Diagnostic Tools for Autism Spectrum Disorders by Gender: Analysis of Current Status and Future Lines
Abstract
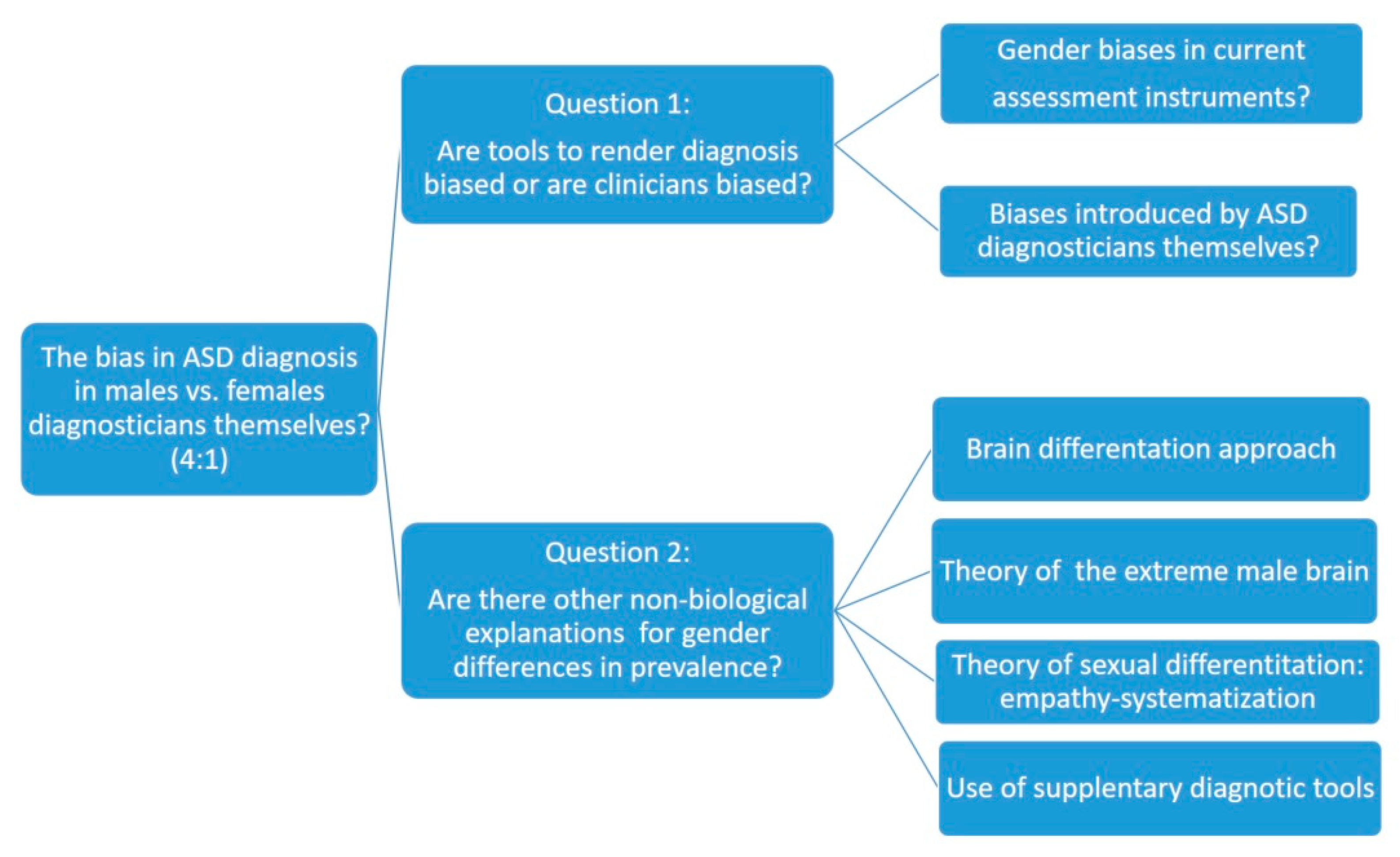
1. Introduction
2. Analysis of Biases in Methods and Tools for the Diagnosis of ASD in Women
2.1. Most Commonly Used Diagnostic Tools
2.2. Differential Results by Sex According to the Diagnostic Tools Used
3. Other Theoretical Approaches
3.1. The Approach of Brain Differentiation
3.2. The Theory of the Extreme Male Brain
3.3. The Theory of Sexual Differentiation Empathy-Systematization
3.4. Use of Complementary Evaluation Questionnaires
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Muhle, R.; Reed, H.; Stratigos, K.; Veemstra-VanderWeele, J. The Emerging Clinical Neuroscience of Autism Spectrum Disorder: A Review. JAMA Psychiatry 2018, 75, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Loomes, R.; Hull, L.; Mandy, W. What is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef]
- Fombonne, E. The prevalence of autism. J. Am. Med Assoc. 2003, 289, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Fombonne, E. Epidemiology of perversive developmental disorders. Pediatric Res. 2009, 65, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Fernell, E.; Gillberg, C. Autism spectrum disorder diagnoses in Stockholm preschoolers. Res. Dev. Disabil. 2010, 31, 680–685. [Google Scholar] [CrossRef]
- Nicholas, J.; Carpenter, L.; King, L.; Jenner, W.; Charles, J. Autism Spectrum Disorders in Preschool-Aged Children: Prevalence and Comparison to a School-Aged Population. Ann. Epidemiol. 2009, 19, 808–814. [Google Scholar] [CrossRef]
- Baio, J.; Wiggins, L.; Chistensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson-Rosenberg, C.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Mmwr Morb. Mortal. Wkly. Rep. Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef]
- Banach, R.; Thompson, A.; Szatmari, P.; Goldberg, J.; Tuff, L.; Zwaigenbaum, L.; Mahoney, W. Brief Report: Relationship Between Non-verbal IQ and Gender in Autism. J. Autism Dev. Disord. 2009, 39, 188–193. [Google Scholar] [CrossRef]
- Werling, D.; Geschwing, D. Sex differences in autism spectrum disorders. Neurology 2013, 26, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Dworzynski, K.; Ronald, A.; Bolton, P.; Happé, F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 788–797. [Google Scholar] [CrossRef]
- Hiller, R.; Young, R.; Weber, N. Sex Differences in Autism Spectrum Disorder based on DSM-5 Criteria: Evidence from Clinician and Teacher Reporting. J. Abnorm. Child Psychol. 2014, 42, 1381–1393. [Google Scholar] [CrossRef]
- Lai, M.C.; Lombardo, M.; Auyeung, B.; Chakrabarti, B.; Baron-Cohen, S. Sex/Gender Differences and Autism: Setting the Scene for Future Research. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 11–24. [Google Scholar] [CrossRef]
- Mandy, W.; Lai, M. Towards sex- and gender-informed autism research. Autism 2017, 2, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Schuch, J.; Mariath, L.; Roman, T.; Schuler-Faccini, L. The Genetic Basis of Autism Spectrum Disorder. In Translational Approaches to Autism Spectrum Disorder; Robinson-Agramonte, M., Ed.; Springer: Cham, Germany, 2015. [Google Scholar] [CrossRef]
