Recipient-Specific Risk Factors Impairing Patient and Graft Outcome after Pediatric Liver Transplantation—Analysis of 858 Transplantations in 38 Years
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design and Setting
2.2. Inclusion and Exclusion Criteria
2.3. Study Outcome Parameters
2.4. Data Retrievement
2.5. Ethical Considerations
2.6. Statistical Methods
3. Results
3.1. Baseline Characeristics
3.1.1. Indications for Pediatric Liver Transplantation
3.1.2. Percentage of Indications Related to Era of Transplantation
3.1.3. Recipient Specific Baseline Characteristics
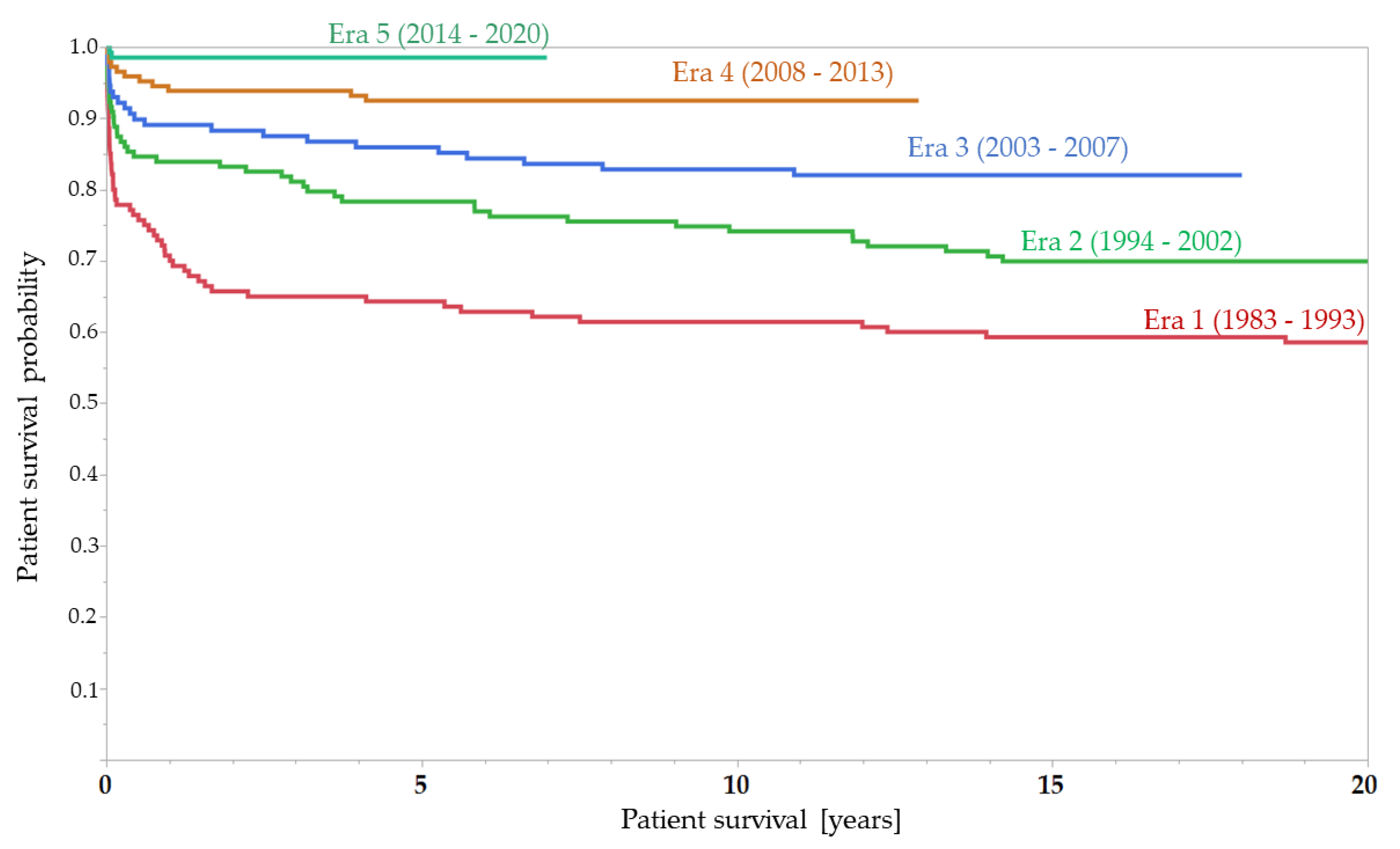
3.2. Patient Survival
3.3. Cause of Death of the Pediatric Liver Recipients
3.4. Graft Survival
3.5. Recipient-Specific Risk Factors of Influence on Patient Survival after Transplantation
3.5.1. Recipient-Specific Factors of Influence on Patient Survival (Univariate Analysis)
3.5.2. Independent Recipient-Specific Factors of Influence on Patient Survival after Transplantation (Multivariate Analysis)
3.6. Risk Factors Influencing Graft Survival
3.6.1. Recipient-Specific Factors Influencing Graft Survival (Univariate Analysis)
3.6.2. Multivariate Analysis for Independent Factors Influencing Graft Survival
4. Discussion
4.1. Patient and Graft Survival
4.2. Changes of Indications for Pediatric Liver Transplantation over Time
4.3. Recipient-Specific Variables Influencing Patient Survival
4.4. Recipient-Specific Variables Influencing Graft Survival
4.5. Limitations and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Starzl, T.E.; Marchioro, T.L.; Vonkaulla, K.N.; Hermann, G.; Brittain, R.S.; Waddell, W.R. Homotransplantation of the Liver in Humans. Surg. Gynecol. Obstet. 1963, 117, 659–676. [Google Scholar] [PubMed]
- Brettschneider, L.; Daloze, P.M.; Huguet, C.; Groth, C.G.; Kashiwagi, N.; Hutchison, D.E.; Starzl, T.E. Successful Orthotopic Transplantation of Liver Homografts after Eight to Twenty-Five Hours Preservation. Surg. Forum 1967, 18, 376–378. [Google Scholar]
- Otte, J.B. Pediatric liver transplantation: Personal perspectives on historical achievements and future challenges. Liver Transpl. 2016, 22, 1284–1294. [Google Scholar] [CrossRef] [Green Version]
- Ringe, B.; Burdelski, M.; Rodeck, B.; Pichlmayr, R. Experience with partial liver transplantation in Hannover. Clin. Transpl. 1990, 135–144. [Google Scholar]
- Ahmed, O.; Brockmeier, D.; Lee, K.; Chapman, W.C.; Doyle, M.B.M. Organ donation during the COVID-19 pandemic. Am. J. Transplant. 2020, 20, 3081–3088. [Google Scholar] [CrossRef] [PubMed]
- Goss, J.A.; Shackleton, C.R.; McDiarmid, S.V.; Maggard, M.; Swenson, K.; Seu, P.; Vargas, J.; Martin, M.; Ament, M.; Brill, J.; et al. Long-term results of pediatric liver transplantation: An analysis of 569 transplants. Ann. Surg. 1998, 228, 411–420. [Google Scholar] [CrossRef]
