticosteroids treatment could represent a risk factor for the infection |
| Burke et al., 2020 [38] | IBD: 5302 COVID-19: 39 (0.7%) | Molecular swab (PCR) | Massachusetts | 01 January 2020–25 April 2020 | 7 hospitalized patients 3 ICU 1 death | To clarify the effect of biologics and immunomodulators on COVID-19 risk | Infection: 0.64% of patients on mesalamine/sulfasalazine therapy, 0.5% of them on immunomodulators, 1% of patients on anti-TNFs, 1% among patients on Vedolizumab therapy. Drug intake does not influence infection risk. Corticosteroids’ or other immunosuppressor’s use have not been associated with a higher infection risk (users 0.37% vs. nonusers 0.36% regarding corticosteroids) |
| Kennedy et al., 2021 [39] | IBD: 6935 (patients ≥ 5 years under Infliximab or Vedolizumab treatment for at least 6 weeks) | Certain cases: molecular swab (PCR) Highly suspected cases: clinic | UK | 22 September 2020–23 December 2020 | Anti-SARS-CoV-2 antibodies: 4.3% (295) | To study if IBD patients under Infliximab treatment show reduced serological response to the infection | Anti-SARS-CoV-2: 3.4% of patients under Infliximab (161/4685) < 6% of patients under Vedolizumab (134/2250), p < 0.0001 Infliximab is associated to a lower seropositivity level compared to Vedolizumab (OR:0.66, p = 0.0027) or other immunomodulators (OR:0.70, p = 0.012) Among patients with COVID-19 confirmed diagnosis, seroconversion regarded: Infliximab (48%, 39/81) < Vedolizumab (83%, 30/36, p = 0.00044) even though the incidence of symptoms was similar in the two groups Failure of seroconversion has been linked to the concomitant use of immunomodulators: in patients treated with Infliximab only the seroconversion rate was 60% (24/40) while in patients treated with infliximab and immunomodulators it was 37% (15/41, p = 0.046) |
| Bossa et al., 2021 [40] | IBD: 259 (27 children) Controls: 214 non-IBD patients | Serologic test | Foggia (Italy) | February 2020–June 2020 | Infection rate (0.77) comparable to general population (0.19), p = 0.5 32 patients (12.3%) developed COVID-like symptoms (1 of them under Infliximab therapy)2 hospitalizations | To understand the impact of SARS-CoV-2 infection in IBD patients and the serum prevalence of antibodies in IBD patients under biologics | Seroprevalence (anti SARS-CoV-2 antibodies) comparable between IBD patients (0.77) and general population (0.9) No risk associated with biologic therapy (34.4% Adalimumab, 24% Infliximab, 22% Vedolizumab, 10.4% Ustekinumab, 7.7% Golimumab, 1.1% experimental therapy, 0.4% thalidomide) |
| Khan et al., 2021 [41] | IBD: 30,911 COVID-19: 649 | Molecular swab (PCR) | USA, VAHS database | 20 January 2020–10 December 2020 | 125 hospitalizations 41 deaths | To understand the role of IBD therapies in the risk of infection and their impact in the infection course | Vedolizumab is associated with a greater infection risk than mesalazine (HR:1.70, p = 0.006) Corticosteroids are associated with an increased risk of infection and of severe forms (hospitalization or death) No differences in terms of outcome between patients on mesalazine and on anti-TNFs Patients who are not under therapy have a significatively higher risk of severe infection compared to patients under mesalazine |
| Berte et al., 2020 [42] | IBD: 354 (biologic therapy) COVID-19 IgG: 8 | Serologic test | Italy and Germany | April 2020–June 2020 | Anti-SARS-CoV-2 IgG have been found in 8 patients (higher incidence of symptoms and contact with positives in these patients) | To determine SARS-CoV-2 infection prevalence in IBD patients under biologics | Seroprevalence (IgG anti SARS-CoV-2) in IBD patients on biologic therapy comparable to that found in general population (Milan 7.5%, Sardinia 0.3% e Germany 0.9%) |
| Agrawal et al., 2021 [43] | IBD and COVID-19: 3647 patients, of which 457 (12.5%) on Vedolizumab therapy | Laboratory diagnosis | Data from SECURE-IBD database | Until 26 January 2021 | 664 hospitalizations 166 severe forms of infection (ICU admission, mechanical ventilation and/or death) | To study Vedolizumab effects in IBD patients who undergo COVID-19 | Vedolizumab is safe and it is not associated with hospitalizations or more severe infections compared to other drugs (aOR:0.87 e 0.95) Hospitalization risk (but not the risk of severe forms) is increased in patients on Vedolizumab monotherapy compared to patients on anti-TNFs (aOR:1.38, aOR:2.92, p = 0.049 e p = 0.055) |
| Brenner et al., 2020 [44] | IBD and COVID-19: 525 (age ≥ 5) | Laboratory diagnosis | Data from SECURE-IBD database | Until May 2020 | Severe forms in 37 patients (ICU, mechanical ventilation, death) Only 3 pediatric patients (10%) hospitalized (none of them in ICU) | To study the clinical course of COVID-19 in IBD patients and to find eventual associations with clinical and demographic characteristics and with immunosuppressant treatment | Factors that have been connected to severe forms: advanced age, comorbidities, use of systemic corticosteroids (aOR:6.9), sulfasalazine (aOR:3.1) Anti-TNFs (43.4% of patients) are not associated with severe forms (aOR:0.9) |