- Jacquemont, S.; Coe, B.; Hersch, M.; Duyzend, M.; Krumm, N.; Bergmann, S.; Beckmann, J.; Rosenfeld, J.; Eichler, E. A Higher Mutational Burden in Females Supports a “Female Protective Model” in Neurodevelopmental Disorders. Am. J. Hum. Genet. Ajhg 2014, 94, 415–425. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.; Wright, C. Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 402–410. [Google Scholar] [CrossRef]
- Werling, D.; Parikshak, N.; Geschwind, D. Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nat. Commun. 2016, 7, 10717. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Li, C.; Zhang, Z.; Teng, H.; Wang, Y.; Zhao, T.; Shi, L.; Zhang, K.; Xia, K.; et al. Genetic evidence of gender difference in autism spectrum disorder supports the female-protective effect. Transl. Psychiatry 2020, 4. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Tsompanidis, A.; Auyeung, A.; Nørgaard-Pedersen, B.; Hougaard, D.; Abdallah, M.; Pohl, A. Foetal oestrogens and autism. Mol. Psychiatry 2020, 25, 2970–2978. [Google Scholar] [CrossRef]
- Chapman, E.; Baron-Cohen, S.; Auyeung, B.; Knickmeyer, R.; Taylor, K.; Hackett, G. Fetal testosterone and empathy: Evidence from the Empathy Quotient (EQ) and the “Reading the Mind in the Eyes” Test. Soc. Neurosci. 2006, 1, 135–148. [Google Scholar] [CrossRef]
- Auyeung, B.; Baron-Cohen, S.; Chapman, E.; Knickmeyer, R.; Taylor, K.; Hackett, G. Foetal testosterone and the child systemizing quotient. Eur. J. Endocrinol. 2006, 155 (Suppl 1), S123–S130. [Google Scholar] [CrossRef]
- Knickmeyer, R.; Wheelwright, S.; Taylor, K.; Raggatt, P.; Hackett, G.; Baron-Cohen, S. Gender-typed play and amniotic testosterone. Dev. Psychol. 2005, 41, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Ingudomnukul, E.; Baron-Cohen, S.; Wheelwright, S.; Knickmeyer, R. Elevated rates of testosterone-related disorders in women with autism spectrum conditions. Horm. Behav. 2007, 51, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Allison, C.; Smith, P.; Baron-Cohen, S.; Booth, T.; Auyeung, B. Investigating Diagnostic Bias in Autism Spectrum Conditions: An Item Response Theory Analysis of Sex Bias in the AQ-10. Autism Res. 2016, 10, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Kanner, L. Autistic disturbance of affective contact. Quaterly J. Psychopathol. Psychother. Ment. Hyg. Guid. Child 1943, 2, 217–250. [Google Scholar]
- Constantino, J.N.; Charman, T. Gender bias, female resilience, and the sex ratio in autism. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 756–758. [Google Scholar] [CrossRef]
- Dean, M.; Harwood, R.; Kasari, C. The art of camouflage: Gender differences in the social behaviors of girls and boys with autism spectrum disorder. Autism 2017, 21, 678–689. [Google Scholar] [CrossRef]
- Lai, M.C.; Lombardo, M.; Chakrabarti, B.; Baron-Cohen, S. Subgrouping the Autism “Spectrum”: Reflections on DSM-5. PLoS Biol. 2013, 11, e1001544. [Google Scholar] [CrossRef]
