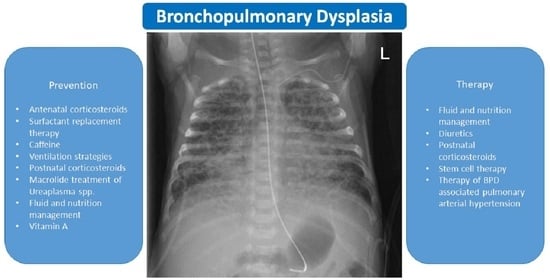
Evidence for the Management of Bronchopulmonary Dysplasia in Very Preterm Infants
Abstract
1. Introduction
2. What Is the Evidence for?
2.1. Antenatal Corticosteroids
2.2. Surfactant Replacement Therapy
2.3. Caffeine
2.4. Ventilation Strategies
2.4.1. High-Frequency Ventilation
2.4.2. Volume-Targeted Ventilation
2.4.3. Permissive Hypercapnia
2.4.4. Supplemental Oxygen
2.4.5. Synchronization
2.4.6. Early Extubation
2.4.7. Tracheostomy Placement
2.5. Postnatal Corticosteroids
2.6. Inhaled Nitric Oxide (iNO)
2.7. Inhaled Bronchodilators
2.8. Macrolides
2.9. Microbiome and Antibiotic Treatment
2.10. Patent Ductus Arteriosus
2.11. Fluid Management and Nutrition
2.12. Vitamin A
2.13. BPD Associated Late Pulmonary Hypertension
2.14. Stem Cell Therapy for BPD—An Outlook to a Present Future
3. Recommendations for the Everyday BPD Management Based on the Presented Evidence
3.1. Antenatal Corticosteroids
3.2. Surfactant-Replacement Therapy
3.3. Caffeine
3.4. Ventilation Strategies
3.4.1. High-Frequency Oscillation Ventilation
3.4.2. Volume-Targeted-Ventilation (VTV)
3.4.3. Permissive Hypercapnia
3.4.4. Supplemental Oxygen
3.5. Tracheostomy Placement
3.6. Postnatal Corticosteroids
3.7. Inhaled Nitric Oxide (iNO)
3.8. Inhaled Bronchodilators
3.9. Macrolides
3.10. Patent Ductus Arteriosus
3.11. Fluid-Management and Nutrition
3.12. Vitamin A
3.13. BPD-Associated Pulmonary Hypertension
3.14. Stem Cell Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Northway, W.H., Jr.; Rosan, R.C.; Porter, D.Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med. 1967, 276, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Shennan, A.T.; Dunn, M.S.; Ohlsson, A.; Lennox, K.; Hoskins, E.M. Abnormal pulmonary outcomes in premature infants: Prediction from oxygen requirement in the neonatal period. Pediatrics 1988, 82, 527–532. [Google Scholar] [PubMed]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; Schmidt, B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res. A Clin. Mol. Teratol. 2014, 100, 145–157. [Google Scholar] [CrossRef]
- Fanaroff, A.A.; Stoll, B.J.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A.; Stark, A.R.; Bauer, C.R.; Donovan, E.F.; Korones, S.B.; Laptook, A.R.; et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am. J. Obstet. Gynecol. 2007, 196, 147.e1–147.e8. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sanchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef]
- Jobe, A.J. The new BPD: An arrest of lung development. Pediatr. Res. 1999, 46, 641–643. [Google Scholar] [CrossRef]
- Thebaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Primers. 2019, 5, 78. [Google Scholar] [CrossRef]
- DeVries, L.B.; Heyne, R.J.; Ramaciotti, C.; Brown, L.S.; Jaleel, M.A.; Kapadia, V.S.; Burchfield, P.J.; Brion, L.P. Mortality among infants with evolving bronchopulmonary dysplasia increases with major surgery and with pulmonary hypertension. J. Perinatol. 2017, 37, 1043–1046. [Google Scholar] [CrossRef]
- Gallini, F.; Coppola, M.; De Rose, D.U.; Maggio, L.; Arena, R.; Romano, V.; Cota, F.; Ricci, D.; Romeo, D.M.; Mercuri, E.M.; et al. Neurodevelopmental outcomes in very preterm infants: The role of severity of Bronchopulmonary Dysplasia. Early Hum. Dev. 2021, 152, 105275. [Google Scholar] [CrossRef]
- Liggins, G.C.; Howie, R.N. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972, 50, 515–525. [Google Scholar]
- Crowley, P.; Chalmers, I.; Keirse, M.J. The effects of corticosteroid administration before preterm delivery: An overview of the evidence from controlled trials. Br. J. Obstet. Gynaecol. 1990, 97, 11–25. [Google Scholar] [CrossRef]
- Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens. Statement 1994, 12, 1–24.
- Gilstrap, L.C.; Christensen, R.; Clewell, W.H.; D’Alton, M.E.; Davidson, E.C.; Escobedo, M.B.; Gjerdingen, D.K.; Goddard-Finegold, J.; Goldenberg, R.L.; Grimes, D.A.; et al. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA 1995, 273, 413–418. [Google Scholar] [CrossRef]
- Roberts, D.; Brown, J.; Medley, N.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2017, 3, CD004454. [Google Scholar] [CrossRef] [PubMed]
- Manktelow, B.N.; Lal, M.K.; Field, D.J.; Sinha, S.K. Antenatal corticosteroids and neonatal outcomes according to gestational age: A cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F95–F98. [Google Scholar] [CrossRef] [PubMed]
- Mori, R.; Kusuda, S.; Fujimura, M.; Neonatal Research Network Japan. Antenatal corticosteroids promote survival of extremely preterm infants born at 22 to 23 weeks of gestation. J. Pediatr. 2011, 159, 110–114.e1. [Google Scholar] [CrossRef] [PubMed]
- Melamed, N.; Shah, J.; Yoon, E.W.; Pelausa, E.; Lee, S.K.; Shah, P.S.; Murphy, K.E.; Canadian Neonatal Network Investigators. The role of antenatal corticosteroids in twin pregnancies complicated by preterm birth. Am. J. Obstet. Gynecol. 2016, 215, 482.e1–482.e9. [Google Scholar] [CrossRef]
- Blankenship, S.A.; Brown, K.E.; Simon, L.E.; Stout, M.J.; Tuuli, M.G. Antenatal corticosteroids in preterm small-for-gestational age infants: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100215. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.; Patole, S. Antenatal corticosteroids in impending preterm deliveries before 25 weeks’ gestation. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F173–F176. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ikeda, T.; Nishimura, K.; Katsuragi, S.; Sengoku, K.; Kusuda, S.; Fujimura, M. Association of antenatal corticosteroids and the mode of delivery with the mortality and morbidity of infants weighing less than 1500g at birth in Japan. Neonatology 2014, 106, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Abdel-Latif, M.; Kent, A.; NICUS Network. Antenatal steroid exposure and outcomes of very premature infants: A regional cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F12–F20. [Google Scholar] [CrossRef] [PubMed]
- Figueras-Aloy, J.; Serrano, M.M.; Rodriguez, J.P.; Perez, C.F.; Serradilla, V.R.; Jimenez, J.Q.; Gonzalez, R.J.; The SEN1500 Spanish Neonatal Network. Antenatal glucocorticoid treatment decreases mortality and chronic lung disease in survivors among 23- to 28-week gestational age preterm infants. Am. J. Perinatol. 2005, 22, 441–448. [Google Scholar] [CrossRef]
- Melamed, N.; Shah, J.; Soraisham, A.; Yoon, E.W.; Lee, S.K.; Shah, P.S.; Murphy, K.E. Association Between Antenatal Corticosteroid Administration-to-Birth Interval and Outcomes of Preterm Neonates. Obstet. Gynecol. 2015, 125, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, N.D.; Kenaley, K.M.; Locke, R.; Paul, D.A. The Joint Effects of Antenatal Steroids and Gestational Age on Improved Outcomes in Neonates. Matern. Child. Health J. 2018, 22, 384–390. [Google Scholar] [CrossRef]
- Battarbee, A.N.; Ros, S.T.; Esplin, M.S.; Biggio, J.; Bukowski, R.; Parry, S.; Zhang, H.; Huang, H.; Andrews, W.; Saade, G.; et al. Optimal timing of antenatal corticosteroid administration and preterm neonatal and early childhood outcomes. Am. J. Obstet. Gynecol. MFM 2020, 2. [Google Scholar] [CrossRef]
- Travers, C.P.; Carlo, W.A.; McDonald, S.A.; Das, A.; Bell, E.F.; Ambalavanan, N.; Jobe, A.H.; Goldberg, R.N.; D’Angio, C.T.; Stoll, B.J.; et al. Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. Am. J. Obstet. Gynecol. 2018, 218, 130.e1–130.e13. [Google Scholar] [CrossRef]
- Crowther, C.A.; McKinlay, C.J.; Middleton, P.; Harding, J.E. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database Syst. Rev. 2015, CD003935. [Google Scholar] [CrossRef]
- Banks, B.A.; Cnaan, A.; Morgan, M.A.; Parer, J.T.; Merrill, J.D.; Ballard, P.L.; Ballard, R.A. Multiple courses of antenatal corticosteroids and outcome of premature neonates. North American Thyrotropin-Releasing Hormone Study Group. Am. J. Obstet. Gynecol. 1999, 181, 709–717. [Google Scholar] [CrossRef]