- Wagenaar, A.E.; Tashiro, J.; Sola, J.E.; Ekwenna, O.; Tekin, A.; Perez, E.A. Pediatric liver transplantation: Predictors of survival and resource utilization. Pediatr. Surg. Int. 2016, 32, 439–449. [Google Scholar] [CrossRef]
- Venick, R.S.; Farmer, D.G.; McDiarmid, S.V.; Duffy, J.P.; Gordon, S.A.; Yersiz, H.; Hong, J.C.; Vargas, J.H.; Ament, M.E.; Busuttil, R.W. Predictors of survival following liver transplantation in infants: A single-center analysis of more than 200 cases. Transplantation 2010, 89, 600–605. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, R.R.; Rela, M.; Girlanda, R.; Bowles, M.J.; Muiesan, P.; Dhawan, A.; Mieli-Vergani, G.; Heaton, N.D. Long-term outcome of liver retransplantation in children. Transplantation 2002, 74, 1124–1130. [Google Scholar] [CrossRef]
- McDiarmid, S.V.; Anand, R.; Martz, K.; Millis, M.J.; Mazariegos, G. A multivariate analysis of pre-, peri-, and post-transplant factors affecting outcome after pediatric liver transplantation. Ann. Surg. 2011, 254, 145–154. [Google Scholar] [CrossRef]
- Rana, A.; Pallister, Z.S.; Guiteau, J.J.; Cotton, R.T.; Halazun, K.; Nalty, C.C.; Khaderi, S.A.; O’Mahony, C.A.; Goss, J.A. Survival Outcomes Following Pediatric Liver Transplantation (Pedi-SOFT) Score: A Novel Predictive Index. Am. J. Transplant. 2015, 15, 1855–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, S.A.; Venkat, V.; Arnon, R.; Gopalareddy, V.V.; Rosenthal, P.; Erinjeri, J.; Anand, R.; Daniel, J.F.; Society of Pediatric Liver Transplantation. Improved Outcomes for Liver Transplantation in Patients with Biliary Atresia Since Pediatric End-Stage Liver Disease Implementation: Analysis of the Society of Pediatric Liver Transplantation Registry. J. Pediatr. 2020, 219, 89–97. [Google Scholar] [CrossRef]
- Chung, P.H.; Chan, S.C.; Mok, V.W.; Tam, P.K.; Lo, C.M. Recipient body size does not matter in pediatric liver transplantation. J. Pediatr. Surg. 2014, 49, 1734–1737. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Oike, F.; Ogura, Y.; Uryuhara, K.; Fujimoto, Y.; Kasahara, M.; Ogawa, K.; Kozaki, K.; Haga, H.; Tanaka, K. Long-term outcomes of 600 living donor liver transplants for pediatric patients at a single center. Liver Transpl. 2006, 12, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Venick, R.S.; Farmer, D.G.; Soto, J.R.; Vargas, J.; Yersiz, H.; Kaldas, F.M.; Agopian, V.G.; Hiatt, J.R.; McDiarmid, S.V.; Busuttil, R.W. One Thousand Pediatric Liver Transplants During Thirty Years: Lessons Learned. J. Am. Coll. Surg. 2018, 226, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waits, S.A.; Wojcik, B.M.; Cai, S.; Mathur, A.K.; Englesbe, M.J. Portal vein thrombosis and outcomes for pediatric liver transplant candidates and recipients in the United States. Liver Transpl. 