Associations between Physical Activity Patterns, Screen Time and Cardiovascular Fitness Levels in Swedish Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment
2.2. Ethical Statement
2.3. Data Collection and Measures
2.3.1. Cardiovascular Fitness
2.3.2. Physical Activity Patterns
2.3.3. Screen Time
2.3.4. Participation in Organised Sports
2.3.5. Anthropometric Measurements
2.3.6. Pubertal Maturation
2.3.7. Demographics
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boreham, C.; Riddoch, C. The physical activity, fitness and health of children. J. Sports Sci. 2001, 19, 915–929. [Google Scholar] [CrossRef]
- Ortega, F.B.; Ruiz, J.R.; Hurtig-Wennlöf, A.; Vicente-Rodriguez, G.; Rizzo, N.S.; Castillo, M.J.; Sjöström, M. Cardiovascular fitness modifies the associations between physical activity and abdominal adiposity in children and adolescents: The European Youth Heart Study. Br. J. Sports Med. 2010, 44, 256–262. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Ortega, F.B.; Rizzo, N.S.; Villa, I.; Hurtig-Wennlöf, A.; Oja, L.; Sjöström, M. High cardiovascular fitness is associated with low metabolic risk score in children: The European Youth Heart Study. Pediatr. Res. 2007, 61, 350. [Google Scholar] [CrossRef] [PubMed]
- Twisk, J.; Kemper, H.; Van Mechelen, W. The relationship between physical fitness and physical activity during adolescence and cardiovascular disease risk factors at adult age. The Amsterdam Growth and Health Longitudinal Study. Int. J. Sports Med. 2002, 23, 8–14. [Google Scholar] [CrossRef]
- Ortega, F.B.; Ruiz, J.R.; Castillo, M.J.; Sjöström, M. Physical fitness in childhood and adolescence: A powerful marker of health. Int. J. Obes. 2008, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tomkinson, G.R.; Carver, K.D.; Atkinson, F.; Daniell, N.D.; Lewis, L.K.; Fitzgerald, J.S.; Lang, J.J.; Ortega, F.B. European normative values for physical fitness in children and adolescents aged 9–17 years: Results from 2 779 165 Eurofit performances representing 30 countries. Br. J. Sports Med. 2018, 52, 1445–1456. [Google Scholar] [CrossRef]
- Rowland, T. Oxygen uptake and endurance fitness in children, revisited. Pediatr. Exerc. Sci. 2013, 25, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Loftin, M.; Sothern, M.; Abe, T.; Bonis, M. Expression of VO 2peak in children and youth, with special reference to allometric scaling. Sports Med. 2016, 46, 1451–1460. [Google Scholar] [CrossRef]
- Sjödin, B.; Svedenhag, J. Oxygen uptake during running as related to body mass in circumpubertal boys: A longitudinal study. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 150–157. [Google Scholar] [CrossRef]
- Schutte, N.M.; Nederend, I.; Hudziak, J.J.; Bartels, M.; de Geus, E.J. Twin-sibling study and meta-analysis on the heritability of maximal oxygen consumption. Physiol. Genom. 2016, 48, 210–219. [Google Scholar] [CrossRef]
