Brain Sparing Effect on Neurodevelopment in Children with Intrauterine Growth Restriction: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion Criteria
2.3. Selection Process
2.4. Data Collection Process
2.5. Quality Assessment
3. Results
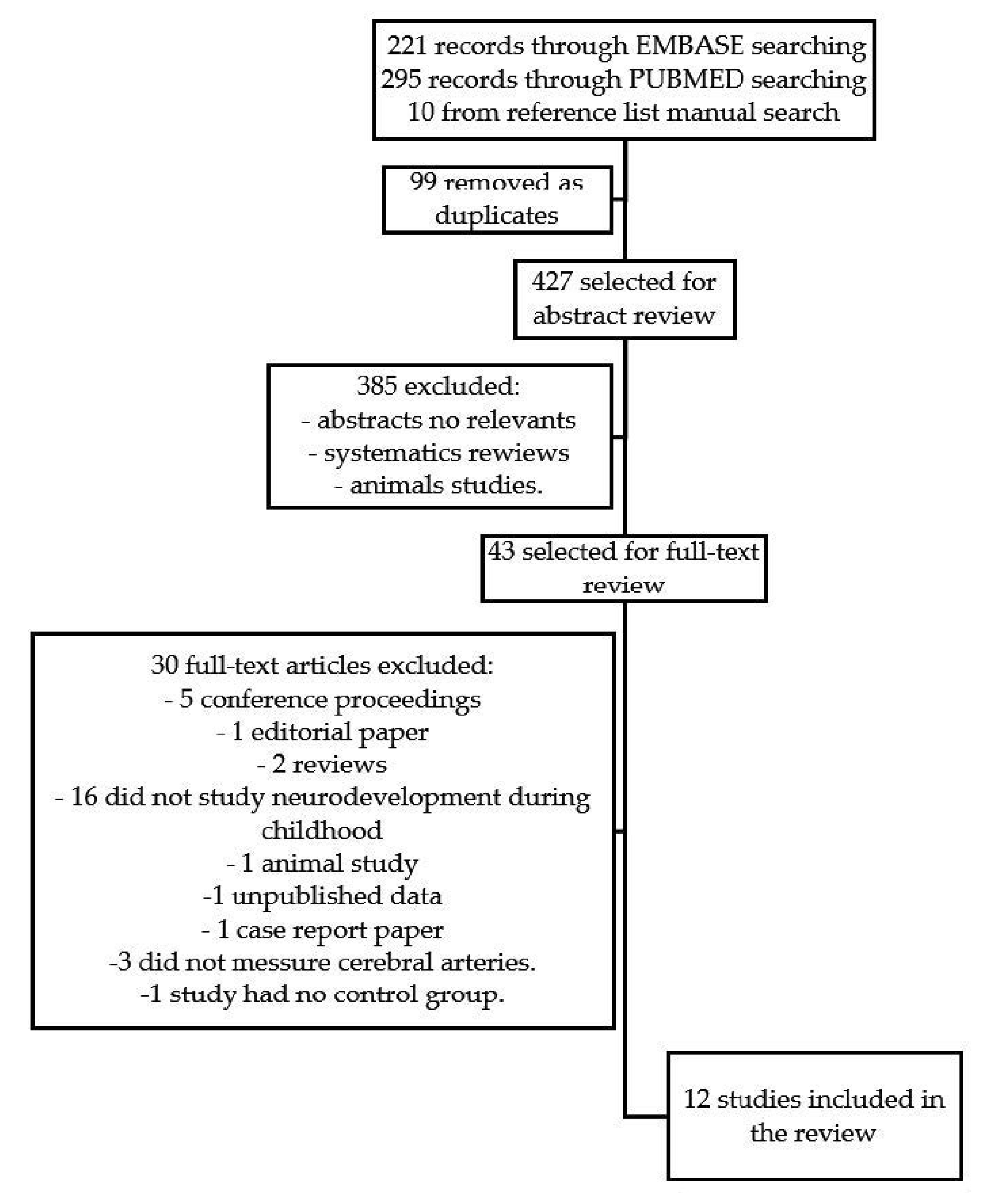
3.1. Overall Search Results
3.2. Descriptions of the Tools Used by the Studies
- -
- Neonatal Behavioral Assessment Scale (NBAS) [30,36]. This test analyzes how newborns control their states and how the transition from one to another progresses. This test evaluates newborns’ habituation to the environment, motor system, social interaction (visual and auditory), organization and regulation state, autonomic system, and attention capacity. The scale evaluates how infants manage these vital tasks that are important for growth and development.