2011, 17, 1066–1072. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.W.; Cameron, A.M.; Maley, W.R.; Segev, D.L.; Montgomery, R.A. Factors affecting graft survival after adult/child split-liver transplantation: Analysis of the UNOS/OPTN data base. Am. J. Transplant. 2008, 8, 1186–1196. [Google Scholar] [CrossRef]
- Ciria, R.; Davila, D.; Khorsandi, S.E.; Dar, F.; Valente, R.; Briceno, J.; Vilca-Melendez, H.; Dhawan, A.; Rela, M.; Heaton, N.D. Predictors of early graft survival after pediatric liver transplantation. Liver Transpl. 2012, 18, 1324–1332. [Google Scholar] [CrossRef]
- Elisofon, S.A.; Magee, J.C.; Ng, V.L.; Horslen, S.P.; Fioravanti, V.; Economides, J.; Erinjeri, J.; Anand, R.; Mazariegos, G.V.; Society of Pediatric Liver Transplantation Research Group. Society of pediatric liver transplantation: Current registry status 2011–2018. Pediatr. Transplant. 2020, 24, e13605. [Google Scholar] [CrossRef]
- McKiernan, P.J.; Ganoza, A.; Squires, J.E.; Squires, R.H.; Vockley, J.; Mazariegos, G.; Soltys, K.; Sun, Q.; Sindhi, R. Evolving Trends in Liver Transplant for Metabolic Liver Disease in the United States. Liver Transpl. 2019, 25, 911–921. [Google Scholar] [CrossRef]
- Kwong, A.; Kim, W.R.; Lake, J.R.; Smith, J.M.; Schladt, D.P.; Skeans, M.A.; Noreen, S.M.; Foutz, J.; Miller, E.; Snyder, J.J.; et al. OPTN/SRTR 2018 Annual Data Report: Liver. Am. J. Transplant. 2020, 20 (Suppl. S1), 193–299. [Google Scholar] [CrossRef]
- Stalke, A.; Skawran, B.; Auber, B.; Illig, T.; Schlegelberger, B.; Junge, N.; Goldschmidt, I.; Leiskau, C.; von Neuhoff, N.; Baumann, U.; et al. Diagnosis of monogenic liver diseases in childhood by next-generation sequencing. Clin. Genet. 2018, 93, 665–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farmer, D.G.; Venick, R.S.; McDiarmid, S.V.; Ghobrial, R.M.; Gordon, S.A.; Yersiz, H.; Hong, J.; Candell, L.; Cholakians, A.; Wozniak, L.; et al. Predictors of outcomes after pediatric liver transplantation: An analysis of more than 800 cases performed at a single institution. J. Am. Coll. Surg. 2007, 204, 904–914; discussion 914–906. [Google Scholar] [CrossRef]
- Martin, S.R.; Atkison, P.; Anand, R.; Lindblad, A.S.; Group, S.R. Studies of Pediatric Liver Transplantation 2002: Patient and graft survival and rejection in pediatric recipients of a first liver transplant in the United States and Canada. Pediatr. Transplant. 2004, 8, 273–283. [Google Scholar] [CrossRef]