- Van Winjngaarden-Cremers, P.; van Eeten, E.; Groen, W.; van Deurzen, P.; Oosterling, I.; van der Gaag, R. Gender and Age Differences in the Core Triad of Impairments in Autism Spectrum Disorders: A Systematic Review and Meta-analysis. J. Autism Dev. Disord. 2014, 44, 627–635. [Google Scholar] [CrossRef]
- Mandy, W.; Lai, M. Annual Research Review: The role of the environment in the developmental psychopathology of autism spectrum condition. J. Child Psychol. Psychiatry 2016, 57, 271–292. [Google Scholar] [CrossRef]
- Falkmer, T.; Anderson, K.; Falkmer, M.; Horlin, C. Diagnostic procedures in autism spectrum disorders: A systematic literature review. Eur. Child Asolescent Psychiatry 2013, 22, 329–340. [Google Scholar] [CrossRef]
- Bryson, S.; Zwaigenbaum, L.; McDermott, C.; Rombough, V.; Brian, J. The autism Observation Scale for Infants: Scale development and reliability data. J. Autism Dev. Disord. 2008, 38, 731–738. [Google Scholar] [CrossRef]
- Cox, A.; Klein, K.; Charman, T.; Baird, G.; Baron-Cohen, S.; Swettenham, J.; Drew, A.; Wheelwright, S. Autism Spectrum Disorders at 20 and 42 Months of Age: Stability of Clinical and ADI-R Diagnosis. J. Child Psychol. Psychiatry 1999, 40, 719–732. [Google Scholar] [CrossRef]
- Dawson, S.; Osterling, J.; Meltzoff, A.; Kuhl, P. Case study of the development of an infant with autism from birth to two years of age. J. Appl. Dev. Psychol. 2000, 21, 299–313. [Google Scholar] [CrossRef]
- Kleinman, J.; Ventola, P.; Pandey, J.; Verbalis, A.; Barton, M.; Hodgson, S.; Green, J.; Dumont-Mathieu, T.; Robins, D.; Fein, D. Diagnostic stability in very young children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2008, 38, 606–615. [Google Scholar] [CrossRef]
- WHO. International Classification of Diseases, 11st ed.; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Constantino, J.; Charman, T. Diagnosis of autism spectrum disorder: Reconciling the syndrome, its diverse origins, and variation in expression. Lancet Neurol. 2016, 15, 279–291. [Google Scholar] [CrossRef]
- Wiggins, L.; Levy, S.; Daniels, J.; Schieve, L.; Croen, L.; DiGuiseppi, C.; Blaskey, L.; Giarelli, E.; Lee, L.; Pinto-Martin, J.; et al. Autism Spectrum Disorder Symptoms Among Children Enrolled in the Study to Explore Early Development (SEED). J. Autism Dev. Disord. 2015, 45, 3183–3194. [Google Scholar] [CrossRef]
- Young, H.; Oreve, M.; Speranza, M. Clinical characteristics and problems diagnosing autism spectrum disorder in girls. Arch. Pédiatrie 2018, 25, 399–403. [Google Scholar] [CrossRef]
- Begeer, S.; Mandell, D.; Wijnker-Holmes, B.; Venderbosch, S.; Rem, D.; Stekelenburg, F.; Koot, H. Sex Differences in the Timing of Identification Among Children and Adults with Autism Spectrum Disorders. J. Autism Dev. Disord. 2013, 43, 1151–1156. [Google Scholar] [CrossRef]
- Giarelli, E.; Wiggins, L.; Rice, C.; Levy, S.; Kirby, R.; Pinto-Martin, J.; Mandell, D. Sex differences in the evaluation and diagnosis of autismspectrum disorders among children. Disabil. Health J. 2010, 3, 107–116. [Google Scholar] [CrossRef]
