The Role of Elevated Wall Shear Stress in Progression of Pulmonary Vein Stenosis: Evidence from Two Case Studies
Abstract
1. Introduction
2. Materials and Methods
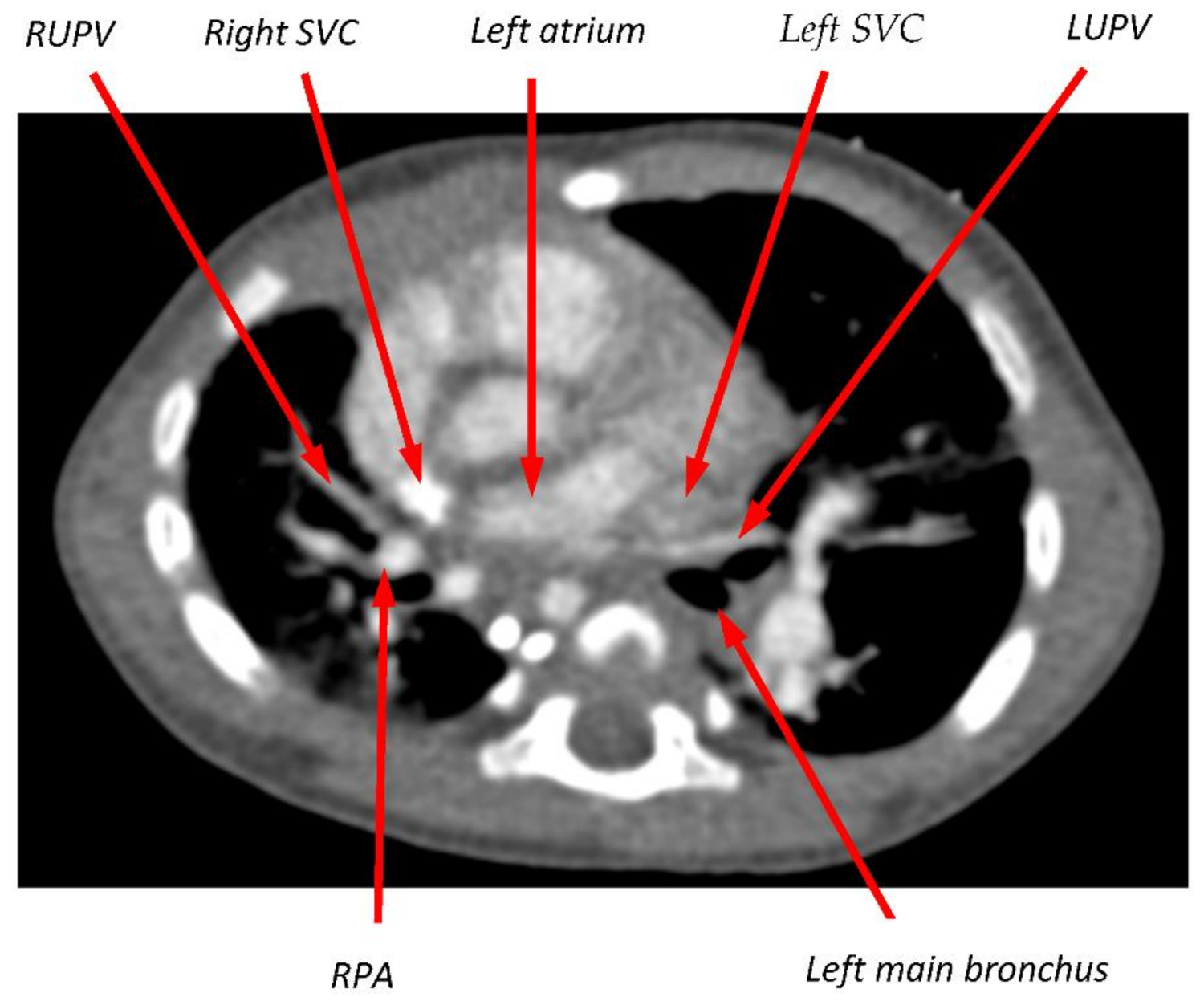
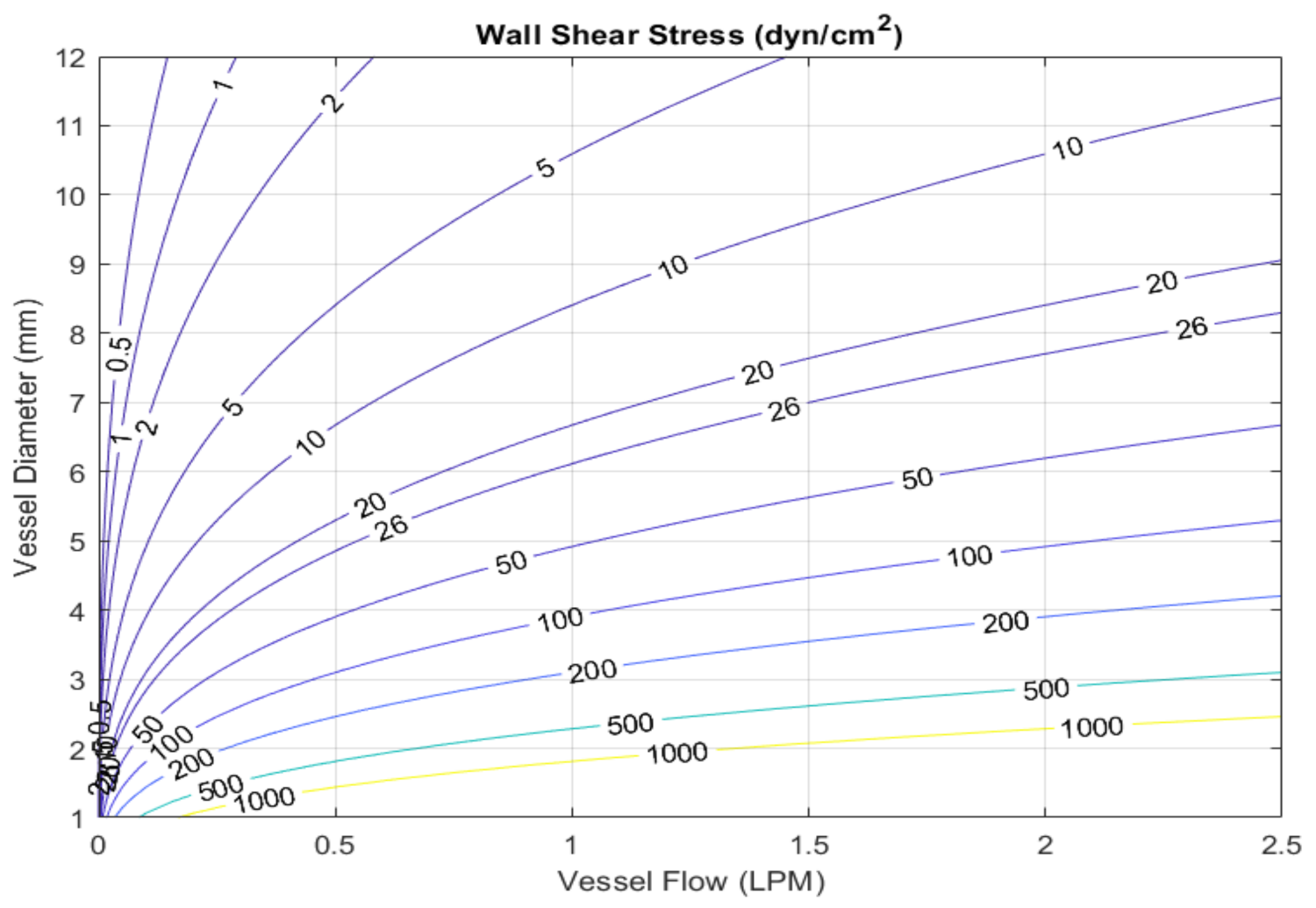
2.1. Calculation of Wall Shear Stress from Clinical Data
2.2. Case 1
2.3. Case 2
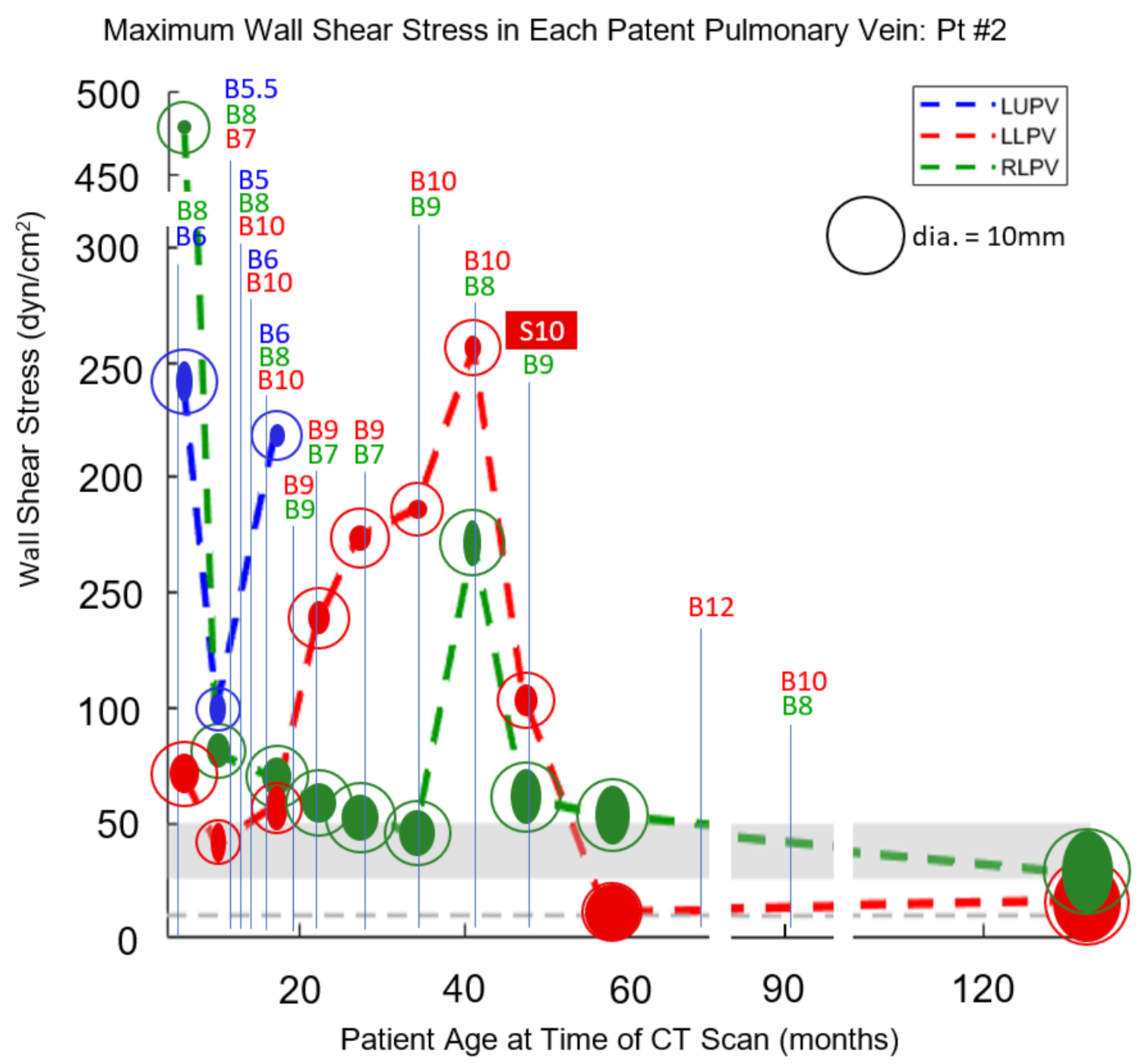
3. Results
3.1. Case 1
3.2. Case 2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Latson, L.A.; Prieto, L.R. Congenital and acquired pulmonary vein stenosis. Circulation 2007, 115, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Sadr, I.M.; Tan, P.E.; Kieran, M.W.; Jenkins, K.J. Mechanism of pulmonary vein stenosis in infants with normally connected veins. Am. J. Cardiol. 2000, 86, 577–579. [Google Scholar] [CrossRef]
- Kovach, A.E.; Magcalas, P.M.; Ireland, C.; McEnany, K.; Oliveira, A.M.; Kieran, M.W.; Baird, C.W.; Jenkins, K.; Vargas, S.O. Paucicellular fibrointimal proliferation characterizes pediatric pulmonary vein stenosis: Clinicopathologic analysis of 213 samples from 97 patients. Am. J. Surg. Pathol. 2017, 41, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.H.; Nealon, E.; Armstrong, A.K.; Cua, C.L.; Mitchell, C.; Krishnan, U.; Vanderlaan, R.D.; Song, M.K.; Viola, N.; Smith, C.V.; et al. Pulmonary vein stenosis in infants: A systematic review, meta-analysis, and meta-regression. J. Pediatr. 2018, 198, 36–45.e3. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Gauvreau, K.; Levy, P.; Callahan, R.; Jenkins, K.J.; Chen, M. Longer exposure to left-to-right shunts is a risk factor for pulmonary vein stenosis in patients with trisomy 21. Children 2021, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Heching, H.J.; Turner, M.; Farkouh-Karoleski, C.; Krishnan, U. Pulmonary vein stenosis and necrotising enterocolitis: Is there a possible link with necrotising enterocolitis? Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F282–F285. [Google Scholar] [CrossRef] [PubMed]
- Drossner, D.M.; Kim, D.W.; Maher, K.O.; Mahle, W.T. Pulmonary vein stenosis: Prematurity and associated conditions. Pediatrics 2008, 122, e656–e661. [Google Scholar] [CrossRef]
- Fick, T.A.; Richards, B.; Backes, C.H.; Ball, M.K. Persistent oxygen requirement beyond prematurity: A case of acquired pulmonary vein stenosis. Case Rep. Pediatr. 2017, 2017, 3106871. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999, 282, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- LaDisa, J.F., Jr.; Olson, L.E.; Molthen, R.C.; Hettrick, D.A.; Pratt, P.F.; Hardel, M.D.; Kersten, J.R.; Warltier, D.C.; Pagel, P.S. Alterations in wall shear stress predict sites of neointimal hyperplasia after stent implantation in rabbit iliac arteries. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2465–H2475. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Sinha, A.K.; Choy, J.S.; Zheng, H.; Sturek, M.; Bigelow, B.; Bhatt, D.L.; Kassab, G.S. Mis-sizing of stent promotes intimal hyperplasia: Impact of endothelial shear and intramural stress. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2254–H2263. [Google Scholar] [CrossRef] [PubMed]