- -
- Bayley Scales of Infants and Toddlers [27,28,38]. This test evaluates cognition, language, and motor development. One study [38] used the second edition, which evaluates the Mental Developmental Index (MDI). The MDI estimates cognitive skills such as memory, problem-solving, vocalization, language, and social skills.
- -
- -
- -
- Revision of the Amsterdam Children’s Intelligence (RAKIT) Test [33]. This test assesses the intelligence quotient. The disk test is a subtest that evaluates the integration of visual recognition and fine motor coordination.
- -
- -
3.3. Summary of the Findings of the Studies
| Study | N | Study Group | Control Group | Definition of Brain Sparing | Exclusion Criteria | Age Assessment | Neurodevelpment Assessment | Results |
|---|---|---|---|---|---|---|---|---|
| Monteith et al. (2019) [27] Ireland Longitudinal multicentre prospective cohort Secondary analysis from PORTO study | 378 | - FGR with normal CPR N = 136 - FGR with abnormal CPR N = 41 GA between 24 + 0 and 36 + 6 | SGA N = 201 | CPR < 1 | - Birthweight < 500 g - major structural and/or chromosomal abnormalities | 3-year-old | Ages and Stages Questionnaire (ASQ) Bayley Scales of Infant and Toddler Development (3rd edition) | - FGR with abnormal CPR value had significantly lower mean scores in ASQ scales and Bayley scales compared with SGA (p < 0.05) - FGR with normal CPR also had lower mean scores compared with SGA, but only significantly in gross and fine motor development (p < 0.05) - When comparing both groups of FGR, only motor score in Bayley Scales reached significance (p = 0.002) |
| Stampalija et al. (2017) [28] United Kingdom Longitudinal multicentre prospective cohort Secondary analysis from TRUFFLE study | 342 | Abnormal neurodevelopment outcome in FGR N = 310 GA between 26 + 0 and 31 + 6 | Normal neurodevelopment outcome in FGR. N = 32 | - delivery planned - major structural abnormality - fetal karyotype abnormality - <18 year-old | 2-year-old | Bayley Scales of Infant and Toddler Development (3rd edition) Gross Motor Function Classification System (GMFCS) Neurodevelopmental impairment was defined as:Bayley score < 85 or cognitive delay >3 months Cerebral Palsy (GMFCS > 1) Hearing loss (hearing aids) Severe visual loss | - MCA PI and UCR Z score at study inclusion were associated with 2-year infant survival without neurodevelopmental impairment (p < 0.05) - CPR Z score at study inclusion, MCA I, UCR, and CPR Z score before birth, and the change of these parameters with the time were not associated with 2-year outcome - Gestational age and birth weight at delivery remained the most important factor in determining 2-year infant outcome without neurodevelopmental impairment (p < 0.05) | |
| Beukers et al. (2017) [29] The NetherlandsLongitudinal prospective cohort | 128 | FGR (NO Doppler criteria in FGR definition) N = 96 GA between 24 + 0 and 34 + 6 at admission | Children with gestational age ≥ 37 weeks and birth weight ≥ 2500 g at delivery. N = 32 | UCR > 0.72 | - Several fetal distress. - Lethal fetal congenital abnormalities. | 12-year-old | Weschler Intelligence Scale. Amsterdam Neuropsychological Task: Visual memory working, set shifting and focusing attention Tower London Test: Planning. Behavior Rating Inventory of Executive Function (parent report). Strengths and Weaknesses of Attention Deficit Hyperactivity Disorder Symptoms and Normal Behavior Scale (parent report) Child Behavior Checklist (parent report) | - 96% of cases had raised UCR, indicating brain sparing - Mean IQ was similar for FGR and control group (101.1 ± 16.7 vs. 105.9 ± 10.0 p = 0.12) - Parents of FGR reported significantly more social problems (p < 0.001) and FGR tend to have more attention problems (p = 0.07) - All executive functions, attention test performances, and parents’ reports did not differ between groups - For attention problems scores there were no significant difference between groups - UCR was not associated with any of the outcome variables - BWR and low SES were both associated with lower IQ |
| Figueras et al. (2011) [30] Spain Longitudinal prospective cohort | 126 | FGR - Normal MCA PI N = 29 - Abnormal MCA PI N = 33 Gestational age at delivery < 34 weeks N = 62 | Singleton AGA N = 64 | MCA PI < 5th percentile | - congenital malformations - congenital infection - chromosomal abnormalities - placental histological chorioamnionitis - infant death before 40 weeks - neurological complication | 40 weeks corrected age | Neonatal Behavioral Assessment Scale (NBAS) | - Neurobehavioral score did not differ between FGR with normal MCA and the control group - Scores were significantly lower in FGR and abnormal MCA, specifically in habituation, motor, social-interactive, and attention areas (p < 0.05) |