- Ekblom, Ö.; Oddsson, K.; Ekblom, B. Health-related fitness in Swedish adolescents between 1987 and 2001. Acta Paediatr. 2004, 93, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Björkman, F.; Eggers, A.; Stenman, A.; Bohman, T.; Ekblom, B.; Ekblom, Ö. Sex and maturity status affected the validity of a submaximal cycle test in adolescents. Acta Paediatr. 2018, 107, 126–133. [Google Scholar] [CrossRef]
- Rowland, T.W.; Boyajian, A. Aerobic response to endurance exercise training in children. Pediatrics 1995, 96, 654–658. [Google Scholar]
- McManus, A.; Armstrong, N.; Williams, C. Effect of training on the aerobic power and anaerobic performance of prepubertal girls. Acta Paediatr. 1997, 86, 456–459. [Google Scholar] [CrossRef]
- Williams, C.A.; Armstrong, N.; Powell, J. Aerobic responses of prepubertal boys to two modes of training. Br. J. Sports Med. 2000, 34, 168–173. [Google Scholar] [CrossRef][Green Version]
- Telama, R.; Yang, X.; Viikari, J.; Välimäki, I.; Wanne, O.; Raitakari, O. Physical activity from childhood to adulthood: A 21-year tracking study. Am. J. Prev. Med. 2005, 28, 267–273. [Google Scholar] [CrossRef]
- US Department of Health Human Services. 2018 Physical Activity Guidelines Advisory Committee Scientific Report; Department of Health and Human Services: Washington, DC, USA, 2018. [Google Scholar]
- Kjønniksen, L.; Anderssen, N.; Wold, B. Organized youth sport as a predictor of physical activity in adulthood. Scand. J. Med. Sci. Sports 2009, 19, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Telama, R.; Yang, X.; Hirvensalo, M.; Raitakari, O. Participation in organized youth sport as a predictor of adult physical activity: A 21-year longitudinal study. Pediatr. Exerc. Sci. 2006, 18, 76–88. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Global trends in insufficient physical activity among adolescents: A pooled analysis of 298 population-based surveys with 1· 6 million participants. Lancet Child Adolesc. Health 2019, 4, 23–35. [Google Scholar] [CrossRef]
- Marques, A.; Santos, R.; Ekelund, U.; Sardinha, L.B. Association between physical activity, sedentary time, and healthy fitness in youth. Med. Sci. Sports Exerc. 2015, 47, 575–580. [Google Scholar] [CrossRef]
- Pate, R.R.; Wang, C.-Y.; Dowda, M.; Farrell, S.W.; O’Neill, J.R. Cardiorespiratory fitness levels among US youth 12 to 19 years of age: Findings from the 1999-2002 National Health and Nutrition Examination Survey. Arch. Pediatr. Adolesc. Med. 2006, 160, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, L.; Syväoja, H.; Kallio, J.; Kulmala, J.; Kujala, U.M.; Tammelin, T.H. Objectively measured physical activity, body composition and physical fitness: Cross-sectional