| Allocca et al., 2020 [20] | IBD: 23,879 COVID-19: 97 | Molecular swab in 64 patients Highly suspected (clinic + contact or radiology) 33 patients | Italy, United Kingdom, France, Spain, Portugal, Malta, Kastoria, Attica, Greece, Russia and Israel | 21 February 2020–30 June 2020 | Symptoms in 90% of positives. Pneumonia in 22%. Hospitalization in 24%. 1 death | To study the incidence of COVID-19 and the eventual effects of immunosuppression on the risk of infection | Corticosteroids treatment is associated with an increased risk of hospitalization (OR 7.69, p = 0.015), while the treatment with monoclonal antibodies is associated with a reduced risk of pneumonia and hospitalization (OR 0.15, p = 0.003 e OR 0.31, p = 0.031). |
| Norsa et al., 2020 [21] | IBD: 522, of which 59 < 18 aa Controls with COVID-19: 479 | Molecular swab | Hospital “Papa Giovanni XXIII”, Bergamo (Italy) | 19 February 2020–23 March 2020 | To report the experience of this IBD Italian center during the pandemic | 0 COVID-19 cases among IBD patients (despite the therapy with immunomodulators in 22% of them and biologics in 16%) | |
| Hormati et al., 2020 [45] | IBD (or AIH): 200 (treated with Azathioprine, anti-TNFs and prednisone) COVID-19: 11 (8 with IBD) | Molecular swab | Iran | Since the beginning of pandemic (publication date): 28 May 2020) | Only mild symptoms that disappears more rapidly compared to general population, as like the RX alterations. 0 deaths | To study the effects of immunosuppressive drugs in SARS-CoV-2 infection | Percentage of positives lower than the general population not receiving immunosuppressors |
| Lukin et al., 2020 [24] | IBD and COVID-19: 80 (considered positive with both a molecular test or highly suspect clinic) COVID-19 non IBD: 160 | Molecular swab or highly suspect clinic | New York | 01 February 2020–30 April 2020 | To notice eventual differences between COVID-19 patients with and without IBD in terms of clinical outcomes and to study risk factors for COVID-19 in IBD patients | Use of corticosteroids is higher among patients with COVID-19 than in patients with IBD but not infected No differences regarding biologics and immunomodulators The proportion of patients receiving Vedolizumab or not receiving biological therapy was numerically higher in patients requiring hospitalization (no biologics: 29%, Vedolizumab: 30%, Ustekinumab 8%, anti TNFs (6%) p = 0.197) compared to others | |
| Khan et al., 2020 [46] | IBD: 37,857 COVID-19: 36 | Laboratory diagnosis | USA, VAHS database | 01 January 2020–15 May 2020 | 2391 patients on thiopurine therapy: 2 COVID-19 4920 patients on anti-TNF therapy: 3 COVID-19 | To study the impact of anti-TNF and thiopurines on SARS-CoV-2 infection | Thiopurines are not connected to an increased risk of infection (OR:0.962, p = 0.9577) Anti-TNFs are not associated with an increased risk of infection (OR:0.581, p = 0.3774) |
| Singh et al., 2020 [27] | IBD: 196,403 COVID-19: 232 (1901 tests) Controls: 19,776 COVID-19 | Laboratory diagnosis or diagnostic code for COVID-19 after hospitalization | USA | 26 January 2020–26 May 2020 | Risk of severe infection (hospitalization and/or death within 30 days after diagnosis) similar between IBD patients and controls | To study clinical presentation and outcomes of COVID-19 among IBD patients and compare them to a large control group | Higher risk of severe forms among patients under corticosteroids treatment for at least 3 months (30.98%) compared to other patients (19.25%) RR:1.6, p = 0.4 (univariate analysis) |
| Allocca, Guidelli et al., 2020 [47] | COVID-19: 41 patients with immune mediated inflammatory diseases (IMID). Among them 12 UC and 9 CD | Molecular swab or highly suspect clinic or chest CT | Italy | N.R. | All patients developed symptoms due to infection: 16 pneumonia (40%), 14 hospitalizations (34%), 0 ICU admissions and 1 death | To report the experience of the Humanitas center (Milan) among patients with IMID | Corticosteroids therapy increases risk of oxygen therapy needing (p = 0.007) Biologics are not associated with hospitalization risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrias, A.; Cortes, G.M.; Bardanzellu, F.; Melis, A.; Fanos, V.; Marcialis, M.A. Risk, Course, and Effect of SARS-CoV-2 Infection in Children and Adults with Chronic Inflammatory Bowel Diseases. Children 2021, 8, 753. https://doi.org/10.3390/children8090753
Corrias A, Cortes GM, Bardanzellu F, Melis A, Fanos V, Marcialis MA. Risk, Course, and Effect of SARS-CoV-2 Infection in Children and Adults with Chronic Inflammatory Bowel Diseases. Children. 2021; 8(9):753. https://doi.org/10.3390/children8090753
Chicago/Turabian StyleCorrias, Angelica, Gian Mario Cortes, Flaminia Bardanzellu, Alice Melis, Vassilios Fanos, and Maria Antonietta Marcialis. 2021. "Risk, Course, and Effect of SARS-CoV-2 Infection in Children and Adults with Chronic Inflammatory Bowel Diseases" Children 8, no. 9: 753. https://doi.org/10.3390/children8090753
APA StyleCorrias, A., Cortes, G. M., Bardanzellu, F., Melis, A., Fanos, V., & Marcialis, M. A. (2021). Risk, Course, and Effect of SARS-CoV-2 Infection in Children and Adults with Chronic Inflammatory Bowel Diseases. Children, 8(9), 753. https://doi.org/10.3390/children8090753