| Richter et al. (2020) [31] The NetherlandsLongitudinal prospective cohort | 25 | FGR (FAC or EFW < 10th percentile or decreased fetal growth more than 30 percentiles) with FBS N = 11 | FGR (FAC or EFW < 10th percentile or decreased fetal growth more than 30 percentiles) without FBS N = 14 | CPR < 1 | - structural or chromosomal abnormalities - multiple pregnancy - intrauterine infection | 4-year-old | Weschler Preschool and Primary Scale Child Behavior Checklist (parent report).Behavior Rating Inventory of Executive Function Preschool Version (parent report) | - FBS was not associated with IQ - FBS was significantly related with better total behavior and better externalizing behavior (p < 0.05) - FBS tended to have better inhibitory self-control (p < 0.1) - Adjusted for gestational age, which is positively correlated with T-score for total behavior, total executive function, and Emergent Metacognition Index (p < 0.05) |
| Scherjon et al. (1998) [32] The Netherlands Longitudinal prospective cohort | 96 | Fetuses with UCR raised N = 34 Gestational age between 26 and 33 weeks at delivery | Fetuses with normal UCR N = 62 | UCR > 0.72 | - structural or chromosomal abnormalities | 3-year-old | Ultrasound findings: intraventricular bleeding or echo densities Hempel neurodevelopmental outcome: motor system, hearing, vision, and eye movements Behavioral Aspects (parent report) | - Lower head circumference was found in infants with raised UCR (p < 0.02) - All infants with abnormal neurological outcomes and all but one middle neurological outcome were found in the normal UCR group (p = 0.23) - Gestational age was lower in abnormal neurological outcomes (p = 0.01) - In the normal UCR group, the association with ultrasound findings and Hempel outcomes was highly significant. No association in raised UCR group (p < 0.0001) - No significant differences in behavioral or language development between groups |
| Scherjon et al. (2000) [33] The Netherlands Longitudinal prospective cohort | 73 | Fetuses with UCR raised N = 28 Gestational age between 26 and 33 weeks at delivery | Fetuses with normal UCR N = 45 | UCR > 0.72 | - structural or chromosomal abnormalities | 5-year-old | Visual Evoked Potentials (VEP) RAKIT Test: intelligent quotient Disk Test: integration of visual recognition and fine motor coordination | - Infants with normal UCR were shortening VEP latencies between 6 to 12 months (decreased 20%) (p = 0.0001). UCR raised group had short VEP latencies at 6 months but remained unchanged at 12 months (decreased 5%) (p = 0.10) - Infants with raised UCR showed a 9-point lower IQ at 5 years compared with normal group (p < 0.02) - 54% of infants with raised UCR were IQ < 85 compared with 20% in normal group (p = 0.003) - There was a positive statistical association between a greater difference in VEP latencies at 6–12 months and higher IQ |
| Van den Broek et al. (2010) [34] The Netherlands Longitudinal prospective cohort | 89 | Fetuses with UCR raised N = 31 Gestational age between 26 and 33 weeks at delivery | Fetuses with normal UCR N = 58 | UCR > 0.72 | - structural or chromosomal abnormalities | 11-year-old | Child Behavior Checklist (parent report) Teacher’s Report Form (teacher report): based in Child Behavior Checklist | - No significant differences in the incidence of behavioral problems between groups - They found a higher incidence of behavioral problems in the cohort compared with general population - No significant difference in not adequate school performance between groups - Birth weight was more important to predictive behavioral problems (p = 0.003) |
| Bellido-Gonzalez et al. (2017) [35] Spain Longitudinal retrospective cohort | 120 | FGR (birth weight < 10th percentile and abnormal MCA PI < 5th percentile): FGR-A: abnormal CPR (<5th percentile) and abnormal UA PI (>95th percentile) N = 32 FGR-B: normal CPR and UA N = 27 Gestational age > 37 weeks | Term AGA | MCA PI < 5th percentile | - parental drugs consumption - multiple gestation - congenital malformation - chromosomopaties - low Apgar score - vision/hearing impairment - cerebral palsy - non-native speaker of Spanish | 6–8-year-old | Wechsler Intelligence Scale for Children IV: III Woodcock–Muñoz Battery: Reading, Written Language, Mathematics Home Observation for Measurement of the Environment (HOME) methods: interview to measure the quality of stimulation and support | WISC-IV: FGR-A presented lower scores than AGA children for all measurements (p < 0.05) Larger differences were observed in working memory FGR-B presented lower scores than AGA only for verbal comprehension and working memory (p < 0.05) Academic achievement: FGR-A presented lower scores than AGA children in reading, written language, and mathematics (p < 0.05) FGR-B presented lower scores than AGA children only in mathematics (p < 0.05) |