- Kasahara, M.; Umeshita, K.; Inomata, Y.; Uemoto, S.; Japanese Liver Transplantation Society. Long-term outcomes of pediatric living donor liver transplantation in Japan: An analysis of more than 2200 cases listed in the registry of the Japanese Liver Transplantation Society. Am. J. Transplant. 2013, 13, 1830–1839. [Google Scholar] [CrossRef] [PubMed]
- Al Sayyed, M.H.; Shamsaeefar, A.; Nikeghbalian, S.; Dehghani, S.M.; Bahador, A.; Dehghani, M.; Rasekh, R.; Gholami, S.; Khosravi, B.; Malek Hosseini, S.A. Single Center Long-Term Results of Pediatric Liver Transplantation. Exp. Clin. Transplant. 2018. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Yi, N.J.; Lee, J.M.; Suh, S.W.; Yoo, T.; Choi, Y.; Ko, J.S.; Seo, J.K.; Kim, H.; Lee, H.W.; et al. Long term outcomes of pediatric liver transplantation according to age. J. Korean Med. Sci. 2014, 29, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Ekong, U.D.; Gupta, N.A.; Urban, R.; Andrews, W.S. 20- to 25-year patient and graft survival following a single pediatric liver transplant-Analysis of the United Network of Organ Sharing database: Where to go from here. Pediatr. Transplant. 2019, 23, e13523. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.P.; Kao, K.; Ko, C.Y.; Farmer, D.G.; McDiarmid, S.V.; Hong, J.C.; Venick, R.S.; Feist, S.; Goldstein, L.; Saab, S.; et al. Long-term patient outcome and quality of life after liver transplantation: Analysis of 20-year survivors. Ann. Surg. 2010, 252, 652–661. [Google Scholar] [CrossRef]
- Cacciarelli, T.V.; Dvorchik, I.; Mazariegos, G.V.; Gerber, D.; Jain, A.B.; Fung, J.J.; Reyes, J. An analysis of pretransplantation variables associated with long-term allograft outcome in pediatric liver transplant recipients receiving primary tacrolimus (FK506) therapy. Transplantation 1999, 68, 650–655. [Google Scholar] [CrossRef]
- Miguel, M.; Andres, A.M.; Lopez-Santamaria, M.; Barrena, S.; Hierro, L.; Hernandez, F.; Ramirez, M.; Frauca, E.; Encinas, J.L.; Lopez-Fernandez, S.; et al. Liver transplantation in children with cystic fibrosis: Experience in our centre and preliminary results with a combined en bloc liver-pancreas graft. Eur. J. Pediatr. Surg. 2012, 22, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; Pelletier, S.J.; Schmitt, T.M.; Porte, R.J.; Northup, P.G. Pre-transplant portal vein thrombosis is an independent risk factor for graft loss due to hepatic artery thrombosis in liver transplant recipients. HPB 2016, 18, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| INDICATIONS for Pediatric Liver Transplantation | n | % |
|---|---|---|
| PRIMARY TRANSPLANTATIONS | 705 | 100 |
| Biliary atresia | 259 | 36.7 |
| Kasai-Op in case of BA (n = 259) | 244 | 94.2 |
| Acute liver failure | 82 | 11.6 |
| PFIC | 60 | 8.5 |