- Fujiwara, T.; Maeta, H.; Chida, S.; Morita, T.; Watabe, Y.; Abe, T. Artificial surfactant therapy in hyaline-membrane disease. Lancet 1980, 1, 55–59. [Google Scholar] [CrossRef]
- Hennes, H.M.; Lee, M.B.; Rimm, A.A.; Shapiro, D.L. Surfactant replacement therapy in respiratory distress syndrome. Meta-analysis of clinical trials of single-dose surfactant extracts. Am. J. Dis. Child. 1991, 145, 102–104. [Google Scholar] [CrossRef]
- Soll, R.F. Synthetic surfactant for respiratory distress syndrome in preterm infants. Cochrane Database Syst. Rev. 2000, CD001149. [Google Scholar] [CrossRef]
- Soll, R.F. Prophylactic natural surfactant extract for preventing morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2000, CD000511. [Google Scholar] [CrossRef]
- Soll, R.; Ozek, E. Prophylactic protein free synthetic surfactant for preventing morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2010, CD001079. [Google Scholar] [CrossRef]
- Seger, N.; Soll, R. Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database Syst. Rev. 2009, CD007836. [Google Scholar] [CrossRef] [PubMed]
- Moya, F.R.; Gadzinowski, J.; Bancalari, E.; Salinas, V.; Kopelman, B.; Bancalari, A.; Kornacka, M.K.; Merritt, T.A.; Segal, R.; Schaber, C.J.; et al. A multicenter, randomized, masked, comparison trial of lucinactant, colfosceril palmitate, and beractant for the prevention of respiratory distress syndrome among very preterm infants. Pediatrics 2005, 115, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Ardell, S.; Pfister, R.H.; Soll, R. Animal derived surfactant extract versus protein free synthetic surfactant for the prevention and treatment of respiratory distress syndrome. Cochrane Database Syst. Rev. 2015, 8, CD000144. [Google Scholar] [CrossRef] [PubMed]
- Pfister, R.H.; Soll, R.F.; Wiswell, T. Protein containing synthetic surfactant versus animal derived surfactant extract for the prevention and treatment of respiratory distress syndrome. Cochrane Database Syst. Rev. 2007, CD006069. [Google Scholar] [CrossRef]
- Singh, N.; Halliday, H.L.; Stevens, T.P.; Suresh, G.; Soll, R.; Rojas-Reyes, M.X. Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst. Rev. 2015, CD010249. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Luna, M.; Bacher, P.; Unnebrink, K.; Martinez-Tristani, M.; Ramos Navarro, C. Beractant and poractant alfa in premature neonates with respiratory distress syndrome: A systematic review of real-world evidence studies and randomized controlled trials. J. Perinatol. 2020, 40, 1121–1134. [Google Scholar] [CrossRef]
- Ramanathan, R.; Rasmussen, M.R.; Gerstmann, D.R.; Finer, N.; Sekar, K.; The North American Study Group. A randomized, multicenter masked comparison trial of poractant alfa (Curosurf) versus beractant (Survanta) in the treatment of respiratory distress syndrome in preterm infants. Am. J. Perinatol. 2004, 21, 109–119. [Google Scholar] [CrossRef]
- Mirzarahimi, M.; Barak, M. Comparison efficacy of Curosurf and Survanta in preterm infants with respiratory distress syndrome. Pak. J. Pharm. Sci. 2018, 31, 469–472. [Google Scholar]
- Najafian, B.; Karimi-Sari, H.; Khosravi, M.H.; Nikjoo, N.; Amin, S.; Shohrati, M. Comparison of efficacy and safety of two available natural surfactants in Iran, Curosurf and Survanta in treatment of neonatal respiratory distress syndrome: A randomized clinical trial. Contemp. Clin. Trials Commun. 2016, 3, 55–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baroutis, G.; Kaleyias, J.; Liarou, T.; Papathoma, E.; Hatzistamatiou, Z.; Costalos, C. Comparison of three treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Eur. J. Pediatr. 2003, 162, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Speer, C.P.; Gefeller, O.; Groneck, P.; Laufkotter, E.; Roll, C.; Hanssler, L.; Harms, K.; Herting, E.; Boenisch, H.; Windeler, J.; et al. Randomised clinical trial of two treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Arch. Dis. Child. Fetal Neonatal Ed. 1995, 72, F8–F13. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, R.; Hamedi, A.; Javadi, A.; Gholami Robatsangi, M.; Dinparvar, S.K. Comparison of side effect of Survanta and Curosurf in decreasing mortality due to respiratory distress syndrome (RDS) in premature infants admitted in NICU of Ghaem Hospital on 2006–2008. Iran. J. Neonatol. 2013, 4, 7–12. [Google Scholar]
- Isayama, T.; Chai-Adisaksopha, C.; McDonald, S.D. Noninvasive Ventilation With vs. Without Early Surfactant to Prevent Chronic Lung Disease in Preterm Infants: A Systematic Review and Meta-analysis. JAMA Pediatr. 2015, 169, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Vento, G.; Ventura, M.L.; Pastorino, R.; van Kaam, A.H.; Carnielli, V.; Cools, F.; Dani, C.; Mosca, F.; Polglase, G.; Tagliabue, P.; et al. Lung recruitment before surfactant administration in extremely preterm neonates with respiratory distress syndrome (IN-REC-SUR-E): A randomised, unblinded, controlled trial. Lancet Respir. Med. 2021, 9, 159–166. [Google Scholar] [CrossRef]
- Aldana-Aguirre, J.C.; Pinto, M.; Featherstone, R.M.; Kumar, M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F17–F23. [Google Scholar] [CrossRef]
- Rigo, V.; Lefebvre, C.; Broux, I. Surfactant instillation in spontaneously breathing preterm infants: A systematic review and meta-analysis. Eur J. Pediatr 2016, 175, 1933–1942. [Google Scholar] [CrossRef]
- Panza, R.; Laforgia, N.; Bellos, I.; Pandita, A. Systematic review found that using thin catheters to deliver surfactant to preterm neonates was associated with reduced bronchopulmonary dysplasia and mechanical ventilation. Acta Paediatr. 2020, 109, 2219–2225. [Google Scholar] [CrossRef]
- Isayama, T.; Iwami, H.; McDonald, S.; Beyene, J. Association of Noninvasive Ventilation Strategies With Mortality and Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review and Meta-analysis. JAMA 2016, 316, 611–624. [Google Scholar] [CrossRef]
- Bellos, I.; Fitrou, G.; Panza, R.; Pandita, A. Comparative efficacy of methods for surfactant administration: A network meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2021. [Google Scholar] [CrossRef]
- Dunwiddie, T.V.; Masino, S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Yang, J.; Wang, Y. Low, but not high, dose caffeine is a readily available probe for adenosine actions. Mol. Asp. Med. 2017, 55, 20–25. [Google Scholar] [CrossRef]
- Bassler, D.; Schmidt, B. Strategies for prevention of apneic episodes in preterm infants: Are respiratory stimulants worth the risk? In The Newborn Lung. Neonatology Questions and Controversies; Bancalari, E., Ed.; Saunders, Elsevier: Philadelphia, PA, USA, 2008; pp. 461–476. [Google Scholar]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W.; Caffeine for Apnea of Prematurity Trial Group. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef]
- Lodha, A.; Entz, R.; Synnes, A.; Creighton, D.; Yusuf, K.; Lapointe, A.; Yang, J.; Shah, P.S.; The investigators of the Canadian Neonatal Network; the Canadian Neonatal Follow-up Network. Early Caffeine Administration and Neurodevelopmental Outcomes in Preterm Infants. Pediatrics 2019, 143. [Google Scholar] [CrossRef]
- Kua, K.P.; Lee, S.W. Systematic review and meta-analysis of clinical outcomes of early caffeine therapy in preterm neonates. Br. J. Clin. Pharmacol. 2017, 83, 180–191. [Google Scholar] [CrossRef]
- Vliegenthart, R.; Miedema, M.; Hutten, G.J.; van Kaam, A.H.; Onland, W. High versus standard dose caffeine for apnoea: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F523–F529. [Google Scholar] [CrossRef]
- Brattstrom, P.; Russo, C.; Ley, D.; Bruschettini, M. High-versus low-dose caffeine in preterm infants: A systematic review and meta-analysis. Acta Paediatr. 2019, 108, 401–410. [Google Scholar] [CrossRef]