- Kreiser, N.W.S. ASD in Females: Are We Overstating the Gender Difference in Diagnosis? Clin. Child Fam. Psychol. Rev. 2014, 17, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Milner, V.; McIntosh, H.; Colvert, E.; Happé, F. A Qualitative Exploration of the Female Experience of Autism Spectrum Disorder (ASD). J. Autism Dev. Disord. 2019, 49, 2389–2402. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.; Shaywitz, S.; Shaywitz, B. Girls with Attention Deficit Disorder: A Silent Minority? A Report on Behavioral and Cognitive Characteristics. Pediatrics 1985, 76, 801–809. [Google Scholar] [PubMed]
- Ruckidge, J.; Tannock, R. Psychiatric, psychosocial and cognitive functioning of female adolescents with ADHD. J. Am. Acad. Child Adolesc. Psychiatry 2001, 5, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Bargiela, S.; Steward, R.; Mandy, W. The Experiences of Late-diagnosed Women with Autism Spectrum Conditions: An Investigation of the Female Autism Phenotype. J. Autism Dev. Disord. 2016, 46, 3281–3294. [Google Scholar] [CrossRef]
- Le Couteur, A.; Lord, C.; Rutter, M. Autism Diagnostic Interview-Revised; Western Psychological Services: Los Angeles, CA, USA, 2003. [Google Scholar]
- Rutter, M.; Le Couteur, A.; Lord, C. Autism Diagnostic Interview Revised; Autism Genetic Resource Exchange: Los Angeles, CA, USA, 2009. [Google Scholar]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Observation Schedule (ADOS) Manual; Western Psychological Services: Los Angeles, CA, USA, 1995. [Google Scholar]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S. Autism Diagnostic Observation Schedule, 2nd ed.; ADOS-2; Western Psychological services: Los Angeles, CA, USA, 2012. [Google Scholar]
- Gotham, K.; Risi, S.; Pickles, A.; Lord, C. The Autism Diagnostic Observation Schedule: Revised Algorithms for Improved Diagnostic Validity. J. Autism Dev. Disord. 2007, 37, 613. [Google Scholar] [CrossRef]
- Zander, E.; Willfors, C.; Berggren, S.; Choque-Olsson, N.; Coco, C.; Elmund, A.; Moretti, A.; Holm, A.; Jifält, I.; Kosiedarzki, R.; et al. The objectivity of the Autism Diagnostic Observation Schedule (ADOS) in naturalistic clinical settings. Eur. Child Adolesc. Psychiatry 2015, 25, 769–780. [Google Scholar] [CrossRef]
- Schopler, E.; Reichler, R.; Renner, B. The Childhood Autism Rating Scale (CARS): For Diagnostic Screening and Classification of Autism; Irvington: New York, NY, USA, 1986. [Google Scholar]
- Schopler, E.; Van Bourgondien, M.; Wellman, G.; Love, S. Chilldhood Autism Rating Scale-Seconf Edition (CARS-2): Manual; Western Psychological Services: Los Angeles, CA, USA, 2010. [Google Scholar]
- Wing, L.; Leekam, S.; Libby, S.; Gould, J.; Larcombe, M. The Diagnostic Interview for Social and Communication Disorders: Background, inter-rater reliability and clinical use. J. Child Psychol. Psychiatry 2002, 43, 307–325. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Wheelwright, S.; Skinner, R.; Martin, J.; Clubley, E. The Autism-Spectrum Quotient (AQ): Evidence from Asperger Syndrome/High-Functioning Autism, Males and Females, Scientists and Mathematicians. J. Autism Dev. Disord. 2001, 31, 5–17. [Google Scholar] [CrossRef]
- Constantino, J.; Gruber, C. Social Responsiveness Scale (SRS); Western Psychological Services: Los Angeles, CA, USA, 2005. [Google Scholar]