| Cruz-Martinez et al. (2009) [36] Spain Longitudinal prospective cohort | 120 | SGA N = 60 Gestational age > 37 weeks | Term AGA N = 60 | MCA PI < 5th percentile Or FMBV > 95th percentile | - Congenital malformations or chromosomopaties - UA PI>95th percentile | 40 weeks corrected age | Neonatal Behavioral Assessment Scale (NBAS) | - SGA showed higher mean frontal FMBV values than AGA. The proportion of FMBV > 95th percentile was 35% in SGA and 5% in AGA (p < 0.001) - The proportion of MCA PI < 5th percentile was 15% in SGA and 1.7% in AGA (p < 0.01) - All neurobehavioral areas had lower scores in SGA group (p < 0.05) - SGA with abnormal FMBV showed lower scores in social-interactive, attention, and organization states. SGA with normal FMBV showed similar scores to AGA (p < 0.05) SGA with abnormal MCA showed lower scores in motor area (p < 0.05) |
| Eixarch et al. (2008) [37] Spain Longitudinal prospective cohort | 222 | SGA Normal MCA PI N = 100 Abnormal MCA PI N = 25 Gestational age > 37 weeks | Term AGA N = 97 | MCA PI < 5th percentile | - Congenital malformations or chromosomopaties - UA PI > 95th percentile | 24 months corrected age | Age and Stage Questionnaire (ASQ) (parent report) | - 24.7% of control group showed abnormal ASQ scores in more than one area, compared with 31% in the non-redistributed SGA and 52% in redistributed SGA groups - Differences between AGA and SGA non-redistributed group was non-significant - Differences between AGA and SGA redistributed group was significant. They showed lower scores in communication and personal-social areas (p < 0.05) - Compared to both SGA groups, redistributed SGA had a lower score in communication and problem-solving (p < 0.05) |
| Leppäpen et al. (2010) [38] Finland Longitudinal multicentre prospective cohort Secondary analysis from PIPARI study | 83 | Preterm delivery < 32 weeks or estimated birth weight < 1500 g In the secondary analysis the antenatal Doppler flow and the relationship with neurodevelopment was studied It was compared: Infants with abnormal UCR N = 16 Infants with normal UCR N = 54 | UCR > 95th percentile | - congenital anomalies or a diagnosed syndrome - non-native speaker of Finnish and ⁄ or Swedish | 2-year-old | Bayley Scales of Infant Development II Hammersmith Infant Neurological Examination (HINE): suboptimal < 74 Cranial nerve function, posture, movements, tone and reflexes, motor functions, behavior | - Abnormal UAPI, UCR, increased Dao PI and DAo/MCA ratio were associated with adverse cognitive performance. When the effect of confounding factor was controlled, only DAo and UCR remained statistically significant (p < 0.05) - When infants with normal and abnormal UCR were compared, no differences in HINE scores were found. The infants with abnormal UCR showed a lower score in MDI compared with normal UCR infants |
3.4. Quality Assessment Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lees, C.; Marlow, N.; Arabin, B.; Bilardo, C.M.; Brezinka, C.; Derks, J.B.; Duvekot, J.; Frusca, T.; Diemert, A.; Ferrazzi, E.; et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: Cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet. Gynecol. 2013, 42, 400–408. [Google Scholar] [CrossRef]
- Simeoni, U.; Armengaud, J.-B.; Siddeek, B.; Tolsa, J.-F. Perinatal Origins of Adult Disease. Neonatology 2018, 113, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Geva, R.; Eshel, R.; Leitner, Y.; Valevski, A.F.; Harel, S. Neuropsychological Outcome of Children With Intrauterine Growth Restriction: A 9-Year Prospective Study. Pediatrics 2006, 118, 91–100. [Google Scholar] [CrossRef]
- Geva, R.; Eshel, R.; Leitner, Y.; Fattal-Valevski, A.; Harel, S. Memory functions of children born with asymmetric intrauterine growth restriction. Brain Res. 2006, 1117, 186–194. [Google Scholar] [CrossRef]
- Morsing, E.; Asard, M.; Ley, D.; Stjernqvist, K.; Marsal, K. Cognitive Function After Intrauterine Growth Restriction and Very Preterm Birth. Pediatrics 2011, 127, e874–e882. [Google Scholar] [CrossRef] [PubMed]
- Guellec, I.; Lapillonne, A.; Renolleau, S.; Charlaluk, M.L.; Roze, J.C.; Marret, S.; Vieux, R.; Monique, K.; Ancel, P.I.; EPIPAGE Study Group. Neurologic Outcomes at School Age in Very Preterm Infants Born With Severe or Mild Growth Restriction. Pediatrics 2011, 127, 2010–2442. [Google Scholar] [CrossRef]