- Chen, J.; Jin, L.; Chen, X. Efficacy and Safety of Different Maintenance Doses of Caffeine Citrate for Treatment of Apnea in Premature Infants: A Systematic Review and Meta-Analysis. Biomed Res. Int. 2018, 2018, 9061234. [Google Scholar] [CrossRef]
- Pakvasa, M.A.; Saroha, V.; Patel, R.M. Optimizing Caffeine Use and Risk of Bronchopulmonary Dysplasia in Preterm Infants: A Systematic Review, Meta-analysis, and Application of Grading of Recommendations Assessment, Development, and Evaluation Methodology. Clin. Perinatol. 2018, 45, 273–291. [Google Scholar] [CrossRef]
- De Rose, D.U.; Cairoli, S.; Dionisi, M.; Santisi, A.; Massenzi, L.; Goffredo, B.M.; Dionisi-Vici, C.; Dotta, A.; Auriti, C. Therapeutic Drug Monitoring Is a Feasible Tool to Personalize Drug Administration in Neonates Using New Techniques: An Overview on the Pharmacokinetics and Pharmacodynamics in Neonatal Age. Int. J. Mol. Sci. 2020, 21, 5898. [Google Scholar] [CrossRef]
- Moschino, L.; Zivanovic, S.; Hartley, C.; Trevisanuto, D.; Baraldi, E.; Roehr, C.C. Caffeine in preterm infants: Where are we in 2020? ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef]
- Bjorklund, L.J.; Ingimarsson, J.; Curstedt, T.; John, J.; Robertson, B.; Werner, O.; Vilstrup, C.T. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatr. Res. 1997, 42, 348–355. [Google Scholar] [CrossRef]
- Dreyfuss, D.; Saumon, G. Ventilator-induced lung injury: Lessons from experimental studies. Am. J. Respir. Crit. Care Med. 1998, 157, 294–323. [Google Scholar] [CrossRef]
- Owen, L.S.; Manley, B.J.; Davis, P.G.; Doyle, L.W. The evolution of modern respiratory care for preterm infants. Lancet 2017, 389, 1649–1659. [Google Scholar] [CrossRef]
- Reiterer, F.; Schwaberger, B.; Freidl, T.; Schmolzer, G.; Pichler, G.; Urlesberger, B. Lung-protective ventilatory strategies in intubated preterm neonates with RDS. Paediatr. Respir. Rev. 2017, 23, 89–96. [Google Scholar] [CrossRef]
- Greenough, A.; Rossor, T.E.; Sundaresan, A.; Murthy, V.; Milner, A.D. Synchronized mechanical ventilation for respiratory support in newborn infants. Cochrane Database Syst. Rev. 2016, 9, CD000456. [Google Scholar] [CrossRef]
- Cools, F.; Offringa, M.; Askie, L.M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst. Rev. 2015, CD000104. [Google Scholar] [CrossRef] [PubMed]
- Bhuta, T.; Henderson-Smart, D.J. Elective high frequency jet ventilation versus conventional ventilation for respiratory distress syndrome in preterm infants. Cochrane Database Syst. Rev. 2000, CD000328. [Google Scholar] [CrossRef] [PubMed]
- Ethawi, Y.H.; Abou Mehrem, A.; Minski, J.; Ruth, C.A.; Davis, P.G. High frequency jet ventilation versus high frequency oscillatory ventilation for pulmonary dysfunction in preterm infants. Cochrane Database Syst. Rev. 2016, CD010548. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.; Wheeler, K.I.; McCallion, N.; Morley, C.J.; Davis, P.G. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst. Rev. 2017, 10, CD003666. [Google Scholar] [CrossRef]
- Bamat, N.; Fierro, J.; Wang, Y.; Millar, D.; Kirpalani, H. Positive end-expiratory pressure for preterm infants requiring conventional mechanical ventilation for respiratory distress syndrome or bronchopulmonary dysplasia. Cochrane Database Syst. Rev. 2019, 2, CD004500. [Google Scholar] [CrossRef] [PubMed]
- Kamlin, C.; Davis, P.G. Long versus short inspiratory times in neonates receiving mechanical ventilation. Cochrane Database Syst. Rev. 2004, CD004503. [Google Scholar] [CrossRef]
- Woodgate, P.G.; Davies, M.W. Permissive hypercapnia for the prevention of morbidity and mortality in mechanically ventilated newborn infants. Cochrane database Syst. Rev. (Online) 2001, CD002061. [Google Scholar] [CrossRef]
- Rossor, T.E.; Hunt, K.A.; Shetty, S.; Greenough, A. Neurally adjusted ventilatory assist compared to other forms of triggered ventilation for neonatal respiratory support. Cochrane Database Syst. Rev. 2017, 10, CD012251. [Google Scholar] [CrossRef]
- Schulzke, S.M.; Pillow, J.; Ewald, B.; Patole, S.K. Flow-cycled versus time-cycled synchronized ventilation for neonates. Cochrane Database Syst. Rev. 2010, CD008246. [Google Scholar] [CrossRef]
- Cools, F.; Henderson-Smart, D.J.; Offringa, M.; Askie, L.M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst. Rev. 2009, CD000104. [Google Scholar] [CrossRef]
- Cools, F.; Askie, L.M.; Offringa, M.; Asselin, J.M.; Calvert, S.A.; Courtney, S.E.; Dani, C.; Durand, D.J.; Gerstmann, D.R.; Henderson-Smart, D.J.; et al. Elective high-frequency oscillatory versus conventional ventilation in preterm infants: A systematic review and meta-analysis of individual patients’ data. Lancet 2010, 375, 2082–2091. [Google Scholar] [CrossRef]
- Ganguly, A.; Makkar, A.; Sekar, K. Volume Targeted Ventilation and High Frequency Ventilation as the Primary Modes of Respiratory Support for ELBW Babies: What Does the Evidence Say? Front. Pediatr. 2020, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Reyes, M.X.; Orrego-Rojas, P.A. Rescue high-frequency jet ventilation versus conventional ventilation for severe pulmonary dysfunction in preterm infants. Cochrane Database Syst. Rev. 2015, CD000437. [Google Scholar] [CrossRef]
- Hernandez, L.A.; Peevy, K.J.; Moise, A.A.; Parker, J.C. Chest wall restriction limits high airway pressure-induced lung injury in young rabbits. J. Appl. Physiol. 1989, 66, 2364–2368. [Google Scholar] [CrossRef]
- Keszler, M. Volume-targeted ventilation: One size does not fit all. Evidence-based recommendations for successful use. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F108–F112. [Google Scholar] [CrossRef]
- Tuzun, F.; Deliloglu, B.; Cengiz, M.M.; Iscan, B.; Duman, N.; Ozkan, H. Volume Guarantee High-Frequency Oscillatory Ventilation in Preterm Infants With RDS: Tidal Volume and DCO2 Levels for Optimal Ventilation Using Open-Lung Strategies. Front. Pediatr. 2020, 8, 105. [Google Scholar] [CrossRef]
- Peng, W.; Zhu, H.; Shi, H.; Liu, E. Volume-targeted ventilation is more suitable than pressure-limited ventilation for preterm infants: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F158–F165. [Google Scholar] [CrossRef]
- Liu, W.Q.; Xu, Y.; Han, A.M.; Meng, L.J.; Wang, J. A comparative study of two ventilation modes in the weaning phase of preterm infants with respiratory distress syndrome. Zhongguo Dang Dai Er Ke Za Zhi 2018, 20, 729–733. [Google Scholar]
- Thome, U.H.; Ambalavanan, N. Permissive hypercapnia to decrease lung injury in ventilated preterm neonates. Semin. Fetal Neonatal Med. 2009, 14, 21–27. [Google Scholar] [CrossRef]
- Kaiser, J.R.; Gauss, C.H.; Pont, M.M.; Williams, D.K. Hypercapnia during the first 3 days of life is associated with severe intraventricular hemorrhage in very low birth weight infants. J. Perinatol. 2006, 26, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Thome, U.H.; Genzel-Boroviczeny, O.; Bohnhorst, B.; Schmid, M.; Fuchs, H.; Rohde, O.; Avenarius, S.; Topf, H.G.; Zimmermann, A.; Faas, D.; et al. Permissive hypercapnia in extremely low birthweight infants (PHELBI): A randomised controlled multicentre trial. Lancet Respir. Med. 2015, 3, 534–543. [Google Scholar] [CrossRef]
- Thome, U.H.; Carroll, W.; Wu, T.J.; Johnson, R.B.; Roane, C.; Young, D.; Carlo, W.A. Outcome of extremely preterm infants randomized at birth to different PaCO2 targets during the first seven days of life. Biol Neonate 2006, 90, 218–225. [Google Scholar] [CrossRef]
- Thome, U.H.; Genzel-Boroviczeny, O.; Bohnhorst, B.; Schmid, M.; Fuchs, H.; Rohde, O.; Avenarius, S.; Topf, H.G.; Zimmermann, A.; Faas, D.; et al. Neurodevelopmental outcomes of extremely low birthweight infants randomised to different PCO2 targets: The PHELBI follow-up study. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F376–F382. [Google Scholar] [CrossRef]