- Binns, R.L.; Ku, D.N.; Stewart, M.T.; Ansley, J.P.; Coyle, K.A. Optimal graft diameter: Effect of wall shear stress on vascular healing. J. Vasc. Surg. 1989, 10, 326–337. [Google Scholar] [CrossRef][Green Version]
- Jenei, C.; Závodszky, G.; Paál, G.; Tar, B.; Kőszegi, Z. High shear stress on the background of clinical restenosis at the site of step-down phenomenon after drug eluting stent implantation. Cor et Vasa 2016, 58, e518–e521. [Google Scholar] [CrossRef]
- Fitts, M.K.; Pike, D.B.; Anderson, K.; Shiu, Y.T. Hemodynamic Shear Stress and Endothelial Dysfunction in Hemodialysis Access. Open Urol. Nephrol. J. 2014, 7 (Suppl. 1 M5), 33–44. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Fu, A.A.; Misra, K.D.; Glockner, J.F.; Mukhopadhyay, D. Wall shear stress measurement using phase contrast magnetic resonance imaging with phase contrast magnetic resonance angiography in arteriovenous polytetrafluoroethylene grafts. Angiology 2009, 60, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Woodrum, D.A.; Homburger, J.; Elkouri, S.; Mandrekar, J.N.; Barocas, V.; Glockner, J.F.; Rajan, D.K.; Mukhopadhyay, D. Assessment of wall shear stress changes in arteries and veins of arteriovenous polytetrafluoroethylene grafts using magnetic resonance imaging. Cardiovasc. Interv. Radiol. 2006, 29, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Fung, Y.C. Biomechanics: Circulation; Springer: New York, NY, USA, 1997. [Google Scholar]