| Cystic fibrosis | 32 | 4.5 |
| Alagille syndrome | 30 | 4.3 |
| Hepatoblastoma | 29 | 4.1 |
| Alpha-1-antitrypsin-deficiency | 26 | 3.7 |
| Wilson’s disease | 18 | 2.6 |
| Primary sclerosing cholangitis | 16 | 2.3 |
| Metabolic disease (other) | 13 | 1.8 |
| Autoimmune hepatitis | 12 | 1.7 |
| Urea Cycle Disorder | 12 | 1.7 |
| ARPKD | 11 | 1.6 |
| Hyperoxaluria | 11 | 1.6 |
| Malignoma other than Hepatoblastoma | 8 | 1.1 |
| Tyrosinemia | 6 | 0.9 |
| GALD | 5 | 0.7 |
| Crigler-Najjar syndrome | 4 | 0.6 |
| Viral hepatitis-related cirrhosis | 4 | 0.6 |
| Cryptogenic biliary or other cirrhosis | 34 | 4.8 |
| Other vascular or biliary liver disease | 27 | 3.8 |
| RE-TRANSPLANTATIONS | 153 | 100 |
| Secondary Transplantation | 133 | 86.9 |
| Tertiary Transplantation | 19 | 12.4 |
| Quartenary Transplantation | 1 | 0.7 |
| Re-PLTx: vascular complications | 58 | 37.9 |
| Re-PLTx: chronic graft failure | 42 | 27.5 |
| Re-PLTx: primary non-function | 34 | 22.2 |
| Re-PLTx: biliary complications | 11 | 7.2 |
| Re-PLTx: chronic rejection | 8 | 5.2 |
| Indication for Primary Pediatric Liver Transplantation | Number of Patients (% of pLTx in Resp. Era) | ||||
|---|---|---|---|---|---|
| Era 1 (1983–1993) | Era 2 (1994–2002) | Era 3 (2003–2007) | Era 4 (2008–2013) | Era 5 (2014–2020) | |
| Acute liver failure | 24 (17.1%) | 18 (12.6%) | 14 (10.9%) | 16 (10.8%) | 15 (10.3%) |
| AIH/PSC | 3 (2.1%) | 6 (4.2%) | 3 (2.3%) | 6 (4.1%) | 2 (1.4%) |
| Alagille syndrome | 7 (5.0%) | 8 (5.6%) | 7 (5.5%) | 5 (3.4%) | 3 (2.1%) |
| α1-antitrypsin-deficiency | 9 (6.4%) | 8 (5.6%) | 4 (3.1%) | 4 (2.7%) | 1 (0.7%) |
| Biliary atresia | 45 (32.1%) | 52 (36.4%) | 61 (47.7%) | 56 (37.8%) | 45 (31.0%) |
| Cryptogenic cirrhosis | 13 (9.3%) | 10 (7.0%) | 7 (5.5%) | 17 (11.5%) | 14 (9.7%) |
| Cystic fibrosis | 0 | 4 (2.8%) | 9 (7.0%) | 8 (5.4%) | 11 (7.6%) |
| Malignoma | 3 (2.1%) | 4 (2.8%) | 1 (0.8%) | 13 (8.8%) | 16 (11.0%) |
| Metabolic disease | 7 (5.0%) | 10 (7.0%) | 0 | 6 (4.1%) | 20 (13.8%) |
| Other | 4 (2.9%) | 7 (4.9%) | 9 (7.0%) | 1 (0.7%) | 2 (1.4%) |
| PFIC | 20 (14.3%) | 11 (7.7%) | 8 (6.3%) | 11 (7.4%) | 10 (6.9%) |
| Wilson’s disease | 3 (2.1%) | 2 (1.4%) | 5 (3.9%) | 4 (2.7%) | 4 (2.8%) |
| Total | 140 | 143 | 128 | 148 | 145 |
| Categorical Variables | n | % |
|---|---|---|
| PEDIATRIC AGE GROUP | ||
| Age group < 1 years | 193 | 22.5 |
| Age group ≥ 1 < 6 years | 310 | 36.1 |
| Age group ≥ 6 < 12 years | 184 | 21.4 |
| Age group ≥ 12 years | 171 | 19.9 |
| Recipient female | 413 | 48.1 |
| Sex mismatch | 390 | 50.5 |
| ERA OF TRANSPLANTATION | ||
| Era 1 (1983–1992) | 175 | 20.4 |
| Era 2 (1993–2002) | 177 | 20.6 |
| Era 3 (2003–2007) | 156 | 18.2 |
| Era 4 (2008–2013) | 188 | 21.9 |