- Askie, L.M.; Henderson-Smart, D.J.; Irwig, L.; Simpson, J.M. Oxygen-saturation targets and outcomes in extremely preterm infants. N. Engl. J. Med. 2003, 349, 959–967. [Google Scholar] [CrossRef]
- Askie, L.M.; Darlow, B.A.; Finer, N.; Schmidt, B.; Stenson, B.; Tarnow-Mordi, W.; Davis, P.G.; Carlo, W.A.; Brocklehurst, P.; Davies, L.C.; et al. Association Between Oxygen Saturation Targeting and Death or Disability in Extremely Preterm Infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. JAMA 2018, 319, 2190–2201. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Singh, B.; El-Naggar, W.; McMillan, D.D. Automated versus manual control of inspired oxygen to target oxygen saturation in preterm infants: A systematic review and meta-analysis. J. Perinatol. 2018, 38, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Kallio, M.; Koskela, U.; Peltoniemi, O.; Kontiokari, T.; Pokka, T.; Suo-Palosaari, M.; Saarela, T. Neurally adjusted ventilatory assist (NAVA) in preterm newborn infants with respiratory distress syndrome-a randomized controlled trial. Eur. J. Pediatr. 2016, 175, 1175–1183. [Google Scholar] [CrossRef]
- Robbins, M.; Trittmann, J.; Martin, E.; Reber, K.M.; Nelin, L.; Shepherd, E. Early extubation attempts reduce length of stay in extremely preterm infants even if re-intubation is necessary. J. Neonatal Perinat. Med. 2015, 8, 91–97. [Google Scholar] [CrossRef]
- Chawla, S.; Natarajan, G.; Shankaran, S.; Carper, B.; Brion, L.P.; Keszler, M.; Carlo, W.A.; Ambalavanan, N.; Gantz, M.G.; Das, A.; et al. Markers of Successful Extubation in Extremely Preterm Infants, and Morbidity After Failed Extubation. J. Pediatr. 2017, 189, 113–119 e112. [Google Scholar] [CrossRef]
- Manley, B.J.; Doyle, L.W.; Owen, L.S.; Davis, P.G. Extubating Extremely Preterm Infants: Predictors of Success and Outcomes following Failure. J. Pediatr. 2016, 173, 45–49. [Google Scholar] [CrossRef]
- Shalish, W.; Latremouille, S.; Papenburg, J.; Sant’Anna, G.M. Predictors of extubation readiness in preterm infants: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F89–F97. [Google Scholar] [CrossRef]
- Ferguson, K.N.; Roberts, C.T.; Manley, B.J.; Davis, P.G. Interventions to Improve Rates of Successful Extubation in Preterm Infants: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017, 171, 165–174. [Google Scholar] [CrossRef]
- Wang, C.S.; Kou, Y.F.; Shah, G.B.; Mitchell, R.B.; Johnson, R.F. Tracheostomy in Extremely Preterm Neonates in the United States: A Cross-Sectional Analysis. Laryngoscope 2020, 130, 2056–2062. [Google Scholar] [CrossRef]
- Donda, K.; Agyemang, C.O.; Adjetey, N.A.; Agyekum, A.; Princewill, N.; Ayensu, M.; Bray, L.; Yagnik, P.J.; Bhatt, P.; Dapaah-Siakwan, F. Tracheostomy trends in preterm infants with bronchopulmonary dysplasia in the United States: 2008–2017. Pediatr. Pulmonol. 2021. [Google Scholar] [CrossRef]
- Kurata, H.; Ochiai, M.; Inoue, H.; Ichiyama, M.; Yasuoka, K.; Fujiyoshi, J.; Matsushita, Y.; Honjo, S.; Sakai, Y.; Ohga, S.; et al. A nationwide survey on tracheostomy for very-low-birth-weight infants in Japan. Pediatr. Pulmonol. 2019, 54, 53–60. [Google Scholar] [CrossRef]
- DeMauro, S.B.; D’Agostino, J.A.; Bann, C.; Bernbaum, J.; Gerdes, M.; Bell, E.F.; Carlo, W.A.; D’Angio, C.T.; Das, A.; Higgins, R.; et al. Developmental outcomes of very preterm infants with tracheostomies. J. Pediatr. 2014, 164, 1303–1310.e2. [Google Scholar] [CrossRef]
- Luo, J.; Shepard, S.; Nilan, K.; Wood, A.; Monk, H.M.; Jensen, E.A.; Harrington, A.T.; Maschhoff, K.; Kirpalani, H.; Feng, Z.; et al. Improved growth and developmental activity post tracheostomy in preterm infants with severe BPD. Pediatr. Pulmonol. 2018, 53, 1237–1244. [Google Scholar] [CrossRef]
- Wood, W.; Wang, C.S.; Mitchell, R.B.; Shah, G.B.; Johnson, R.F. A Longitudinal Analysis of Outcomes in Tracheostomy Placement Among Preterm Infants. Laryngoscope 2021, 131, 417–422. [Google Scholar] [CrossRef]
- Com, G.; Kuo, D.Z.; Bauer, M.L.; Lenker, C.V.; Melguizo-Castro, M.M.; Nick, T.G.; Makris, C.M. Outcomes of children treated with tracheostomy and positive-pressure ventilation at home. Clin. Pediatr. (Phila) 2013, 52, 54–61. [Google Scholar] [CrossRef]
- Cristea, A.I.; Carroll, A.E.; Davis, S.D.; Swigonski, N.L.; Ackerman, V.L. Outcomes of children with severe bronchopulmonary dysplasia who were ventilator dependent at home. Pediatrics 2013, 132, e727–e734. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Halliday, H.L.; Ehrenkranz, R.A.; Davis, P.G.; Sinclair, J.C. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: Effect modification by risk of bronchopulmonary dysplasia. J. Pediatr. 2014, 165, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Cheong, J.L.; Ehrenkranz, R.A.; Halliday, H.L. Late (>7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017, 10, CD001145. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Cheong, J.L.; Ehrenkranz, R.A.; Halliday, H.L. Early (<8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017, 10, CD001146. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Tian, J.; Song, F.; Li, W.; Jiang, L.; Gui, G.; Zhang, Y.; Ge, L.; Shi, J.; Sun, X.; et al. Corticosteroids for the prevention of bronchopulmonary dysplasia in preterm infants: A network meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F506–F511. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, M.L.; Baud, O.; Lacaze-Masmonteil, T.; Peltoniemi, O.M.; Bonsante, F.; Watterberg, K.L. Effect of Prophylaxis for Early Adrenal Insufficiency Using Low-Dose Hydrocortisone in Very Preterm Infants: An Individual Patient Data Meta-Analysis. J. Pediatr. 2019, 207, 136–142.e5. [Google Scholar] [CrossRef] [PubMed]
- Onland, W.; Cools, F.; Kroon, A.; Rademaker, K.; Merkus, M.P.; Dijk, P.H.; van Straaten, H.L.; Te Pas, A.B.; Mohns, T.; Bruneel, E.; et al. Effect of Hydrocortisone Therapy Initiated 7 to 14 Days After Birth on Mortality or Bronchopulmonary Dysplasia Among Very Preterm Infants Receiving Mechanical Ventilation: A Randomized Clinical Trial. JAMA 2019, 321, 354–363. [Google Scholar] [CrossRef]
- Venkataraman, R.; Kamaluddeen, M.; Hasan, S.U.; Robertson, H.L.; Lodha, A. Intratracheal Administration of Budesonide-Surfactant in Prevention of Bronchopulmonary Dysplasia in Very Low Birth Weight Infants: A Systematic Review and Meta-Analysis. Pediatr. Pulmonol. 2017, 52, 968–975. [Google Scholar] [CrossRef]
- Shinwell, E.S.; Portnov, I.; Meerpohl, J.J.; Karen, T.; Bassler, D. Inhaled Corticosteroids for Bronchopulmonary Dysplasia: A Meta-analysis. Pediatrics 2016, 138. [Google Scholar] [CrossRef]
- Bassler, D.; Plavka, R.; Shinwell, E.S.; Hallman, M.; Jarreau, P.H.; Carnielli, V.; Van den Anker, J.N.; Meisner, C.; Engel, C.; Schwab, M.; et al. Early Inhaled Budesonide for the Prevention of Bronchopulmonary Dysplasia. N. Engl. J. Med. 2015, 373, 1497–1506. [Google Scholar] [CrossRef]
- Bassler, D.; Shinwell, E.S.; Hallman, M.; Jarreau, P.H.; Plavka, R.; Carnielli, V.; Meisner, C.; Engel, C.; Koch, A.; Kreutzer, K.; et al. Long-Term Effects of Inhaled Budesonide for Bronchopulmonary Dysplasia. N. Engl. J. Med. 2018, 378, 148–157. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiu, W.; Lin, Y.; Ren, Y.; Zhang, B.; Yang, C. Long-term effects of the intratracheal administration of corticosteroids for the prevention of bronchopulmonary dysplasia: A meta-analysis. Pediatr. Pulmonol. 2019, 54, 1722–1734. [Google Scholar] [CrossRef]
- Jakkula, M.; Le Cras, T.D.; Gebb, S.; Hirth, K.P.; Tuder, R.M.; Voelkel, N.F.; Abman, S.H. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L600–L607. [Google Scholar] [CrossRef] [PubMed]
