High Mortality among Premature Neonates with Positive Blood Culture Neonatal Sepsis in a Tertiary Hospital, Tanzania: A Call for Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Duration and Study Area
2.2. Sample Size Estimation, Sampling Technique
2.3. Study Population and Data Collection
2.4. Microbiological Analysis
2.5. Data Management and Analysis
3. Results
3.1. Socio Demographic Characteristics of the Enrolled Preterm Neonates
3.2. Preterm Neonates’ Characteristics Based on Blood Culture
3.3. Maternal and Clinical Characteristics during Delivery
3.4. Factors Associated with Confirmed Sepsis (Positive Blood Culture)
3.5. Pathogens and Resistance Profiles
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPM | Breath Per Minute |
| BMC | Bugando Medical Centre |
| C/S | Caesarian Section |
| CUHAS | Catholic University of Health and Allied Sciences |
| EOS | Early Onset sepsis |
| GA | Gestational Age |
| IPC | Infection Prevention and Control |
| KCMC | Kilimanjaro Christian Medical University College |
| LOS | Late Onset sepsis |
| NICU | Neonatal Intensive Care Unit |
| PROM | Premature Rapture of Membrane |
| SVD | Spontaneous Vaginal Delivery |
| WHO | World Health Organization |
References
- Mekonnen, Y.; Tensou, B.; Telake, D.S.; Degefie, T.; Bekele, A. Neonatal mortality in Ethiopia: Trends and determinants. BMC Public Health 2013, 13, 483. [Google Scholar] [CrossRef] [Green Version]
- Wynn, J.L. Defining neonatal sepsis. Curr. Opin. Pediatrics 2016, 28, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Debelew, G.T.; Afework, M.F.; Yalew, A.W. Determinants and causes of neonatal mortality in Jimma Zone, Southwest Ethiopia: A multilevel analysis of prospective follow up study. PLoS ONE 2014, 9, e107184. [Google Scholar] [CrossRef] [PubMed]
- Shane, A.L.; Sánchez, P.J.; Stoll, B.J. Neonatal sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef]
- Molloy, E.J.; Wynn, J.L.; Bliss, J.; Koenig, J.M.; Keij, F.M.; McGovern, M.; Kuester, H.; Turner, M.A.; Giannoni, E.; Mazela, J.; et al. Neonatal sepsis: Need for consensus definition, collaboration and core outcomes. Pediatric Res. 2020, 88, 2–4. [Google Scholar] [CrossRef] [Green Version]
- Polin, R.A.; Denson, S.; Brady, M.T.; The Committee on Fetus and Newborn; The Committee on Infectious Diseases. Epidemiology and Diagnosis of Health Care–Associated Infections in the NICU. Pediatrics 2012, 129, e1104. [Google Scholar] [CrossRef] [Green Version]
- Benitz, W.E.; Wynn, J.L.; Polin, R.A. Reappraisal of guidelines for management of neonates with suspected early-onset sepsis. J. Pediatrics 2015, 166, 1070–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, C.R.; Cavanagh, M.M.M.; Tembo, B.; Chiume, M.; Lufesi, N.; Goldfarb, D.M.; Kissoon, N.; Lavoie, P.M. Neonatal sepsis in low-income countries: Epidemiology, diagnosis and prevention. Expert Rev. Anti-Infect. Ther. 2020, 18, 443–452. [Google Scholar] [CrossRef]
- Bangi, V.; Devi, S. Neonatal sepsis: A risk approach. J. Dr. NTR Univ. Health Sci. 2014, 3, 254–258. [Google Scholar] [CrossRef]
- Marando, R.; Seni, J.; Mirambo, M.M.; Falgenhauer, L.; Moremi, N.; Mushi, M.F.; Kayange, N.; Manyama, F.; Imirzalioglu, C.; Chakraborty, T. Predictors of the extended-spectrum-beta lactamases producing Enterobacteriaceae neonatal sepsis at a tertiary hospital, Tanzania. Int. J. Med. Microbiol. 2018, 308, 803–811. [Google Scholar] [CrossRef]
- Kayange, N.; Kamugisha, E.; Mwizamholya, D.L.; Jeremiah, S.; Mshana, S.E. Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza-Tanzania. BMC Pediatrics 2010, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Narang, A.; Bhakoo, O.N. Predictive Perinatal Score in the Diagnosis of Neonatal Sepsis. J. Trop. Pediatrics 1994, 40, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.D.; McMullan, C.; Mills, B.M. Early warning systems: The next level of rapid response. Nursing 2012, 42, 38–44; quiz 45. [Google Scholar] [CrossRef] [PubMed]
- Adam, A. Sample Size Determination in Survey Research. J. Sci. Res. Rep. 2020, 26, 90–97. [Google Scholar] [CrossRef]
- The WHO Young Infants Study Group. Clinical prediction of serious bacterial infections in young infants in developing countries. Pediatric Infect. Dis. J. 1999, 18 (Suppl. 10), S23–S31. [Google Scholar] [CrossRef] [PubMed]
- WHO. Preterm Birth. 2018. Available online: www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 18 September 2021).