associations in 9-to 15-year-old children. Eur. J. Sport Sci. 2018, 18, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Ekelund, U.; Sardinha, L.B. Associations between organized sports participation and objectively measured physical activity, sedentary time and weight status in youth. J. Sci. Med. Sport 2016, 19, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Golle, K.; Granacher, U.; Hoffmann, M.; Wick, D.; Muehlbauer, T. Effect of living area and sports club participation on physical fitness in children: A 4 year longitudinal study. BMC Public Health 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Martinez-Gomez, D.; Ortega, F.B.; Ruiz, J.R.; Vicente-Rodriguez, G.; Veiga, O.L.; Widhalm, K.; Manios, Y.; Béghin, L.; Valtueña, J.; Kafatos, A. Excessive sedentary time and low cardiorespiratory fitness in European adolescents: The HELENA study. Arch. Dis. Child. 2011, 96, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Cabanas-Sánchez, V.; Martínez-Gómez, D.; Esteban-Cornejo, I.; Pérez-Bey, A.; Castro Piñero, J.; Veiga, O.L. Associations of total sedentary time, screen time and non-screen sedentary time with adiposity and physical fitness in youth: The mediating effect of physical activity. J. Sports Sci. 2019, 37, 839–849. [Google Scholar] [CrossRef]
- Porter, A.K.; Matthews, K.J.; Salvo, D.; Kohl, H.W. Associations of physical activity, sedentary time, and screen time with cardiovascular fitness in United States adolescents: Results from the NHANES National Youth Fitness Survey. J. Phys. Act. Health 2017, 14, 506–512. [Google Scholar] [CrossRef]
- Nyberg, G.; Ekblom, Ö.; Kjellenberg, K.; Wang, R.; Larsson, H.; Thedin Jakobsson, B.; Helgadóttir, B. Associations between the School Environment and Physical Activity Pattern during School Time in Swedish Adolescents. Int. J. Environ. Res. Public Health 2021, 18, 10239. [Google Scholar] [CrossRef]
- Björkman, F.; Ekblom-Bak, E.; Ekblom, Ö.; Ekblom, B. Validity of the revised Ekblom Bak cycle ergometer test in adults. Eur. J. Appl. Physiol. 2016, 116, 1627–1638. [Google Scholar] [CrossRef]
- Evenson, K.R.; Catellier, D.J.; Gill, K.; Ondrak, K.S.; McMurray, R.G. Calibration of two objective measures of physical activity for children. J. Sports Sci. 2008, 26, 1557–1565. [Google Scholar] [CrossRef]
- Corder, K.; Ekelund, U.; Steele, R.M.; Wareham, N.J.; Brage, S. Assessment of physical activity in youth. J. Appl. Physiol. 2008, 105, 977–987. [Google Scholar] [CrossRef]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, J.; Luo, Z.; Albertsson-Wikland, K. Body mass index reference values (mean and SD) for Swedish children. Acta Paediatr. 2001, 90, 1427–1434. [Google Scholar] [CrossRef]
- Kabiri, L.S.; Hernandez, D.C.; Mitchell, K. Reliability, validity, and diagnostic value of a pediatric bioelectrical impedance analysis scale. Child. Obes. 2015, 11, 650–655. [Google Scholar] [CrossRef]