- Mourani, P.M.; Mandell, E.W.; Meier, M.; Younoszai, A.; Brinton, J.T.; Wagner, B.D.; Arjaans, S.; Poindexter, B.B.; Abman, S.H. Early Pulmonary Vascular Disease in Preterm Infants Is Associated with Late Respiratory Outcomes in Childhood. Am. J. Respir. Crit. Care Med. 2019, 199, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Afshar, S.; Gibson, L.L.; Yuhanna, I.S.; Sherman, T.S.; Kerecman, J.D.; Grubb, P.H.; Yoder, B.A.; McCurnin, D.C.; Shaul, P.W. Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L749–L758. [Google Scholar] [CrossRef] [PubMed]
- Askie, L.M.; Ballard, R.A.; Cutter, G.R.; Dani, C.; Elbourne, D.; Field, D.; Hascoet, J.M.; Hibbs, A.M.; Kinsella, J.P.; Mercier, J.C.; et al. Inhaled nitric oxide in preterm infants: An individual-patient data meta-analysis of randomized trials. Pediatrics 2011, 128, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Cole, F.S.; Alleyne, C.; Barks, J.D.; Boyle, R.J.; Carroll, J.L.; Dokken, D.; Edwards, W.H.; Georgieff, M.; Gregory, K.; Johnston, M.V.; et al. NIH Consensus Development Conference statement: Inhaled nitric-oxide therapy for premature infants. Pediatrics 2011, 127, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Donohue, P.K.; Gilmore, M.M.; Cristofalo, E.; Wilson, R.F.; Weiner, J.Z.; Lau, B.D.; Robinson, K.A.; Allen, M.C. Inhaled nitric oxide in preterm infants: A systematic review. Pediatrics 2011, 127, e414–e422. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.C.; Donohue, P.; Gilmore, M.; Cristofalo, E.; Wilson, R.F.; Weiner, J.Z.; Robinson, K. Inhaled nitric oxide in preterm infants. Evid. Rep. Technol. Assess. (Full Rep.) 2010, 195, 1–135. [Google Scholar]
- Barrington, K.J.; Finer, N.; Pennaforte, T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst. Rev. 2017, 1, CD000509. [Google Scholar] [CrossRef]
- Hasan, S.U.; Potenziano, J.; Konduri, G.G.; Perez, J.A.; Van Meurs, K.P.; Walker, M.W.; Yoder, B.A.; The Newborns Treated With Nitric Oxide (NEWNO) Trial Group. Effect of Inhaled Nitric Oxide on Survival Without Bronchopulmonary Dysplasia in Preterm Infants: A Randomized Clinical Trial. JAMA Pediatr. 2017, 171, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Askie, L.M.; Davies, L.C.; Schreiber, M.D.; Hibbs, A.M.; Ballard, P.L.; Ballard, R.A. Race Effects of Inhaled Nitric Oxide in Preterm Infants: An Individual Participant Data Meta-Analysis. J. Pediatr. 2018, 193, 34–39.e2. [Google Scholar] [CrossRef] [PubMed]
- Rotschild, A.; Solimano, A.; Puterman, M.; Smyth, J.; Sharma, A.; Albersheim, S. Increased compliance in response to salbutamol in premature infants with developing bronchopulmonary dysplasia. J. Pediatr. 1989, 115, 984–991. [Google Scholar] [CrossRef]
- Khalaf, M.N.; Hurley, J.F.; Bhandari, V. A prospective controlled trial of albuterol aerosol delivered via metered dose inhaler-spacer device (MDI) versus jet nebulizer in ventilated preterm neonates. Am. J. Perinatol. 2001, 18, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Pfenninger, J.; Aebi, C. Respiratory response to salbutamol (albuterol) in ventilator-dependent infants with chronic lung disease: Pressurized aerosol delivery versus intravenous injection. Intensive Care Med. 1993, 19, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Ng, G.; da Silva, O.; Ohlsson, A. Bronchodilators for the prevention and treatment of chronic lung disease in preterm infants. Cochrane Database Syst. Rev. 2016, 12, CD003214. [Google Scholar] [CrossRef] [PubMed]
- Armanian, A.M.; Badiee, Z.; Afghari, R.; Salehimehr, N.; Hassanzade, A.; Sheikhzadeh, S.; Shariftehrani, M.; Rezvan, G. Reducing the incidence of chronic lung disease in very premature infants with aminophylline. Int. J. Prev. Med. 2014, 5, 569–576. [Google Scholar]
- Denjean, A.; Paris-Llado, J.; Zupan, V.; Debillon, T.; Kieffer, F.; Magny, J.F.; Desfreres, L.; Llanas, B.; Guimaraes, H.; Moriette, G.; et al. Inhaled salbutamol and beclomethasone for preventing broncho-pulmonary dysplasia: A randomised double-blind study. Eur. J. Pediatr. 1998, 157, 926–931. [Google Scholar] [CrossRef]
- Clouse, B.J.; Jadcherla, S.R.; Slaughter, J.L. Systematic Review of Inhaled Bronchodilator and Corticosteroid Therapies in Infants with Bronchopulmonary Dysplasia: Implications and Future Directions. PLoS ONE 2016, 11, e0148188. [Google Scholar] [CrossRef]
- Euteneuer, J.C.; Kerns, E.; Leiting, C.; McCulloh, R.J.; Peeples, E.S. Inhaled bronchodilator exposure in the management of bronchopulmonary dysplasia in hospitalized infants. J. Perinatol. 2021, 41, 53–61. [Google Scholar] [CrossRef]
- Chu, A.; de St Maurice, A.; Sim, M.S.; Kallapur, S.G. Neonatal Mycoplasma and Ureaplasma Infections. Pediatr. Ann. 2020, 49, e305–e312. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.E.; Ohlsson, A.; Kellner, J.D. Association of Ureaplasma urealyticum colonization with chronic lung disease of prematurity: Results of a metaanalysis. J. Pediatr. 1995, 127, 640–644. [Google Scholar] [CrossRef]
- Schelonka, R.L.; Katz, B.; Waites, K.B.; Benjamin, D.K., Jr. Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr. Infect. Dis. J. 2005, 24, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Lal, C.V.; Wagner, B.D.; Mourani, P.M.; Lohmann, P.; Luna, R.A.; Sisson, A.; Shivanna, B.; Hollister, E.B.; Abman, S.H.; et al. Airway Microbiome and Development of Bronchopulmonary Dysplasia in Preterm Infants: A Systematic Review. J. Pediatr. 2019, 204, 126–133.e2. [Google Scholar] [CrossRef] [PubMed]
- Glaser, K.; Gradzka-Luczewska, A.; Szymankiewicz-Breborowicz, M.; Kawczynska-Leda, N.; Henrich, B.; Waaga-Gasser, A.M.; Speer, C.P. Perinatal Ureaplasma Exposure Is Associated With Increased Risk of Late Onset Sepsis and Imbalanced Inflammation in Preterm Infants and May Add to Lung Injury. Front. Cell. Infect. Microbiol. 2019, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.; Watkins, W.J.; Edwards, M.O.; Spiller, O.B.; Jacqz-Aigrain, E.; Kotecha, S.J.; Kotecha, S. Association between pulmonary ureaplasma colonization and bronchopulmonary dysplasia in preterm infants: Updated systematic review and meta-analysis. Pediatr. Infect. Dis. J. 2014, 33, 697–702. [Google Scholar] [CrossRef]
- Viscardi, R.M.; Kallapur, S.G. Role of Ureaplasma Respiratory Tract Colonization in Bronchopulmonary Dysplasia Pathogenesis: Current Concepts and Update. Clin. Perinatol. 2015, 42, 719–738. [Google Scholar] [CrossRef]
- Mabanta, C.G.; Pryhuber, G.S.; Weinberg, G.A.; Phelps, D.L. Erythromycin for the prevention of chronic lung disease in intubated preterm infants at risk for, or colonized or infected with Ureaplasma urealyticum. Cochrane Database Syst. Rev. 2003, CD003744. [Google Scholar] [CrossRef]
- Nair, V.; Loganathan, P.; Soraisham, A.S. Azithromycin and other macrolides for prevention of bronchopulmonary dysplasia: A systematic review and meta-analysis. Neonatology 2014, 106, 337–347. [Google Scholar] [CrossRef]
- Razak, A.; Alshehri, N. Azithromycin for preventing bronchopulmonary dysplasia in preterm infants: A systematic review and meta-analysis. Pediatr. Pulmonol. 2020, 31, 31. [Google Scholar] [CrossRef]
- Lowe, J.; Gillespie, D.; Hubbard, M.; Zhang, L.; Kirby, N.; Pickles, T.; Thomas-Jones, E.; Turner, M.A.; Klein, N.; Marchesi, J.R.; et al. Study protocol: Azithromycin therapy for chronic lung disease of prematurity (AZTEC)—A randomised, placebo-controlled trial of azithromycin for the prevention of chronic lung disease of prematurity in preterm infants. BMJ Open 2020, 10, e041528. [Google Scholar] [CrossRef] [PubMed]