| Era 5 (2014–2020) | 162 | 18.9 |
| On high-urgency list [y/n] | 189 | 22.1 |
| Pre-existing portal vein thrombosis | 21 | 3.6 |
| Combined pLTx to other organ | 21 | 2.4 |
| Kidney | 17 | 81.0 |
| Lung | 3 | 14.3 |
| Bone marrow | 1 | 4.8 |
| Hepaticojejunostomy as biliary anastomosis | 492 | 63.8 |
| Graft loss due to death or re-transplantation | 294 | 34.8 |
| Blood mismatch | 28 | 3.7 |
| Subsequent re-transplantation | 153 | 17.8 |
| Death after pLTx (of patients, n = 705) | 141 | 20.0 |
| Recipient COD: infection | 61 | 43.9 |
| Recipient COD: cerebral event | 20 | 14.4 |
| Recipient COD: cardiopulmonal event | 19 | 13.7 |
| Recipient COD: liver graft related | 17 | 12.2 |
| Recipient COD: malignancy | 13 | 9.4 |
| Continuous variables | Mean (Median) | Standard deviation (Range) |
| Age at pLTx | 6.03 (4.02) | 5.47 (0.05–17.98) |
| Weight [kg] | 22.3 (15.8) | 17.99 (2.7–92) |
| Height [cm] | 105.7 (98) | 37.7 (45–192) |
| BMI [kg/m2] | 16.99 (16.41) | 2.95 (11.46–35.34) |
| Creatinine at pLTx (µmol/L) | 52.1 (28) | 103.7 (6–815) |
| Bilirubine at pLTx [µmol/L] | 188.5 (103) | 209.4 (2–977) |
| Albumine at pLTx (g/L) | 33.4 (33) | 8.0 (12–67) |
| INR at pLTx | 1.31 (1.66) | 0.87 (0.9–6.4) |
| Lab-MELD | 9.6 (6) | 7.66 (6–40) |
| Ped-MELD (since 2006) | 29.3 (28) | 5.84 (22–40) |
| Waiting time for pLTx all status [days] | 158.9 (94) | 194.4 (1–1141) |
| Waiting time for pLTx on high urgency list [days] | 6.15 (4) | 6.22 (1–41) |
| Waiting time for pLTx (non-HU list) [days] | 199.5 (146] | 202.5 (1–1141) |
| ICU stay post pLtx [days] | 9 (15) | 17.4 (1–153) |
| NON-ICU stay post Ltx [days] | 28 (30) | 27.8 (0–295) |
| PATIENT SURVIVAL | GRAFT SURVIVAL | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95%-CI | p-Value | HR | 95%-CI | p-Value |
| ANTHROPOMETRIC DATA | ||||||
| Recipient Age at tx | 1.038 | 1.009–1.068 | 0.0117 | 1.002 | 0.981–1.023 | 0.8561 |
| Age group < 1 year | 0.645 | 0.399–0.996 | 0.0477 | 0.855 | 0.633–1.137 | 0.2874 |
| Age group ≥ 1/< 6 years | 1.089 | 0.770–1.525 | 0.625 | 1.235 | 0.972–1.563 | 0.0838 |
| Age group ≥ 6/< 12 years | 0.764 | 0.485–1.156 | 0.209 | 0.704 | 0.510–0.951 | 0.0214 |
| Age group ≥ 12 years | 1.627 | 1.120–2.322 | 0.0114 | 1.205 | 0.908–1.577 | 0.1926 |
| Weight [kg] | 1.008 | 0.999–1.017 | 0.0581 | 1.001 | 0.995–1.008 | 0.6818 |
| Height [cm] | 1.002 | 0.997–1.006 | 0.4503 | 0.999 | 0.996–1.003 | 0.7332 |
| BMI [kg/m2] | 1.030 | 0.973–1.084 | 0.2970 | 0.997 | 0.956–1.037 | 0.8941 |
| Sex [female] | 0.785 | 0.586–1.046 | 0.0990 | 0.898 | 0.710–1.134 | 0.3674 |
| LABARATORY VALUES | ||||||
| Creatinine at pLTx (µmol/L) | 1.001 | 0.996–1.003 | 0.6528 | 1.000 | 0.997–1.002 | 0.9365 |