- Korzeniewski, S.J.; Allred, E.N.; Joseph, R.M.; Heeren, T.; Kuban, K.C.K.; O’Shea, T.M.; Leviton, A.; ELGAN Study Investigators. Neurodevelopment at Age 10 Years of Children Born ˂ 28 Weeks with Fetal Growth Restriction. Pediatrics 2017, 140, e20170697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz, I.; Gale, R.; Laor, A.; Danon, Y.; Stevenson, D.; Seidman, D. The cognitive outcome of full-term small for gestational age infants at late adolescence. Obstet. Gynecol. 1995, 85, 452–456. [Google Scholar]
- Paz, I.; Laor, A.; Gale, R.; Harlap, S.; Stevenson, D.K.; Seidman, D.S. Term infants with fetal growth restriction are not at increased risk for low intelligence scores at age 17 years. J. Pediatr. 2001, 138, 87–91. [Google Scholar] [CrossRef]
- Sommerfelt, K. Cognitive development of term small for gestational age children at five years of age. Arch. Dis. Child. 2000, 83, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulseng, S.; Jennekens-Schinkel, A.; Naess, P.; Romundstad, P.; Indredavik, M.; Vik, T.; Brubakk, A.-M. Very-low-birthweight and term small-for-gestational-age adolescents: Attention revisited. Acta Paediatr. 2007, 95, 224–230. [Google Scholar] [CrossRef]
- Theodore, R.F.; Thompson, J.M.D.; Waldie, K.E.; Becroft, D.M.O.; Robinson, E.; Wild, C.J.; Clark, P.; Mitchell, E.A. Determinants of cognitive ability at 7 years: A longitudinal case–control study of children born small-for-gestational age at term. Eur. J. Pediatr. 2009, 168, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Scherjon, S.A.; Smolders-DeHaas, H.; Kok, J.H.; Zondervan, H.A. The “brain-sparing” effect: Antenatal cerebral Doppler findings in relation to neurologic outcome in very preterm infants. Am. J. Obstet. Gynecol. 1993, 169, 169–175. [Google Scholar] [CrossRef]
- Maunu, J.; Ekholm, E.; Parkkola, R.; Palo, P.; Rikalainen, H.; Lapinleimu, H.; Haataja, L.; Lehtonen, L.; PIPARI Study Group. Antenatal Doppler Measurements and Early Brain Injury in Very Low Birth Weight Infants. J. Pediatr. 2007, 150, 51–56.e1. [Google Scholar] [CrossRef]
- Mallard, C.; Loeliger, M.; Copolov, D.; Rees, S. Reduced number of neurons in the hippocampus and the cerebellum in the postnatal guinea-pig following intrauterine growth-restriction. Neuroscience 2000, 100, 327–333. [Google Scholar] [CrossRef]
- Dieni, S.; Rees, S. Dendritic morphology is altered in hippocampal neurons following prenatal compromise. J. Neurobiol. 2003, 55, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Tolcos, M.; Bateman, E.; O’Dowd, R.; Markwick, R.; Vrijsen, K.; Rehn, A.; Rees, S. Intrauterine growth restriction affects the maturation of myelin. Exp. Neurol. 2011, 232, 53–65. [Google Scholar] [CrossRef]
- Eixarch, E.; Batalle, D.; Illa, M.; Muñoz-Moreno, E.; Arbat-Plana, A.; Amat-Roldan, I.; Figueras, F.; Gratacos, E. Neonatal Neurobehavior and Diffusion MRI Changes in Brain Reorganization Due to Intrauterine Growth Restriction in a Rabbit Model. PLoS ONE 2012, 7, e31497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodygensky, G.A.; Seghier, M.L.; Warfield, S.K.; Tolsa, C.B.; Sizonenko, S.; Lazeyras, F.; Hüppi, P.S. Intrauterine Growth Restriction Affects the Preterm Infant’s Hippocampus. Pediatr. Res. 2008, 63, 438–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolsa, C.B.; Zimine, S.; Warfield, S.K.; Freschi, M.; Rossignol, A.S.; Lazeyras, F.; Hanquinet, S.; Pfizenmaier, M.; Huppi, P.S. Early Alteration of Structural and Functional Brain Development in Premature Infants Born with Intrauterine Growth Restriction. Pediatr. Res. 2004, 56, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Bruno, C.J.; Bengani, S.; Gomes, W.A.; Brewer, M.; Vega, M.; Xie, X.; Kim, M.; Fuloria, M. MRI Differences Associated with Intrauterine Growth Restriction in Preterm Infants. Neonatology 2017, 111, 317–323. [Google Scholar] [CrossRef]
- Eixarch, E.; Muñoz-Moreno, E.; Bargallo, N.; Batalle, D.; Gratacos, E. Motor and cortico-striatal-thalamic connectivity alterations in intrauterine growth restriction. Am. J. Obstet. Gynecol. 2016, 214, e1–e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilla, N.; Junqué, C.; Figueras, F.; Sanz-Cortes, M.; Bargalló, N.; Arranz, A.; Donaire, A.; Figueras, J.; Gratacos, E. Differential vulnerability of gray matter and white matter to intrauterine growth restriction in preterm infants at 12 months corrected age. Brain Res. 2014, 1545, 1–11. [Google Scholar] [CrossRef]
- Dubois, J.; Benders, M.; Borradori-Tolsa, C.; Cachia, A.; Lazeyras, F.; Ha-Vinh Leuchter, R.; Sizonenko, S.V.; Warfield, S.K.; Mangin, J.F.; Hüppi, P.S. Primary cortical folding in the human newborn: An early marker of later functional development. Brain 2008, 131, 2028–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Mc Master University. Effective Health Practice Project: Quality Assessment tool for Quantitative Studies. 2010. Available online: https://merst.ca/ephpp-tools/ (accessed on 4 August 2021).