- Piersigilli, F.; Van Grambezen, B.; Hocq, C.; Danhaive, O. Nutrients and Microbiota in Lung Diseases of Prematurity: The Placenta-Gut-Lung Triangle. Nutrients 2020, 12, 469. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.Y.; Synnes, A.; Roberts, A.; Deshpandey, A.; Dow, K.; Yoon, E.W.; Lee, K.S.; Dobson, S.; Lee, S.K.; Shah, P.S.; et al. Association Between Antibiotic Use and Neonatal Mortality and Morbidities in Very Low-Birth-Weight Infants Without Culture-Proven Sepsis or Necrotizing Enterocolitis. JAMA Pediatr. 2016, 170, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Sengupta, S.; Puopolo, K.M. Challenges and opportunities for antibiotic stewardship among preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F327–F332. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.I.; Chang, Y.S.; Kim, J.; Choi, J.H.; Ahn, S.Y.; Park, W.S. Natural evolution of ductus arteriosus with noninterventional conservative management in extremely preterm infants born at 23–28 weeks of gestation. PLoS ONE 2019, 14, e0212256. [Google Scholar] [CrossRef]
- Hamrick, S.E.G.; Sallmon, H.; Rose, A.T.; Porras, D.; Shelton, E.L.; Reese, J.; Hansmann, G. Patent Ductus Arteriosus of the Preterm Infant. Pediatrics 2020, 146. [Google Scholar] [CrossRef]
- Mitra, S.; McNamara, P.J. Patent Ductus Arteriosus-Time for a Definitive Trial. Clin. Perinatol. 2020, 47, 617–639. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, X.; Li, D.; Shi, Y. Related Factors of Patent Ductus Arteriosus in Preterm Infants: A Systematic Review and Meta-Analysis. Front. Pediatr. 2020, 8, 605879. [Google Scholar] [CrossRef] [PubMed]
- Hundscheid, T.; Onland, W.; van Overmeire, B.; Dijk, P.; van Kaam, A.; Dijkman, K.P.; Kooi, E.M.W.; Villamor, E.; Kroon, A.A.; Visser, R.; et al. Early treatment versus expectative management of patent ductus arteriosus in preterm infants: A multicentre, randomised, non-inferiority trial in Europe (BeNeDuctus trial). BMC Pediatr. 2018, 18, 262. [Google Scholar] [CrossRef]
- Fowlie, P.W.; Davis, P.G.; McGuire, W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst. Rev. 2010, CD000174. [Google Scholar] [CrossRef]
- Evans, P.; O’Reilly, D.; Flyer, J.N.; Soll, R.; Mitra, S. Indomethacin for symptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst. Rev. 2021, 1, CD013133. [Google Scholar] [CrossRef]
- Ohlsson, A.; Shah, P.S. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2020, 1, CD010061. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Shah, S.S. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst. Rev. 2020, 1, CD004213. [Google Scholar] [CrossRef]
- Ohlsson, A.; Walia, R.; Shah, S.S. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst. Rev. 2020, 2, CD003481. [Google Scholar] [CrossRef]
- Mitra, S.; Florez, I.D.; Tamayo, M.E.; Mbuagbaw, L.; Vanniyasingam, T.; Veroniki, A.A.; Zea, A.M.; Zhang, Y.; Sadeghirad, B.; Thabane, L. Association of Placebo, Indomethacin, Ibuprofen, and Acetaminophen With Closure of Hemodynamically Significant Patent Ductus Arteriosus in Preterm Infants: A Systematic Review and Meta-analysis. JAMA 2018, 319, 1221–1238. [Google Scholar] [CrossRef]
- Marconi, E.; Bettiol, A.; Ambrosio, G.; Perduca, V.; Vannacci, A.; Troiani, S.; Dani, C.; Mugelli, A.; Lucenteforte, E. Efficacy and safety of pharmacological treatments for patent ductus arteriosus closure: A systematic review and network meta-analysis of clinical trials and observational studies. Pharm. Res. 2019, 148, 104418. [Google Scholar] [CrossRef] [PubMed]
- Bixler, G.M.; Powers, G.C.; Clark, R.H.; Walker, M.W.; Tolia, V.N. Changes in the Diagnosis and Management of Patent Ductus Arteriosus from 2006 to 2015 in United States Neonatal Intensive Care Units. J. Pediatr. 2017, 189, 105–112. [Google Scholar] [CrossRef]
- Weisz, D.E.; More, K.; McNamara, P.J.; Shah, P.S. PDA ligation and health outcomes: A meta-analysis. Pediatrics 2014, 133, e1024–e1046. [Google Scholar] [CrossRef]
- Weisz, D.E.; Mirea, L.; Rosenberg, E.; Jang, M.; Ly, L.; Church, P.T.; Kelly, E.; Kim, S.J.; Jain, A.; McNamara, P.J.; et al. Association of Patent Ductus Arteriosus Ligation With Death or Neurodevelopmental Impairment Among Extremely Preterm Infants. JAMA Pediatr. 2017, 171, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.H.; Rivera, B.K.; Bridge, J.A.; Armstrong, A.K.; Boe, B.A.; Berman, D.P.; Fick, T.; Holzer, R.J.; Hijazi, Z.M.; Abadir, S.; et al. Percutaneous Patent Ductus Arteriosus (PDA) Closure During Infancy: A Meta-analysis. Pediatrics 2017, 139. [Google Scholar] [CrossRef]
- Sathanandam, S.K.; Gutfinger, D.; O’Brien, L.; Forbes, T.J.; Gillespie, M.J.; Berman, D.P.; Armstrong, A.K.; Shahanavaz, S.; Jones, T.K.; Morray, B.H.; et al. Amplatzer Piccolo Occluder clinical trial for percutaneous closure of the patent ductus arteriosus in patients >/=700 grams. Catheter. Cardiovasc. Interv. 2020, 96, 1266–1276. [Google Scholar] [CrossRef]
- Semberova, J.; Sirc, J.; Miletin, J.; Kucera, J.; Berka, I.; Sebkova, S.; O’Sullivan, S.; Franklin, O.; Stranak, Z. Spontaneous Closure of Patent Ductus Arteriosus in Infants </=1500 g. Pediatrics 2017, 140. [Google Scholar] [CrossRef]
- Mitra, S.; Scrivens, A.; von Kursell, A.M.; Disher, T. Early treatment versus expectant management of hemodynamically significant patent ductus arteriosus for preterm infants. Cochrane Database Syst. Rev. 2020, 12, CD013278. [Google Scholar] [CrossRef]
- Sung, S.I.; Chang, Y.S.; Ahn, S.Y.; Jo, H.S.; Yang, M.; Park, W.S. Conservative Non-intervention Approach for Hemodynamically Significant Patent Ductus Arteriosus in Extremely Preterm Infants. Front. Pediatr. 2020, 8, 605134. [Google Scholar] [CrossRef]
- Letshwiti, J.B.; Semberova, J.; Pichova, K.; Dempsey, E.M.; Franklin, O.M.; Miletin, J. A conservative treatment of patent ductus arteriosus in very low birth weight infants. Early Hum. Dev. 2017, 104, 45–49. [Google Scholar] [CrossRef]
- Clyman, R.I.; Hills, N.K.; Liebowitz, M.; Johng, S. Relationship between Duration of Infant Exposure to a Moderate-to-Large Patent Ductus Arteriosus Shunt and the Risk of Developing Bronchopulmonary Dysplasia or Death Before 36 Weeks. Am. J. Perinatol. 2020, 37, 216–223. [Google Scholar] [CrossRef]
- Stephens, B.E.; Gargus, R.A.; Walden, R.V.; Mance, M.; Nye, J.; McKinley, L.; Tucker, R.; Vohr, B.R. Fluid regimens in the first week of life may increase risk of patent ductus arteriosus in extremely low birth weight infants. J. Perinatol. 2008, 28, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.F.; Acarregui, M.J. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2014, CD000503. [Google Scholar] [CrossRef] [PubMed]
- Barrington, K.J.; Fortin-Pellerin, E.; Pennaforte, T. Fluid restriction for treatment of preterm infants with chronic lung disease. Cochrane Database Syst. Rev. 2017, 2, CD005389. [Google Scholar] [CrossRef]
- Oh, W.; Poindexter, B.B.; Perritt, R.; Lemons, J.A.; Bauer, C.R.; Ehrenkranz, R.A.; Stoll, B.J.; Poole, K.; Wright, L.L.; Neonatal Research Network. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J. Pediatr. 2005, 147, 786–790. [Google Scholar] [CrossRef]
- Marshall, D.D.; Kotelchuck, M.; Young, T.E.; Bose, C.L.; Kruyer, L.; O’Shea, T.M. Risk factors for chronic lung disease in the surfactant era: A North Carolina population-based study of very low birth weight infants. North Carolina Neonatologists Association. Pediatrics 1999, 104, 1345–1350. [Google Scholar] [CrossRef]
- Wemhoner, A.; Ortner, D.; Tschirch, E.; Strasak, A.; Rudiger, M. Nutrition of preterm infants in relation to bronchopulmonary dysplasia. BMC Pulm. Med. 2011, 11, 7. [Google Scholar] [CrossRef]
- Uberos, J.; Jimenez-Montilla, S.; Molina-Oya, M.; Garcia-Serrano, J.L. Early energy restriction in premature infants and bronchopulmonary dysplasia: A cohort study. Br. J. Nutr. 2020, 123, 1024–1031. [Google Scholar] [CrossRef]
- Greenberg, J.M.; Poindexter, B.B.; Shaw, P.A.; Bellamy, S.L.; Keller, R.L.; Moore, P.E.; McPherson, C.; Ryan, R.M. Respiratory medication use in extremely premature (<29 weeks) infants during initial NICU hospitalization: Results from the prematurity and respiratory outcomes program. Pediatr. Pulmonol. 2020, 55, 360–368. [Google Scholar] [CrossRef]