| Albumine at pLTx (g/L) | 0.991 | 0.932–1.049 | 0.7511 | 0.981 | 0.950–1.013 | 0.2446 |
| INR at pLTx | 1.673 | 1.167–2.285 | 0.0007 | 1.370 | 1.085–1.682 | 0.0096 |
| Bilirubine at pLTx [µmol/L] | 1.002 | 1.001–1.003 | 0.0037 | 1.001 | 1.000–1.002 | 0.0619 |
| Pediatric MELD-Score | 0.809 | 0.720–0.893 | <0.0001 | 0.896 | 0.840–0.959 | 0.0004 |
| INDICATIONS | ||||||
| Biliary atresia | 0.629 | 0.441–0.878 | 0.0059 | 0.772 | 0.588–1.003 | 0.0525 |
| Kasai procedure in BA | 0.655 | 0.283–1.899 | 0.3983 | 0.833 | 0.411–1.993 | 0.6523 |
| Acute liver failure | 2.536 | 1.729–3.615 | <0.0001 | 2.046 | 1.464–2.790 | <0.0001 |
| PFIC | 0.457 | 0.194–0.899 | 0.0211 | 0.781 | 0.460–1.235 | 0.3057 |
| Alagille syndrome | 0.698 | 0.248–1.524 | 0.401 | 0.798 | 0.380–1.458 | 0.4900 |
| Alpha-1-antitrypsin-deficiency | 1.423 | 0.643–2.701 | 0.3540 | 1.542 | 0.840–2.579 | 0.1521 |
| Cryptogenic biliary or other cirrhosis | 0.662 | 0.235–1.445 | 0.3305 | 0.780 | 0.372–1.424 | 0.4452 |
| Cystic fibrosis | 0.693 | 0.246–1.514 | 0.3909 | 0.413 | 0.147–0.896 | 0.0225 |
| Vascular or other liver disease | 0.647 | 0.199–1.526 | 0.3559 | 1.077 | 0.513–1.968 | 0.8275 |
| Primary sclerosing cholangitis | 1.052 | 0.324–2.481 | 0.920 | 1.286 | 0.549–2.517 | 0.5276 |
| Wilson’s disease | 1.295 | 0.460–2.827 | 0.5837 | 1.008 | 0.398–2.066 | 0.9842 |
| Hepatoblastoma | 0.765 | 0.235–1.816 | 0.5831 | 0.393 | 0.121–0.922 | 0.0293 |
| Autoimmune hepatitis | 0.809 | 0.134–2.537 | 0.7580 | 0.696 | 0.172–1.820 | 0.5067 |
| Re-transplantation | 1.781 | 1.274–2.448 | 0.0010 | 1.318 | 0.985–1.737 | 0.0562 |
| ERA of TRANSPLANTATION | ||||||
| Era 1 (1983–1992) | 3.186 | 2.268–4.451 | <0.0001 | 2.552 | 1.997–3.245 | <0.0001 |
| Era 2 (1993–2002) | 1.466 | 1.009–2.094 | 0.045 | 1.262 | 0.961–1.639 | 0.0930 |
| Era 3 (2003–2007) | 0.808 | 0.503–1.241 | 0.3405 | 0.872 | 0.635–1.173 | 0.3731 |
| Era 4 (2008–2013) | 0.347 | 0.186–0.591 | <0.0001 | 0.584 | 0.415–0.803 | 0.0007 |
| Era 5 (2014–2020) | 0.073 | 0.012–0.231 | <0.0001 | 0.246 | 0.137–0.406 | <0.0001 |
| Waiting time for Tx [days] | 0.998 | 0.996–0.999 | 0.0002 | 0.999 | 0.998–1.000 | 0.00163 |
| Waiting time for pLTx [days] non-HU | 0.999 | 0.998–1.000 | 0.1195 | 0.999 | 0.999–1.000 | 0.0880 |
| On high urgency list [y/n] | 2.160 | 1.600–2.896 | <0.0001 | 1.686 | 1.231–2.295 | 0.0013 |
| Waiting time for Tx on HU list [days] | 1.057 | 1.016–1.091 | 0.0089 | 1.018 | 0.984–1.047 | 0.2832 |
| Pre-transplant portal vein thrombosis | 1.992 | 0.899–3.786 | 0.0570 | 1.575 | 0.781–2.805 | 0.1593 |
| Blood mismatch | 1.409 | 0.964–2.002 | 0.0753 | 1.204 | 0.869–1.632 | 0.2563 |
| Sex mismatch | 0.805 | 0.604–1.072 | 0.1367 | 0.847 | 0.671–1.069 | 0.1618 |