- Monteith, C.; Flood, K.; Pinnamaneni, R.; Levine, T.A.; Alderdice, F.A.; Unterscheider, J.; McAuliffe, F.M.; Dicker, P.; Tully, E.C.; Malone, F.D.; et al. An abnormal cerebroplacental ratio (CPR) is predictive of early childhood delayed neurodevelopment in the setting of fetal growth restriction. Am. J. Obstet. Gynecol. 2019, 221, 273.e1–273.e9. [Google Scholar] [CrossRef] [PubMed]
- Stampalija, T.; Arabin, B.; Wolf, H.; Bilardo, C.M.; Lees, C.; Brezinka, C. Is middle cerebral artery Doppler related to neonatal and 2-year infant outcome in early fetal growth restriction? Am. J. Obstet. Gynecol. 2017, 216, 521.e1–521.e13. [Google Scholar] [CrossRef] [PubMed]
- Beukers, F.; Aarnoudse-Moens, C.S.H.; van Weissenbruch, M.M.; Ganzevoort, W.; van Goudoever, J.B.; van Wassenaer-Leemhuis, A.G. Fetal Growth Restriction with Brain Sparing: Neurocognitive and Behavioral Outcomes at 12 Years of Age. J. Pediatr. 2017, 188, 103–109.e2. [Google Scholar] [CrossRef]
- Figueras, F.; Cruz-Martinez, R.; Sanz-Cortes, M.; Arranz, A.; Illa, M.; Botet, F.; Costas-Moragas, C.; Gratacos, E. Neurobehavioral outcomes in preterm, growth-restricted infants with and without prenatal advanced signs of brain-sparing. Ultrasound Obstet. Gynecol. 2011, 38, 288–294. [Google Scholar] [CrossRef]
- Richter, A.E.; Salavati, S.; Kooi, E.M.W.; Heijer AE den Foreman, A.B.; Schoots, M.H.; Bilardo, C.M.; Scherjon, S.A.; Tanis, J.C.; Bos, A.F. Fetal Brain-Sparing, Postnatal Cerebral Oxygenation, and Neurodevelopment at 4 Years of Age Following Fetal Growth Restriction. Front. Pediatr. 2020, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Scherjon, S.A.; Oosting, H.; Smolders-DeHaas, H.; Zondervan, H.A.; Kok, J.H. Neurodevelopmental outcome at three years of age after fetal ‘brain-sparing’. Early Hum. Dev. 1998, 52, 67–79. [Google Scholar] [CrossRef]
- Scherjon, S.; Briet, J.; Oosting, H.; Kok, J. The Discrepancy between Maturation of Visual-Evoked Potentials and Cognitive Outcome at Five Years in Very Preterm Infants with and Without Hemodynamic Signs of Fetal Brain-Sparing. Pediatrics 2000, 105, 385–391. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, A.J.M.; Kok, J.H.; Houtzager, B.A.; Scherjon, S.A. Behavioural problems at the age of eleven years in preterm-born children with or without fetal brain sparing: A prospective cohort study. Early Hum. Dev. 2010, 86, 379–384. [Google Scholar] [CrossRef]
- Bellido-González, M.; Díaz-López, M.Á.; López-Criado, S.; Maldonado-Lozano, J. Cognitive Functioning and Academic Achievement in Children Aged 6–8 Years, Born at Term after Intrauterine Growth Restriction and Fetal Cerebral Redistribution. J. Pediatr. Psychol. 2017, 42, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Martinez, R.; Figueras, F.; Oros, D.; Padilla, N.; Meler, E.; Hernandez-Andrade, E.; Gratacos, E. Cerebral blood perfusion and neurobehavioral performance in full-term small-for-gestational-age fetuses. Am. J. Obstet. Gynecol. 2009, 201, 474.e1–474.e7. [Google Scholar] [CrossRef]