- Constantino, J.; Gruber, C. The Social Responsiveness Scale Manual, 2nd ed.; SRS-2; Western Psychological Services: Los Angeles, CA, USA, 2012. [Google Scholar]
- Lam, K.; Aman, M. The Repetitive Behavior Scale-Revised: Independent validation in individuals with autism spectrum disorders. J. Autism Dev. Disord. 2007, 37, 855–866. [Google Scholar] [CrossRef]
- Sparrow, S.; Balla, D.; Cicchetti, D. Vineland-II Adaptive Behavior Scales; AGS Publishing: Circle Pines, MN, USA, 2005. [Google Scholar]
- Achenbach, T.; Edelbrock, C. Manual for the Child Behavior Checklist and Revised Child Behavior Profile; University of Vermont, Departament of Psychiatry: Burlington, NJ, USA, 1983. [Google Scholar]
- Aman, M.; Singh, N.; Stewart, A.; Field, C. Psychometric characteristics of the aberrant behavior checklist. Am. J. Ment. Defic. 1985, 89, 485–491. [Google Scholar]
- Frazier, T.; Georgiades, S.; Bishop, S.; Hardan, A. Behavioral and Cognitive Characteristics of Females and Males with Autism in the Simons Simplex Collection. J. Od. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 329–340. [Google Scholar] [CrossRef]
- Tillmann, J.; Ashwood, K.; Absoud, M.; Bölte, S.; Bonnet-Brilhault, F.; Buitelaar, J.; Calderoni, S.; Calvo, R.; Canal-Bedia, R.; Canitano, R.; et al. Evaluating Sex and Age Differences in ADI-R and ADOS Scores in a Large European Multi-site Sample of Individuals with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 2490–2505. [Google Scholar] [CrossRef]
- Adamou, M.; Johnson, M.; Alty, B. Autism Diagnostic Observation Schedule (ADOS) scores in males and females diagnosed with autism: A naturalistic study. Adv. Autism 2018, 4, 49–55. [Google Scholar] [CrossRef]
- Kumazaki, H.; Muramatsu, T.; Kosaka, H.; Fujisawa, T.; Iwata, K.; Tomoda, A.; Tsuchiya, K.; Mimura, M. Sex differences in cognitive and symptom profiles in children with high functioning autism spectrum disorders. Res. Autism Spectr. Disord. 2015, 13–14, 1–7. [Google Scholar] [CrossRef]
- Duvekot, J.; van der Ende, J.; Verhulst, F.; Slappendel, G.; van Daalen, E.; Maras, A.; Greaves-Lord, K. Factors influencing the probability of a diagnosis of autism spectrum disorder in girls versus boys. Autism 2017, 6, 646–658. [Google Scholar] [CrossRef]
- Ratto, A.; Kenworthy, L.; Yerys, B.; Bascom, J.; Wieckowski, A.; White, S.; Wallace, G.; Pugliese, C.; Schultz, R.; Ollendick, T.; et al. What About the Girls? Sex-Based Differences in Autistic Traits and Adaptative Skills. J. Autism Dev. Disord. 2018, 48, 1698–1711. [Google Scholar] [CrossRef]
- Lai, M.C.; Lombardo, M.; Pasco, G.; Ruigrok, A.; Wheelwright, S.; Sadek, S.; Chakrabarti, B.; MRC AIMS Consortium and Baron-Cohen, S. A Behavioral Comparison of Male and Female Adults with High Functioning Autism Spectrum Conditions. PLoS ONE 2011, 6, e20836. [Google Scholar] [CrossRef]
- Kaat, A.; Shui, A.; Ghods, S.; Farmer, C.; Esler, A.; Thurm, A.; Georgiades, S.; Kanne, S.; Lord, C.; Kim, Y.; et al. Sex differences in scores on standardized measures of autism symptoms: A multisite integrative data analysis. J. Child Psychol. Psychiatry 2021, 62, 97–106. [Google Scholar] [CrossRef]