- Goff, D.; Gauvreau, K.; del Nido, P.; Kieran, M.; Roth, S.; Jenkins, K. Host factor vulnerability and development of progressive intraluminal pulmonary vein stenosis after congenital heart surgery. Congenit. Heart Dis. 2009, 4, 86–90. [Google Scholar] [CrossRef]
- Patel, J.D.; Briones, M.; Mandhani, M.; Jones, S.; Suthar, D.; Gray, R.; Pettus, J.; McCracken, C.; Thomas, A.; Petit, C.J. Systemic Sirolimus therapy for infants and children with pulmonary vein stenosis. J. Am. Coll. Cardiol. 2021, 77, 2807–2818. [Google Scholar] [CrossRef] [PubMed]
- Callahan, R.; Esch, J.J.; Wang, G.; Ireland, C.M.; Gauvreau, K.; Jenkins, K.J. Systemic Sirolimus to prevent in-stent stenosis in pediatric pulmonary vein stenosis. Pediatr. Cardiol. 2020, 41, 282–289. [Google Scholar] [CrossRef] [PubMed]

| Age Months | BSA m2 | CI L/min/m2 | Qp:Qs | QL/QR | 2a mm | 2b mm | Τ dyn/cm2 | Re | dτ = 10 mm | |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 0.22 | 3.2 | 1.8 | 58/42 | 3.3 | 1.8 | 204 | 898 | 6.0 | LUPV |
| 3.0 | 2.6 | 108 | 835 | 6.0 | LLPV | |||||
| 4.8 | 3.9 | 43 | 777 | 6.8 | RLPV | |||||
| 10 | 0.30 | 2.8 | 1.8 | 35/65 | 2.9 | 1.4 | 277 | 760 | 5.4 | LUPV |
| 2.7 | 1.9 | 161 | 727 | 5.4 | LLPV | |||||
| 5.8 | 4.4 | 52 | 1221 | 8.4 | RLPV | |||||
| 14 | 0.33 | 4.0 | 1.0 | 40/60 | 1.9 | 1.8 | 255 | 908 | 5.4 | LUPV |
| 4.3 | 2.5 | 58 | 486 | 5.4 | LLPV | |||||
| 5.1 | 4.7 | 42 | 1029 | 7.8 | RLPV | |||||
| 22 | 0.45 | 3.4 | 1.0 | 44/56 | 2.8 | 2.4 | 124 | 823 | 5.8 | LUPV |
| 4.7 | 4.4 | 22 | 471 | 5.8 | LLPV | |||||