- Rasmussen, A.R.; Wohlfahrt-Veje, C.; de Renzy-Martin, K.T.; Hagen, C.P.; Tinggaard, J.; Mouritsen, A.; Mieritz, M.G.; Main, K.M. Validity of self-assessment of pubertal maturation. Pediatrics 2015, 135, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.M. Growth at Adolescence: With a General Consideration of the Effects of Hereditary and Environmental Factors upon Growth and Maturation from Birth to Maturity; Blackwell Scientific Publications Ltd.: Oxford, UK, 1962; p. xiii + 325p. [Google Scholar]
- StataCorp LCC. Stata Statistical Software. Release 16. [Software]; Stata Press: College Station, TX, USA, 2021; Available online: https://www.stata.com/ (accessed on 13 September 2021).
- Ortega, F.B.; Ruiz, J.R.; Hurtig-Wennlöf, A.; Sjöström, M. Physically active adolescents are more likely to have a healthier cardiovascular fitness level independently of their adiposity status. The European youth heart study. Rev. Española Cardiol. (Engl. Ed.) 2008, 61, 123–129. [Google Scholar] [CrossRef]
- Telford, R.M.; Telford, R.D.; Cochrane, T.; Cunningham, R.B.; Olive, L.S.; Davey, R. The influence of sport club participation on physical activity, fitness and body fat during childhood and adolescence: The LOOK longitudinal study. J. Sci. Med. Sport 2016, 19, 400–406. [Google Scholar] [CrossRef]
| All | Girls | Boys | Sig. | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | p | |
| Demographics | 1139 (100) | 580 (51.0) | 558 (49.0) | |
| Age (year) | 13.4 ± 0.3 | 13.4 ± 0.3 | 13.4 ± 0.4 | 0.147 |
| Parental education, ≥12 years n (%) | 695 (71.6) | 353 (71.0) | 341 (72.2) | 0.674 |
| Participant country of birth, Sweden n (%) | 967 (85.7) | 490 (84.9) | 476 (86.4) | 0.758 |
| Height | n = 1137 | n = 580 | n = 557 | |
| Height (cm) | 161.7 | 161.1 | 162.3 | 0.007 |
| Weight | n = 1134 | n = 580 | n = 554 | |
| Weight (kg) | 52.8 | 53.1 | 52.6 | 0.491 |
| BMI | n = 1134 | n = 580 | n = 554 | |
| BMI | 20.10 ± 3.6 | 20.40 ± 3.5 | 19.80 ± 3.6 | 0.005 |
| BMI sds | 0.36 ± 1.23 | 0.45 ± 1.11 | 0.26 ± 1.35 | 0.012 |
| BMI Categories | 0.203 | |||
| Underweight n (%) | 89 (7.8) | 38 (6.6) | 51 (9.2) | |
| Normal weight n (%) | 815 (71.8) | 430 (74.1) | 384 (69.3) | |
| Overweight n (%) | 179 (15.8) | 89 (15.3) | 90 (16.2) | |
| Obese n (%) | 52 (4.6) | 23 (4.0) | 29 (5.2) | |
| Body composition | n = 1131 | n = 580 | n = 551 | <0.001 |
| Body fat (%) | 22.6 | 25.7 | 19.3 | |
| Physical activity | n = 903 | n = 490 | n = 413 | |
| SED (min/day) | 602.0 ± 66.6 | 608.9 ± 62.65 | 593.7 ± 70.2 | <0.001 |
| MVPA (min/day) | 52.0 ± 19.0 | 49.5 ± 17.7 | 54.9 ± 20.1 | <0.001 |
| Light (min/day) | 138.6 ± 30.2 | 137.5 ± 27.1 | 139.9 ± 33.6 | 0.231 |