- Dewinter, J.; De Graaf, H.; Begeer, S. Sexual Orientation, Gender Identity, and Romantic Relationships in Adolescents and Adults with Autism Spectrum Disorder. J. Autism Dev. Disord. Vol. 2017, 47, 2927–2934. [Google Scholar] [CrossRef]
- Taylor, J.; Seltzed, M. Changes in the Autism Behavioral Phenotype During the Transition to Adulthood. J. Autism Dev. Disord. 2010, 40, 1431–1446. [Google Scholar] [CrossRef]
- Lockwood-Estrin, G.; Milner, V.; Spain, D.; Happé, F.; Colvert, E. Barriers to Autism Spectrum Disorder Diagnosis for Young Women and Girls: A Systematic Review. Rev. J. Autism Dev. Disord. 2020. [Google Scholar] [CrossRef]
- Beggiato, A.; Peyre, H.; Maruani, A.; Scheid, I.; Rastam, M.; Amsellem, F.; Gillberg, C.; Leboyer, M.; Bourgeron, T.; Gillberg, C.; et al. Gender differences in autism spectrum disorders: Divergence among specific core symptoms. Autism Res. 2017, 10, 680–689. [Google Scholar] [CrossRef]
- Bölte, S.; Duketis, E.; Poustka, F.; Holtmann, M. Sex differences in cognitive domains and their clinical correlates in higher-functioning autism spectrum disorders. Autism 2011, 15, 497–511. [Google Scholar] [CrossRef]
- Holtmann, M.; Bölte, S.; Poustka, F. Autism spectrum disorders: Sex differences in autistic behaviour domains and coexisting psychopathology. Dev. Med. Child Neurol. 2007, 49, 361–366. [Google Scholar] [CrossRef] [PubMed]
- May, T.; Cornish, K.; Rinehart, N. Gender Profiles of Behavioral Attention in Children with Autism Spectrum Disorder. J. Atten. Disord. 2012, 20, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, V.; Wetherby, A.; Schatschneider, C.; Lord, C. Examination of Sex Differences in a Large Sample of Young Children with Autism Spectrum Disorder and Typical Development. J. Autism Dev. Disord. 2015, 45, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Szatmari, P.; Liu, X.; Goldberg, J.; Zwaigenbaum, L.; Paterson, A.; Woodbury-Smith, M.; Georgiades, S.; Duku, E.; Thompson, A. Sex differences in repetitive stereotyped behaviors in autism: Implications for genetic liability. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2012, 159, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Hull, L.; Petrides, K.; Allison, C.; Smith, P.; Baron-Cohen, S.; Lai, M.; Mandy, W. “Putting on My Best Normal”: Social Camouflaging in Adults with Autism Spectrum Conditions. J. Autism Dev. Disord. 2017, 47, 2519–2534. [Google Scholar] [CrossRef]
- Gould, J.; Ashton-Smith, J. Missed diagnosis or misdiagnosis? Girls and women on the autism spectrum. Good Autism Pract. 2011, 12, 34–41. [Google Scholar]
- Head, A.; McGillivray, J.; Stokes, M. Gender differences in emotionality and sociability in children with autism spectrum disorders. Mol. Autism 2014, 5, 19. [Google Scholar] [CrossRef]
- Boorse, J.; Cola, M.; Plate, S.; Yankowitz, L.; Pandey, J.; Schultz, R.; Parish-Morris, J. Linguistic markers of autism in girls: Evidence of a “blended phenotype” during storytelling. Mol. Autism 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Chojnicka, I.; Wawer, A. Social language in autism spectrum disorder: A computational analysis of sentiment and linguistic abstraction. PLoS ONE 2020, 15, e0229985. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Cola, M.; Plate, S.; Petrulla, V.; Yankowitz, L.; Pandey, J.; Schultz, R.; Parish-Morris, J. Natural language markers of social phenotype in girls with autism. J. Child Psychol. Psychiatry 2020. [Google Scholar] [CrossRef]