| 4.7 | 4.3 | 59 | 1212 | 8.0 | RLPV | |||||
| 23.5 | 0.46 | 3.4 | 1.0 | 44/56 | 3.1 | 2.7 | 90 | 754 | 5.9 | LUPV |
| 5.5 | 4.1 | 22 | 454 | 5.9 | LLPV | |||||
| 4.7 | 4.2 | 63 | 1252 | 8.0 | RLPV |
| Age Months | BSA m2 | CI L/min/m2 | Qp:Qs | QL/QR | 2a mm | 2b mm | τ dyn/cm2 | Re | dτ=10 mm | |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 0.28 | 3.2 | 2.6 | 80/20 | 5.2 | 2.1 | 241 | 1554 | 8.2 | LUPV |
| 5.1 | 3.9 | 71 | 1312 | 8.2 | LLPV | |||||
| 1.8 | 1.8 | 475 | 1648 | 6.5 | RLPV | |||||
| 10 | 0.33 | 3.3 | 1.0 | 52/48 | 4.2 | 2.0 | 100 | 564 | 5.5 | LUPV |
| 5.2 | 2.0 | 40 | 435 | 5.5 | LLPV | |||||
| 4.3 | 3.0 | 80 | 905 | 6.8 | RLPV | |||||
| 17 | 0.39 | 4.2 | 1.0 | 52/48 | 2.9 | 2.0 | 218 | 1097 | 6.3 | LUPV |
| 5.6 | 2.8 | 58 | 628 | 6.3 | LLPV | |||||
| 4.9 | 3.7 | 70 | 1158 | 7.8 | RLPV | |||||
| 22 | 0.42 | 5.2 | 0.8 | 44/56 | - | - | - | - | - | LUPV |
| 4.3 | 2.8 | 135 | 1363 | 7.7 | LLPV | |||||
| 5.0 | 4.5 | 57 | 1310 | 8.3 | RLPV | |||||
| 27 | 0.45 | 4.1 | 1.0 | 37/63 | - | - | - | - | - | LUPV |
| 3.2 | 2.7 | 174 | 1471 | 7.4 | LLPV | |||||
| 5.8 | 4.7 | 54 | 1406 | 8.8 | RLPV | |||||
| - | - | - | - | - | LUPV | |||||
| 34 | 0.48 | 3.0 | 1.0 | 34/66 | 2.5 | 2.5 | 186 | 1247 | 6.6 | LLPV |
| 5.9 | 4.6 | 45 | 1148 | 8.3 | RLPV | |||||
| - | - | - | - | - | LUPV | |||||
| 41 | 0.52 | 2.9 | 1.0 | 39/61 | 3.1 | 2.1 | 256 | 1427 | 7.0 | LLPV |
| 6.0 | 2.3 | 172 | 1344 | 8.2 | RLPV | |||||
| - | - | - | - | - | LUPV | |||||
| 47 | 0.55 | 3.1 | 1.0 | 35/65 | 4.0 | 2.9 | 105 | 1094 | 7.1 | LLPV |