| Biliodigestive anastomosis | 0.735 | 0.546–0.994 | 0.0462 | 0.826 | 0.647–1.059 | 0.1311 |
| Subsequent re-Tx | 1.934 | 1.400–2.637 | <0.0001 | n/a | n/a | n/a |
| ICU stay | 1.010 | 1.004–1.017 | 0.0041 | 1.009 | 1.003–1.014 | 0.0029 |
| Non-ICU stay | 0.960 | 0.952–0.968 | <0.0001 | 0.970 | 0.963–0.976 | <0.0001 |
| Combined liver transplant with other organ | 0.801 | 0.198–2.105 | 0.6923 | 0.690 | 0.213–1.618 | 0.4328 |
| Variable | Log-Ranking | Hazard Ratio | 95%-CI | p-Value |
|---|---|---|---|---|
| Era of pLTx [1,2,3,4,5] | 7378 | n/a | n/a | <0.00001 |
| Waiting time on high urgency list [d] | 2588 | 1.073 | 1.028–1.112 | 0.00258 |
| Indication: Acute liver Failure | 1944 | 2.015 | 1.181–3.296 | 0.01139 |
| Subsequent re-transplantation | 1755 | 1.744 | 1.106–2.674 | 0.01757 |
| Indication: Biliary atresia | 1502 | 0.575 | 0.332–0.954 | 0.03151 |
| Variable | Log-Ranking | Hazard Ratio | 95%-CI | p-Value |
|---|---|---|---|---|
| Era of pLTx | 9657 | n/a | n/a | <0.0001 |
| Indication: Acute liver failure | 3923 | 1.935 | 1.364–2.677 | 00001 |
| Pretransplant portal vein thrombosis | 1482 | 2.016 | 1.009–3.643 | 0.0330 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiskau, C.; Junge, N.; Pfister, E.-D.; Goldschmidt, I.; Mutschler, F.; Laue, T.; Ohlendorf, J.; Nasser, H.; Beneke, J.; Richter, N.; et al. Recipient-Specific Risk Factors Impairing Patient and Graft Outcome after Pediatric Liver Transplantation—Analysis of 858 Transplantations in 38 Years. Children 2021, 8, 641. https://doi.org/10.3390/children8080641
Leiskau C, Junge N, Pfister E-D, Goldschmidt I, Mutschler F, Laue T, Ohlendorf J, Nasser H, Beneke J, Richter N, et al. Recipient-Specific Risk Factors Impairing Patient and Graft Outcome after Pediatric Liver Transplantation—Analysis of 858 Transplantations in 38 Years. Children. 2021; 8(8):641. https://doi.org/10.3390/children8080641
Chicago/Turabian StyleLeiskau, Christoph, Norman Junge, Eva-Doreen Pfister, Imeke Goldschmidt, Frauke Mutschler, Tobias Laue, Johanna Ohlendorf, Hamoud Nasser, Jan Beneke, Nicolas Richter, and et al. 2021. "Recipient-Specific Risk Factors Impairing Patient and Graft Outcome after Pediatric Liver Transplantation—Analysis of 858 Transplantations in 38 Years" Children 8, no. 8: 641. https://doi.org/10.3390/children8080641
APA StyleLeiskau, C., Junge, N., Pfister, E.-D., Goldschmidt, I., Mutschler, F., Laue, T., Ohlendorf, J., Nasser, H., Beneke, J., Richter, N., Vondran, F., & Baumann, U. (2021). Recipient-Specific Risk Factors Impairing Patient and Graft Outcome after Pediatric Liver Transplantation—Analysis of 858 Transplantations in 38 Years. Children, 8(8), 641. https://doi.org/10.3390/children8080641