- Eixarch, E.; Meler, E.; Iraola, A.; Illa, M.; Crispi, F.; Hernandez-Andrade, E.; Gratacos, E.; Figueras, F. Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet. Gynecol. 2008, 32, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, M.; Ekholm, E.; Palo, P.; Maunu, J.; Munck, P.; Parkkola, R.; Matomäki, J.; Lapinleimu, H.; Haataja, L.; Lehtonen, L.; et al. Abnormal antenatal Doppler velocimetry and cognitive outcome in very-low-birth-weight infants at 2 years of age. Ultrasound Obstet. Gynecol. 2010, 36, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Visser, G.H.A.; Bilardo, C.M.; Derks, J.B.; Ferrazzi, E.; Fratelli, N.; Frusca, T.; Ganzevoort, W.; Lees, C.C.; Napolitano, R.; Todros, T.; et al. Fetal monitoring indications for delivery and 2-year outcome in 310 infants with fetal growth restriction delivered before 32 weeks’ gestation in the TRUFFLE study. Ultrasound Obstet. Gynecol. 2017, 50, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Ganzevoort, W.; Rep, A.; Bonsel, G.J.; Fetter, W.P.F.; Sonderen, L.; Vries, J.I.P.; Wolf, H.; PETRA investigators. A randomised controlled trial comparing two temporising management strategies, one with and one without plasma volume expansion, for severe and early onset pre-eclampsia. BJOG 2005, 112, 1358–1368. [Google Scholar] [CrossRef] [Green Version]
- Unterscheider, J.; Daly, S.; Geary, M.P.; Kennelly, M.M.; McAuliffe, F.M.; O’Donoghue, K.; Hunter, A.; Morrison, J.J.; Burke, G.; Dicker, P.; et al. Optimizing the definition of intrauterine growth restriction: The multicenter prospective PORTO Study. Am. J. Obstet. Gynecol. 2013, 208, 290.e1–290.e6. [Google Scholar] [CrossRef]
- Samuelsen, G.B.; Pakkenberg, B.; Bogdanović, N.; Gundersen, H.J.G.; Larsen, J.F.; Græm, N.; Laursen, H. Severe cell reduction in the future brain cortex in human growth–restricted fetuses and infants. Am. J. Obstet. Gynecol. 2007, 197, 56.e1–56.e7. [Google Scholar] [CrossRef]
- Padilla, N.; Perapoch, J.; Carrascosa, A.; Acosta-Rojas, R.; Botet, F.; Gratacós, E. Twelve-month neurodevelopmental outcome in preterm infants with and without intrauterine growth restriction. Acta Paediatr. 2010, 99, 1498–1503. [Google Scholar] [CrossRef]
- Egaña-Ugrinovic, G.; Sanz-Cortes, M.; Figueras, F.; Bargalló, N.; Gratacós, E. Differences in cortical development assessed by fetal MRI in late-onset intrauterine growth restriction. Am. J. Obstet. Gynecol. 2013, 209, 126.e1–126.e8. [Google Scholar] [CrossRef] [PubMed]
- Egaña-Ugrinovic, G.; Sanz-Cortés, M.; Couve-Pérez, C.; Figueras, F.; Gratacós, E. Corpus callosum differences assessed by fetal MRI in late-onset intrauterine growth restriction and its association with neurobehavior. Prenat. Diagn. 2014, 34, 843–849. [Google Scholar] [CrossRef]
- Sanz-Cortes, M.; Egaña-Ugrinovic, G.; Simoes, R.V.; Vazquez, L.; Bargallo, N.; Gratacos, E. Association of brain metabolism with sulcation and corpus callosum development assessed by MRI in late-onset small fetuses. Am. J. Obstet. Gynecol. 2015, 212, 804.e1–804.e8. [Google Scholar] [CrossRef]
- Egaña-Ugrinovic, G.; Savchev, S.; Bazán-Arcos, C.; Puerto, B.; Gratacós, E.; Sanz-Cortés, M. Neurosonographic Assessment of the Corpus Callosum as Imaging Biomarker of Abnormal Neurodevelopment in Late-Onset Fetal Growth Restriction. Fetal Diagn. Ther. 2015, 37, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Torrance, H.L.; Bloemen, M.C.T.; Mulder, E.J.H.; Nikkels, P.G.J.; Derks, J.B.; de Vries, L.S.; Visser, G.H.A. Predictors of outcome at 2 years of age after early intrauterine growth restriction. Ultrasound Obstet. Gynecol. 2010, 36, 171–177. [Google Scholar] [CrossRef]
- Baschat, A.A.; Viscardi, R.M.; Hussey-Gardner, B.; Hashmi, N.; Harman, C. Infant neurodevelopment following fetal growth restriction: Relationship with antepartum surveillance parameters. Ultrasound Obstet. Gynecol. 2009, 33, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Dubiel, M.; Gunnarsson, G.Ö.; Gudmundsson, S. Blood redistribution in the fetal brain during chronic hypoxia. Ultrasound Obstet. Gynecol. 2002, 20, 117–121. [Google Scholar] [CrossRef]