| 7.1 | 3.9 | 61 | 1256 | 8.7 | RLPV | |||||
| - | - | - | - | - | LUPV | |||||
| 57 | 0.64 | 3.0 | 1.0 | 35/65 | 7.2 | 7.0 | 11 | 603 | 7.4 | LLPV |
| 7.5 | 4.3 | 53 | 1322 | 9.1 | RLPV | |||||
| - | - | - | - | - | LUPV | |||||
| 132 | 1.36 | 3.1 | 1.0 | 49/51 | 10.0 | 8.6 | 17 | 1412 | 10.7 | LLPV |
| 10.4 | 6.6 | 28 | 1591 | 10.9 | RLPV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammer, P.E.; McEnaney, K.; Callahan, R.; Baird, C.W.; Hoganson, D.M.; Jenkins, K.J. The Role of Elevated Wall Shear Stress in Progression of Pulmonary Vein Stenosis: Evidence from Two Case Studies. Children 2021, 8, 729. https://doi.org/10.3390/children8090729
Hammer PE, McEnaney K, Callahan R, Baird CW, Hoganson DM, Jenkins KJ. The Role of Elevated Wall Shear Stress in Progression of Pulmonary Vein Stenosis: Evidence from Two Case Studies. Children. 2021; 8(9):729. https://doi.org/10.3390/children8090729
Chicago/Turabian StyleHammer, Peter E., Kerry McEnaney, Ryan Callahan, Christopher W. Baird, David M. Hoganson, and Kathy J. Jenkins. 2021. "The Role of Elevated Wall Shear Stress in Progression of Pulmonary Vein Stenosis: Evidence from Two Case Studies" Children 8, no. 9: 729. https://doi.org/10.3390/children8090729
APA StyleHammer, P. E., McEnaney, K., Callahan, R., Baird, C. W., Hoganson, D. M., & Jenkins, K. J. (2021). The Role of Elevated Wall Shear Stress in Progression of Pulmonary Vein Stenosis: Evidence from Two Case Studies. Children, 8(9), 729. https://doi.org/10.3390/children8090729