- Bamat, N.A.; Nelin, T.D.; Eichenwald, E.C.; Kirpalani, H.; Laughon, M.M.; Jackson, W.M.; Jensen, E.A.; Gibbs, K.A.; Lorch, S.A. Loop Diuretics in Severe Bronchopulmonary Dysplasia: Cumulative Use and Associations with Mortality and Age at Discharge. J. Pediatr. 2020. [Google Scholar] [CrossRef]
- Brion, L.P.; Primhak, R.A.; Yong, W. Aerosolized diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst. Rev. 2006, CD001694. [Google Scholar] [CrossRef]
- Stewart, A.; Brion, L.P. Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst. Rev. 2011, CD001453. [Google Scholar] [CrossRef]
- Stewart, A.; Brion, L.P.; Ambrosio-Perez, I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst. Rev. 2011, CD001817. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.G.; Gayam, S.; Savage, D.; Tong, A.; Gorham, D.; Sholomon, A.; Clark, R.H.; Benjamin, D.K.; Laughon, M.; Smith, P.B.; et al. Furosemide Exposure and Prevention of Bronchopulmonary Dysplasia in Premature Infants. J. Pediatr. 2019, 208, 134–140.e2. [Google Scholar] [CrossRef]
- Pacifici, G.M. Clinical pharmacology of furosemide in neonates: A review. Pharmaceuticals (Basel) 2013, 6, 1094–1129. [Google Scholar] [CrossRef] [PubMed]
- de Meer, K.; Westerterp, K.R.; Houwen, R.H.; Brouwers, H.A.; Berger, R.; Okken, A. Total energy expenditure in infants with bronchopulmonary dysplasia is associated with respiratory status. Eur. J. Pediatr. 1997, 156, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Malikiwi, A.I.; Lee, Y.M.; Davies-Tuck, M.; Wong, F.Y. Postnatal nutritional deficit is an independent predictor of bronchopulmonary dysplasia among extremely premature infants born at or less than 28weeks gestation. Early Hum. Dev. 2019, 131, 29–35. [Google Scholar] [CrossRef]
- Lai, N.M.; Rajadurai, S.V.; Tan, K.H. Increased energy intake for preterm infants with (or developing) bronchopulmonary dysplasia/chronic lung disease. Cochrane Database Syst. Rev. 2006, CD005093. [Google Scholar] [CrossRef] [PubMed]
- Milanesi, B.G.; Lima, P.A.; Villela, L.D.; Martins, A.S.; Gomes-Junior, S.C.S.; Moreira, M.E.L.; Meio, M. Assessment of early nutritional intake in preterm infants with bronchopulmonary dysplasia: A cohort study. Eur. J. Pediatr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, H.; Geng, H.; Cui, N.; Huang, F.; Zhu, X.; Zhu, X. Prediction of Bronchopulmonary Dysplasia in Preterm Infants Using Postnatal Risk Factors. Front. Pediatr. 2020, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Moschino, L.; Duci, M.; Fascetti Leon, F.; Bonadies, L.; Priante, E.; Baraldi, E.; Verlato, G. Optimizing Nutritional Strategies to Prevent Necrotizing Enterocolitis and Growth Failure after Bowel Resection. Nutrients 2021, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Villamor-Martinez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Kramer, B.W.; Villamor, E. Donor Human Milk Protects against Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 238. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Tang, J.; Shi, J.; Qu, Y.; Xiong, T.; Mu, D. Human milk as a protective factor for bronchopulmonary dysplasia: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F128–F136. [Google Scholar] [CrossRef]
- Villamor-Martinez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Villamor, E. Mother’s Own Milk and Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Front. Pediatr. 2019, 7, 224. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Boquien, C.Y.; King, C.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of Human Milk for Preterm Infants: Update and Recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front. Pediatr. 2019, 7, 76. [Google Scholar] [CrossRef]
- Fabrizio, V.; Trzaski, J.M.; Brownell, E.A.; Esposito, P.; Lainwala, S.; Lussier, M.M.; Hagadorn, J.I. Individualized versus standard diet fortification for growth and development in preterm infants receiving human milk. Cochrane Database Syst. Rev. 2020, 11, CD013465. [Google Scholar] [CrossRef]
- Jain, D.; Bancalari, E. Prevention of bronchopulmonary dysplasia: Current strategies. Zhongguo Dang Dai Er Ke Za Zhi 2017, 19, 841–851. [Google Scholar]
- Shenai, J.P.; Kennedy, K.A.; Chytil, F.; Stahlman, M.T. Clinical trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia. J. Pediatr. 1987, 111, 269–277. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Z.; Lu, Y. Vitamin A supplementation prevents the bronchopulmonary dysplasia in premature infants: A systematic review and meta-analysis. Medicine (Baltimore) 2021, 100, e23101. [Google Scholar] [CrossRef]
- Hustead, V.A.; Gutcher, G.R.; Anderson, S.A.; Zachman, R.D. Relationship of vitamin A (retinol) status to lung disease in the preterm infant. J. Pediatr. 1984, 105, 610–615. [Google Scholar] [CrossRef]
- Darlow, B.A.; Graham, P.J.; Rojas-Reyes, M.X. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database Syst. Rev. 2016, CD000501. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Kato, S.; Namba, F.; Ota, E. Vitamin A to prevent bronchopulmonary dysplasia in extremely low birth weight infants: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0207730. [Google Scholar] [CrossRef]
- Garg, B.D.; Bansal, A.; Kabra, N.S. Role of vitamin A supplementation in prevention of bronchopulmonary dysplasia in extremely low birth weight neonates: A systematic review of randomized trials. J. Matern. Fetal Neonatal Med. 2019, 32, 2608–2615. [Google Scholar] [CrossRef] [PubMed]
- Tolia, V.N.; Murthy, K.; McKinley, P.S.; Bennett, M.M.; Clark, R.H. The effect of the national shortage of vitamin A on death or chronic lung disease in extremely low-birth-weight infants. JAMA Pediatrics 2014, 168, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Rakshasbhuvankar, A.A.; Simmer, K.; Patole, S.K.; Stoecklin, B.; Nathan, E.A.; Clarke, M.W.; Pillow, J.J. Enteral Vitamin A for Reducing Severity of Bronchopulmonary Dysplasia: A Randomized Trial. Pediatrics 2021, 147. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Khanna, P.; Srivastava, R.; Kumar, A. Oral vitamin A supplementation in very low birth weight neonates: A randomized controlled trial. Eur. J. Pediatr. 2019, 178, 1255–1265. [Google Scholar] [CrossRef]
- Tyson, J.E.; Wright, L.L.; Oh, W.; Kennedy, K.A.; Mele, L.; Ehrenkranz, R.A.; Stoll, B.J.; Lemons, J.A.; Stevenson, D.K.; Bauer, C.R.; et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N. Engl. J. Med. 1999, 340, 1962–1968. [Google Scholar] [CrossRef]
- Sun, H.; Cheng, R.; Wang, Z. Early Vitamin a Supplementation Improves the Outcome of Retinopathy of Prematurity in Extremely Preterm Infants. Retina 2020, 40, 1176–1184. [Google Scholar] [CrossRef]
- Meyer, S.; Gortner, L.; NeoVitaA Trial investigators. Up-date on the NeoVitaA Trial: Obstacles, challenges, perspectives, and local experiences. Wien. Med. Wochenschr. 2017, 167, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Calisici, E.; Yarci, E.; Degirmencioglu, H.; Oncel, M.; Oguz, S.; Uras, N.; Dilmen, U. PO-0731 The Effects of Early Oral Vitamin a Treatment on the Prevention of Bronchopulmonary Displasia in the Low Birth Weight Infants. Arch. Dis. Child. 2014, 99, A494. [Google Scholar] [CrossRef]
- Meyer, S.; Kronfeld, K.; Graber, S.; Butzer, R.; Wahl, H.; Gortner, L. Vitamin A to prevent bronchopulmonary dysplasia: The NeoVitaA trial. J. Matern. Fetal Neonatal Med. 2013, 26, 544–545. [Google Scholar] [CrossRef]
- Kumar, V.H.S. Diagnostic Approach to Pulmonary Hypertension in Premature Neonates. Children (Basel) 2017, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Arjaans, S.; Zwart, E.A.H.; Ploegstra, M.J.; Bos, A.F.; Kooi, E.M.W.; Hillege, H.L.; Berger, R.M.F. Identification of gaps in the current knowledge on pulmonary hypertension in extremely preterm infants: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2018, 32, 258–267. [Google Scholar] [CrossRef]
- Al-Ghanem, G.; Shah, P.; Thomas, S.; Banfield, L.; El Helou, S.; Fusch, C.; Mukerji, A. Bronchopulmonary dysplasia and pulmonary hypertension: A meta-analysis. J. Perinatol. 2017, 37, 414–419. [Google Scholar] [CrossRef]