- Baron-Cohen, S. The extreme male brain theory of autism. Trends Cogn. Sci. 2002, 6, 248–254. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Knickmeyer, R.; Belmonte, M. Sex differences in the brain: Implications for explaining autism. Science 2005, 310, 819–823. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Bowen, D.; Holt, R.; Allison, C.; Auyeung, B.; Lombardo, M.; Smith, P.; Lai, M.C. The “Reading the Mind in the Eyes” Test: Complete Absence of Typical Sex Difference in 400 Men and Women with Autism. PLoS ONE 2015, 10, e0136521. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Leslie, A.; Frith, U. Does the autistic child have a “theory of mind”? Cognition 1985, 21, 37–46. [Google Scholar] [CrossRef]
- Greenberg, D.; Warrier, V.; Allison, C.; Baron-Cohen, S. Testing the Empathizing–Systemizing theory of sex differences and the Extreme Male Brain theory of autism in half a million people. Proc. Natl. Acad. Sci. USA 2018, 115, 12152–12157. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Wheelwright, S. The Friendship Questionnaire: An Investigation of Adults with Asperger Syndrome or High-Functioning Autism, and Normal Sex Differences. J. Autism Dev. Disord. 2003, 33, 509–517. [Google Scholar] [CrossRef]
- Strang, J.; van der Miesen, A.; Caplan, R.; Hughes, C.; DaVanport, S.; Lai, M. Both sex- and gender-related factors should be considered in autism research and clinical practice. Autism 2020, 24, 539–543. [Google Scholar] [CrossRef]
- Ostan, R.; Monti, D.; Gueresi, P.; Bussolotto, M.; Franceschi, C. Gender, aging and longevity in humans: An update of an intriguing/neglected scenario paving the way to a gender-specific medicine. Clin. Sci. 2016, 130, 1711–1725. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Pardo, E.; López-Ramón, M.F.; Alonso-Esteban, Y.; Alcantud-Marín, F. Diagnostic Tools for Autism Spectrum Disorders by Gender: Analysis of Current Status and Future Lines. Children 2021, 8, 262. https://doi.org/10.3390/children8040262
Navarro-Pardo E, López-Ramón MF, Alonso-Esteban Y, Alcantud-Marín F. Diagnostic Tools for Autism Spectrum Disorders by Gender: Analysis of Current Status and Future Lines. Children. 2021; 8(4):262. https://doi.org/10.3390/children8040262
Chicago/Turabian StyleNavarro-Pardo, Esperanza, Maria Fernanda López-Ramón, Yurena Alonso-Esteban, and Francisco Alcantud-Marín. 2021. "Diagnostic Tools for Autism Spectrum Disorders by Gender: Analysis of Current Status and Future Lines" Children 8, no. 4: 262. https://doi.org/10.3390/children8040262
APA StyleNavarro-Pardo, E., López-Ramón, M. F., Alonso-Esteban, Y., & Alcantud-Marín, F. (2021). Diagnostic Tools for Autism Spectrum Disorders by Gender: Analysis of Current Status and Future Lines. Children, 8(4), 262. https://doi.org/10.3390/children8040262