- Figueroa-Diesel, H.; Hernandez-Andrade, E.; Acosta-Rojas, R.; Cabero, L.; Gratacos, E. Doppler changes in the main fetal brain arteries at different stages of hemodynamic adaptation in severe intrauterine growth restriction. Ultrasound Obstet. Gynecol. 2007, 30, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Andrade, E.; Figueroa-Diesel, H.; Jansson, T.; Rangel-Nava, H.; Gratacos, E. Changes in regional fetal cerebral blood flow perfusion in relation to hemodynamic deterioration in severely growth-restricted fetuses. Ultrasound Obstet. Gynecol. 2008, 32, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M. Frontal lobe and cognitive development. J. Neurocytol. 2002, 31, 373–385. [Google Scholar] [CrossRef] [PubMed]
| Study | Selection Bias | Study Design | Confounders | Blinding | Data Collection Method | Withdrawals and Dropouts | Global Rating |
|---|---|---|---|---|---|---|---|
| Monteith et al. (2019) [27] | Moderate | Moderate | Strong | Moderate | Strong | Weak | Moderate |
| Stampalija et al. (2017) [28] | Moderate | Moderate | Moderate | Moderate | Strong | Moderate | Strong |
| Beukers et al. (2017) [29] | Moderate | Moderate | Strong | Moderate | Strong | Weak | Moderate |
| Figueras et al. (2011) [30] | Moderate | Moderate | Moderate | Moderate | Strong | Strong | Strong |
| Richter et al. (2020) [31] | Moderate | Moderate | Weak | Moderate | Strong | Weak | Weak |
| Scherjon et al. (1998) [32] | Moderate | Moderate | Weak | Moderate | Strong | Strong | Moderate |
| Scherjon et al. (2000) [33] | Moderate | Moderate | Weak | Moderate | Strong | Weak | Weak |
| Van den Broek et al. (2010) [34] | Moderate | Moderate | Weak | Moderate | Strong | Strong | Moderate |
| Bellido-Gonzalez et al. (2016) [35] | Moderate | Moderate | Strong | Moderate | Strong | Strong | Strong |
| Cruz-Martinez et al. (2009) [36] | Moderate | Moderate | Strong | Moderate | Strong | Strong | Strong |
| Eixarch et al. (2008) [37] | Moderate | Moderate | Strong | Moderate | Strong | Weak | Moderate |
| Leppäpen et al. (2010) [38] | Moderate | Moderate | Strong | Moderate | Strong | Strong | Strong |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez-Marín, M.J.; Marín-Clavijo, J.; Blanco-Elena, J.A.; Jiménez-López, J.; González-Mesa, E. Brain Sparing Effect on Neurodevelopment in Children with Intrauterine Growth Restriction: A Systematic Review. Children 2021, 8, 745. https://doi.org/10.3390/children8090745
Benítez-Marín MJ, Marín-Clavijo J, Blanco-Elena JA, Jiménez-López J, González-Mesa E. Brain Sparing Effect on Neurodevelopment in Children with Intrauterine Growth Restriction: A Systematic Review. Children. 2021; 8(9):745. https://doi.org/10.3390/children8090745
Chicago/Turabian StyleBenítez-Marín, María José, Jesús Marín-Clavijo, Juan Antonio Blanco-Elena, Jesús Jiménez-López, and Ernesto González-Mesa. 2021. "Brain Sparing Effect on Neurodevelopment in Children with Intrauterine Growth Restriction: A Systematic Review" Children 8, no. 9: 745. https://doi.org/10.3390/children8090745
APA StyleBenítez-Marín, M. J., Marín-Clavijo, J., Blanco-Elena, J. A., Jiménez-López, J., & González-Mesa, E. (2021). Brain Sparing Effect on Neurodevelopment in Children with Intrauterine Growth Restriction: A Systematic Review. Children, 8(9), 745. https://doi.org/10.3390/children8090745