- Lagatta, J.M.; Hysinger, E.B.; Zaniletti, I.; Wymore, E.M.; Vyas-Read, S.; Yallapragada, S.; Nelin, L.D.; Truog, W.E.; Padula, M.A.; Porta, N.F.M.; et al. The Impact of Pulmonary Hypertension in Preterm Infants with Severe Bronchopulmonary Dysplasia through 1 Year. J. Pediatr. 2018, 203, 218–224 e213. [Google Scholar] [CrossRef]
- Arjaans, S.; Haarman, M.G.; Roofthooft, M.T.R.; Fries, M.W.F.; Kooi, E.M.W.; Bos, A.F.; Berger, R.M.F. Fate of pulmonary hypertension associated with bronchopulmonary dysplasia beyond 36 weeks postmenstrual age. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 45–50. [Google Scholar] [CrossRef]
- Altit, G.; Bhombal, S.; Hopper, R.K.; Tacy, T.A.; Feinstein, J. Death or resolution: The “natural history” of pulmonary hypertension in bronchopulmonary dysplasia. J. Perinatol. 2019, 39, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Mourani, P.M.; Sontag, M.K.; Younoszai, A.; Miller, J.I.; Kinsella, J.P.; Baker, C.D.; Poindexter, B.B.; Ingram, D.A.; Abman, S.H. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2015, 191, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Hilgendorff, A.; Apitz, C.; Bonnet, D.; Hansmann, G. Pulmonary hypertension associated with acute or chronic lung diseases in the preterm and term neonate and infant. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016, 102 (Suppl. 2), ii49–ii56. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, G.; Koestenberger, M.; Alastalo, T.P.; Apitz, C.; Austin, E.D.; Bonnet, D.; Budts, W.; D’Alto, M.; Gatzoulis, M.A.; Hasan, B.S.; et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J. Heart Lung Transplant. 2019, 38, 879–901. [Google Scholar] [CrossRef] [PubMed]
- Baczynski, M.; Kelly, E.; McNamara, P.J.; Shah, P.S.; Jain, A. Short and long-term outcomes of chronic pulmonary hypertension in preterm infants managed using a standardized algorithm. Pediatr. Pulmonol. 2020. [Google Scholar] [CrossRef]
- Laliberte, C.; Hanna, Y.; Ben Fadel, N.; Lemyre, B.; Bijelic, V.; Barrowman, N.; Hoey, L.; Thebaud, B.; Katz, S.L. Target oxygen saturation and development of pulmonary hypertension and increased pulmonary vascular resistance in preterm infants. Pediatr. Pulmonol. 2019, 54, 73–81. [Google Scholar] [CrossRef]
- Chandrasekharan, P.; Lakshminrusimha, S. Oxygen therapy in preterm infants with pulmonary hypertension. Semin. Fetal Neonatal Med. 2020, 25, 101070. [Google Scholar] [CrossRef]
- Laux, D.; Rocchisani, M.A.; Boudjemline, Y.; Gouton, M.; Bonnet, D.; Ovaert, C. Pulmonary Hypertension in the Preterm Infant with Chronic Lung Disease can be Caused by Pulmonary Vein Stenosis: A Must-Know Entity. Pediatr. Cardiol. 2016, 37, 313–321. [Google Scholar] [CrossRef]
- van der Graaf, M.; Rojer, L.A.; Helbing, W.; Reiss, I.; Etnel, J.R.G.; Bartelds, B. EXPRESS: Sildenafil for bronchopulmonary dysplasia and pulmonary hypertension: A meta-analysis. Pulm. Circ. 2019, 2045894019837875. [Google Scholar] [CrossRef]
- FDA. Drug Safety Communication: FDA Recommends against Use of Revatio (Sildenafil) in Children With pulmonary Hypertension; FDA: Silver Spring, MD, USA, 2012. [Google Scholar]
- Unegbu, C.; Noje, C.; Coulson, J.D.; Segal, J.B.; Romer, L. Pulmonary Hypertension Therapy and a Systematic Review of Efficacy and Safety of PDE-5 Inhibitors. Pediatrics 2017, 139. [Google Scholar] [CrossRef]
- Nitkin, C.R.; Rajasingh, J.; Pisano, C.; Besner, G.E.; Thebaud, B.; Sampath, V. Stem cell therapy for preventing neonatal diseases in the 21st century: Current understanding and challenges. Pediatr. Res. 2020, 87, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Augustine, S.; Cheng, W.; Avey, M.T.; Chan, M.L.; Lingappa, S.M.C.; Hutton, B.; Thebaud, B. Are all stem cells equal? Systematic review, evidence map, and meta-analyses of preclinical stem cell-based therapies for bronchopulmonary dysplasia. Stem. Cells Transl. Med. 2020, 9, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Namba, F. Mesenchymal stem cells for the prevention of bronchopulmonary dysplasia. Pediatr. Int. 2019, 61, 945–950. [Google Scholar] [CrossRef]
- Augustine, S.; Avey, M.T.; Harrison, B.; Locke, T.; Ghannad, M.; Moher, D.; Thebaud, B. Mesenchymal Stromal Cell Therapy in Bronchopulmonary Dysplasia: Systematic Review and Meta-Analysis of Preclinical Studies. Stem. Cells Transl. Med. 2017, 6, 2079–2093. [Google Scholar] [CrossRef]
- Obendorf, J.; Fabian, C.; Thome, U.H.; Laube, M. Paracrine stimulation of perinatal lung functional and structural maturation by mesenchymal stem cells. Stem. Cell Res. Ther. 2020, 11, 525. [Google Scholar] [CrossRef]
- Chang, Y.S.; Ahn, S.Y.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Oh, W.I.; Park, W.S. Mesenchymal stem cells for bronchopulmonary dysplasia: Phase 1 dose-escalation clinical trial. J. Pediatr. 2014, 164, 966–972.e6. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Chang, Y.S.; Kim, J.H.; Sung, S.I.; Park, W.S. Two-Year Follow-Up Outcomes of Premature Infants Enrolled in the Phase I Trial of Mesenchymal Stem Cells Transplantation for Bronchopulmonary Dysplasia. J. Pediatr. 2017, 185, 49–54.e2. [Google Scholar] [CrossRef] [PubMed]
- van Kaam, A.H.; De Jaegere, A.P.; Rimensberger, P.C.; Neovent Study Group. Incidence of hypo- and hyper-capnia in a cross-sectional European cohort of ventilated newborn infants. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F323–F326. [Google Scholar] [CrossRef]
- Bronsky, J.; Campoy, C.; Braegger, C.; The ESPGHAN/ESPEN/ESPR/CSPEN Working Group on Pediatric Parenteral Nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Vitamins. Clin. Nutr. 2018, 37, 2366–2378. [Google Scholar] [CrossRef]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellof, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
| BPD Severity | Criteria |
|---|---|
| mild | Supplemental oxygen on 28th day of life and no supplemental oxygen at 36 weeks PMA |
| moderate | Supplemental oxygen of >21% but <30% at 36 weeks PMA |
| severe | Supplemental oxygen >30% or positive airway pressure or mechanical ventilation at 36 weeks PMA |
| Evidence-Based Preventive Strategies for BPD | |
| Antenatal corticosteroids |
|
| Surfactant replacement therapy |
|
| Caffeine |
|
| Ventilation strategies |
|
| Systemic postnatal corticosteroids |
|
| Fluid management and nutrition |
|
| Preventive strategies for BPD with unclear evidence | |
| High-frequency oscillation ventilation |
|
| Permissive hypercapnia |
|
| Topic corticosteroids |
|
| Macrolides |
|
| Patent ductus arteriosus |
|
| Preventive strategies for BPD with a lack of evidence | |
| Inhaled nitric oxide |
|
| Inhaled bronchodilators |
|
| Evidence-Based Therapeutic Strategies for BPD | |
| Fluid management and nutrition |
|
| Systemic postnatal corticosteroids |
|
| Therapeutic strategies for BPD with unclear evidence | |
| Diuretic therapy |
|
| Stem cell therapy |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muehlbacher, T.; Bassler, D.; Bryant, M.B. Evidence for the Management of Bronchopulmonary Dysplasia in Very Preterm Infants. Children 2021, 8, 298. https://doi.org/10.3390/children8040298
Muehlbacher T, Bassler D, Bryant MB. Evidence for the Management of Bronchopulmonary Dysplasia in Very Preterm Infants. Children. 2021; 8(4):298. https://doi.org/10.3390/children8040298
Chicago/Turabian StyleMuehlbacher, Tobias, Dirk Bassler, and Manuel B. Bryant. 2021. "Evidence for the Management of Bronchopulmonary Dysplasia in Very Preterm Infants" Children 8, no. 4: 298. https://doi.org/10.3390/children8040298
APA StyleMuehlbacher, T., Bassler, D., & Bryant, M. B. (2021). Evidence for the Management of Bronchopulmonary Dysplasia in Very Preterm Infants. Children, 8(4), 298. https://doi.org/10.3390/children8040298