| Accelerometer wear time (min/day) | 792.6 ± 60.8 | 796.0 ± 58.1 | 788.5 ± 63.8 | 0.067 |
| Total valid days included for accelerometer | 6.0 ± 1.1 | 6.2 ± 1.0 | 5.8 ± 1.1 | <0.001 |
| Reached the PA recommendations N (%) | 273 (30.2) | 121 (24.7) | 152 (36.8) | <0.001 |
| Organised sports | ||||
| Participated in organised sports N (%) | 787 (72.0) | 396 (71.4) | 391 (72.7) | 0.626 |
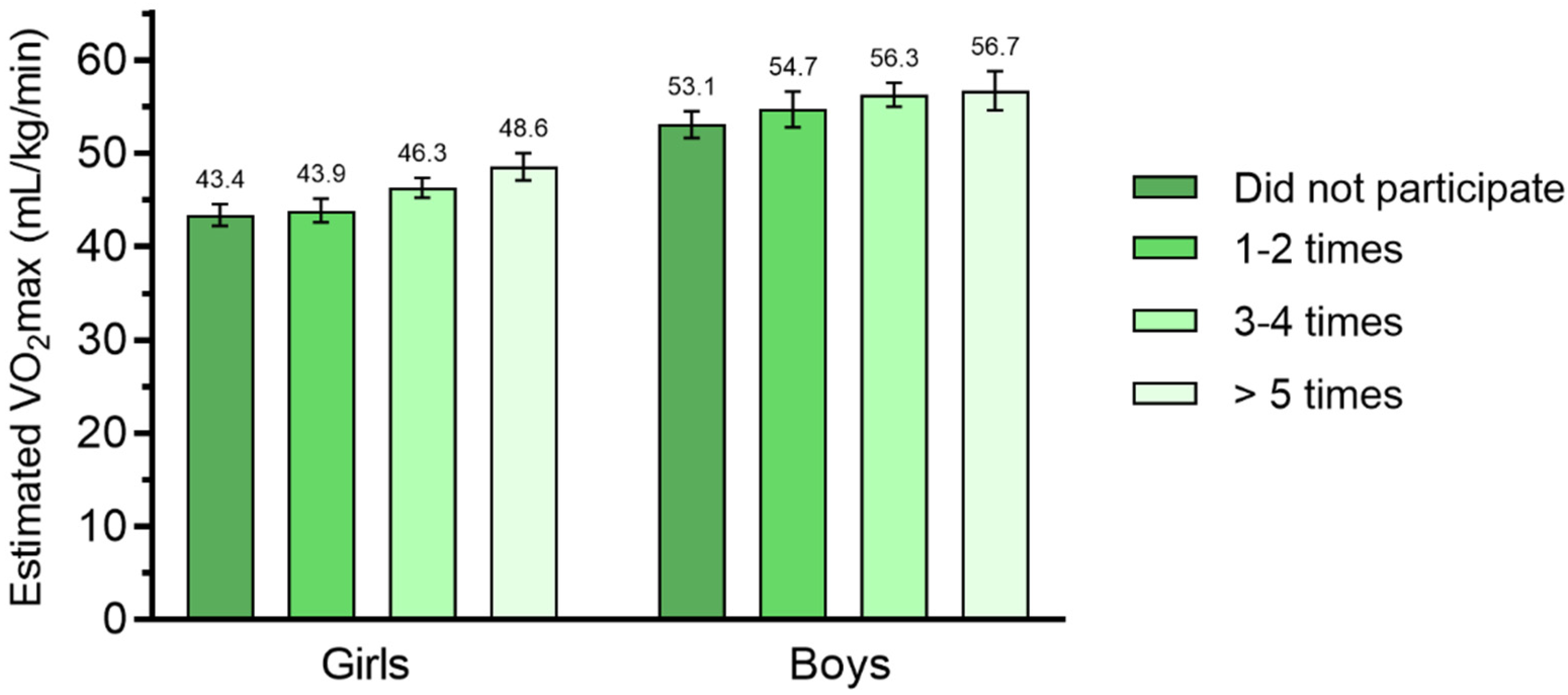
| Participation rate (per week) | 0.064 | |||
| 1–2 times N (%) | 217 (28.0) | 121 (30.9) | 96 (25.0) | |
| 3–4 times N (%) | 356 (45.9) | 181 (46.2) | 175 (45.6) | |
| ≥5 times or more N (%) | 203 (26.2) | 90(23.0) | 113 (29.4) | |
| Screen time weekdays | n = 1125 | n = 575 | n = 550 | 0.842 |
| ≤2 h N (%) | 359 (31.9) | 179 (31.1) | 180 (32.7) | |
| 3–4 h N (%) | 515 (46.0) | 267 (46.4) | 248 (45.1) | |
| ≥5 h N (%) | 251 (22.3) | 129 (22.4) | 122 (22.2) | |
| Screen time weekend | n = 1122 | n = 576 | n = 546 | <0.001 |
| ≤2 h N (%) | 178 (15.9) | 70 (12.2) | 108 (19.8) | |
| 3–4 h N (%) | 411 (36.6) | 238 (41.3) | 173 (31.7) | |
| ≥5 h N (%) | 533 (47.5) | 268 (46.5) | 265 (48.5) | |
| Fitness | n = 1020 | n = 569 | n = 451 | |
| Estimated Vo2max (mL/kg/min) | 49.5 ± 10.1 | 44.8 ± 8.5 | 55.5 ± 8.9 | <0.001 |
| Estimated Vo2max (L/min) | 2.6 ± 0.02 | 2.3 ± 0.01 | 2.9 ± 0.03 | <0.001 |
| Puberty | n = 1041 | n = 537 | n = 504 | 0.346 |
| Tanner score | 3.26 ± 0.85 | 3.28 ± 0.79 | 3.23 ± 0.91 |
| All | Girls | Boys | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vo2max (mL) | Sig. | Vo2max (mL) | Sig. | Vo2max (mL) | Sig. | ||||
| n | Mean ± SD | p | n | Mean ± SD | p | n | Mean ± SD | p | |
| BMI status | <0.001 | <0.001 | <0.001 | ||||||
| Underweight/normal weight | 800 | 51.5 ± 9.4 | 460 | 46.8 ± 7.7 | 340 | 57.8 ± 7.6 | |||
| Overweight/obese | 220 | 42.5 ± 9.8 | 109 | 36.4 ± 6.2 | 111 | 48.5 ± 8.9 | |||
| Parental education | 0.012 | 0.168 | 0.009 | ||||||
| ≤12 years | 249 | 48.6 ± 9.6 | 141 | 44.6 ± 7.7 | 108 | 53.8 ± 9.4 | |||
| >12 years | 624 | 50.4 ± 9.9 | 348 | 45.8 ± 8.4 | 276 | 56.3 ± 8.4 | |||
| Country of Birth | 0.031 | 0.010 | 0.868 | ||||||
| Sweden | 872 | 49.8 ± 10.0 | 481 | 45.2 ± 8.4 | 391 | 55.5 ± 8.9 | |||
| Outside Sweden | 141 | 47.8 ± 10.5 | 85 | 42.7 ± 8.3 | 56 | 55.7 ± 8.4 | |||
| Puberty quartiles | 0.001 | 0.037 | |||||||
| 1 | 258 | 46.1 ± 8.6 | 144 | 53.8 ± 8.3 | |||||
| 2 | 115 | 43.5 ± 8.4 | 75 | 56.5 ± 9.4 | |||||
| 3 | 93 | 44.2 ± 8.1 | 139 | 56.60 ± 9.03 | |||||
| 4 | 62 | 42.0 ± 7.3 | 63 | 55.5 ± 8.5 | |||||
| Participated in organised sports | <0.001 | <0.001 | <0.001 | ||||||
| Yes | 700 | 51.0 ± 0.59 | 391 | 46.3 ± 0.4 | 309 | 57.1 ± 0.5 | |||
| No | 280 | 46.0 ± 0.37 | 153 | 41.4 ± 0.6 | 127 | 51.6 ± 0.8 | |||
| Reached the PA recommendations | <0.001 | <0.001 | 0.001 | ||||||
| Yes | 305 | 53.1 ± 0.6 | 144 | 48.0 ± 0.4 | 161 | 57.7 ± 0.6 | |||
| No | 682 | 47.9 ± 0.4 | 410 | 43.8 ± 0.7 | 272 | 54.1 ± 0.5 | |||
| Model | Girls | Boys | ||||
|---|---|---|---|---|---|---|
| n | β | 95% CI | n | β | 95% CI | |
| 1. Physical activity | 482 | 346 | ||||
| 1.1 MVPA whole week | 0.074 | 0.038, 0.110 | 0.069 | 0.029, 0.108 | ||
| 1.2 SED whole week | −0.039 | −0.057, 0.022 | −0.030 | −0.046, −0.014 | ||
| 1.3 SED:MVPA ratio | −0.473 | −0.699, −0.248 | −0.407 | −0.638, −0.175 | ||
| 1.4 Light PA whole week | 0.043 | 0.020, 0.066 | 0.035 | 0.013, 0.057 | ||
| 1.5 Reached the PA recommendations | 3.313 | 1.962, 4.664 | 1.874 | 0.262, 3.486 | ||
| 2. Organised sports | ||||||
| 2.1 Participated in organised sports | 461 | 2.269 | 0.853, 3.686 | 341 | 2.870 | 1.141, 4.599 |
| 3. Rate of participation (per week) | ||||||
| Do not participate at all | REF | REF | ||||
| 1–2 times | 0.486 | −1.197, 2.170 | 1.635 | −0.763, 4.032 | ||
| 3–4 times | 2.937 | 1.302, 4.572 | 3.120 | 1.242, 5.154 | ||
| ≥5 times or more | 5.188 | 3.262, 7.115 | 3.615 | 1.099, 6.130 | ||
| 4. Screen time weekdays | 458 | 428 | ||||
| ≤2 h | REF | REF | ||||
| 3–4 h | −1.824 | −3.209, −0.440 | −1.579 | −3.356, 0.198 | ||
| ≥5 h | −2.315 | −3.922, −0.708 | −2.821 | −5.140, −0.502 | ||
| 5. Screen time weekend | 479 | 341 | ||||
| ≤2 h | REF | REF | ||||
| 3–4 h | 0.582 | −1.543, 2.708 | 1.308 | −0.836, 3.452 | ||
| ≥5 h | −1.266 | −3.362, 0.831 | −1.138 | −3.238, 0.963 | ||
| 6. Body composition | 569 | 448 | ||||
| Body fat (%) | −0.520 | −0.671, −0.369 | −0.714 | −0.887, −0.541 | ||
| 7. Puberty | 528 | 421 | ||||
| Tanner | −0.621 | −1.349, 0.108 | 2.613 | 1.860, 3.366 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kjellenberg, K.; Ekblom, Ö.; Stålman, C.; Helgadóttir, B.; Nyberg, G. Associations between Physical Activity Patterns, Screen Time and Cardiovascular Fitness Levels in Swedish Adolescents. Children 2021, 8, 998. https://doi.org/10.3390/children8110998
Kjellenberg K, Ekblom Ö, Stålman C, Helgadóttir B, Nyberg G. Associations between Physical Activity Patterns, Screen Time and Cardiovascular Fitness Levels in Swedish Adolescents. Children. 2021; 8(11):998. https://doi.org/10.3390/children8110998
Chicago/Turabian StyleKjellenberg, Karin, Örjan Ekblom, Cecilia Stålman, Björg Helgadóttir, and Gisela Nyberg. 2021. "Associations between Physical Activity Patterns, Screen Time and Cardiovascular Fitness Levels in Swedish Adolescents" Children 8, no. 11: 998. https://doi.org/10.3390/children8110998
APA StyleKjellenberg, K., Ekblom, Ö., Stålman, C., Helgadóttir, B., & Nyberg, G. (2021). Associations between Physical Activity Patterns, Screen Time and Cardiovascular Fitness Levels in Swedish Adolescents. Children, 8(11), 998. https://doi.org/10.3390/children8110998