- Bekhof, J.; Reitsma, J.B.; Kok, J.H.; Van Straaten, I.H. Clinical signs to identify late-onset sepsis in preterm infants. Eur. J. Pediatrics 2013, 172, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.M. Manual of Methods for General Bacteriology. J. Clin. Pathol. 1981, 34, 1069. [Google Scholar] [CrossRef] [Green Version]
- Moremi, N.; Claus, H.; Mshana, S.E. Antimicrobial resistance pattern: A report of microbiological cultures at a tertiary hospital in Tanzania. BMC Infect. Dis. 2016, 16, 756. [Google Scholar] [CrossRef] [Green Version]
- Silago, V.; Kovacs, D.; Msanga, D.R.; Seni, J.; Matthews, L.; Oravcová, K.; Zadoks, R.N.; Lupindu, A.M.; Hoza, A.S.; Mshana, S.E. Bacteremia in critical care units at Bugando Medical Centre, Mwanza, Tanzania: The role of colonization and contaminated cots and mothers’ hands in cross-transmission of multidrug resistant Gram-negative bacteria. Antimicrob. Resist. Infect. Control 2020, 9, 58. [Google Scholar] [CrossRef]
- Geyt, J.L.; Hauck, S.G. G272 Epidemiological trends of neonatal sepsis in a county referral hospital in central Kenya. Arch. Dis. Child. 2016, 101, A154. [Google Scholar] [CrossRef]
- Fleischmann, C.; Reichert, F.; Cassini, A.; Horner, R.; Harder, T.; Markwart, R.; Tröndle, M.; Savova, Y.; Kissoon, N.; Schlattmann, P.; et al. Global incidence and mortality of neonatal sepsis: A systematic review and meta-analysis. Arch. Dis. Child. 2021, 106, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Jefferies, A.L.; Yoon, E.W.; Lee, S.K.; Shah, P.S.; on behalf of the Canadian Neonatal Network. Risk Factors and Outcomes of Late-Onset Bacterial Sepsis in Preterm Neonates Born at <32 Weeks’ Gestation. Am. J. Perinatol. 2015, 32, 675–682. [Google Scholar] [PubMed]
- WHO. Integrated Management of Childhood Illness; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E.; Committee on Fetus and Newborn; Committee on Infectious Diseases. Management of Neonates Born at ≤34 6/7 Weeks’ Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018, 142, e20182896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoll, B.J.; Hansen, N.I.; Sánchez, P.J.; Faix, R.G.; Poindexter, B.B.; Van Meurs, K.P.; Bizzarro, M.J.; Goldberg, R.N.; Frantz, I.D., 3rd; Hale, E.C.; et al. Early onset neonatal sepsis: The burden of group B Streptococcal and E. coli disease continues. Pediatrics 2011, 127, 817–826. [Google Scholar] [CrossRef] [Green Version]
- Sgro, M.; Shah, P.S.; Campbell, D.; Tenuta, A.; Shivananda, S.; Lee, S.K. Early-onset neonatal sepsis: Rate and organism pattern between 2003 and 2008. J. Perinatol. 2011, 31, 794–798. [Google Scholar] [CrossRef] [Green Version]
- Martius, J.A.; Roos, T.; Gora, B.; Oehler, M.K.; Schrod, L.; Papadopoulos, T.; Groß, U. Risk factors associated with early-onset sepsis in premature infants. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 85, 151–158. [Google Scholar] [CrossRef]
- Yapıcıoğlu, H.; Özlü, F.; Sertdemir, Y. Are vital signs indicative for bacteremia in newborns? J. Matern.-Fetal Neonatal Med. 2015, 28, 2244–2249. [Google Scholar] [CrossRef]
- Belachew, A.; Tewabe, T. Neonatal sepsis and its association with birth weight and gestational age among admitted neonates in Ethiopia: Systematic review and meta-analysis. BMC Pediatrics 2020, 20, 55. [Google Scholar] [CrossRef]
- Eyeberu, A.; Shore, H.; Getachew, T.; Atnafe, G.; Dheresa, M. Neonatal mortality among neonates admitted to NICU of Hiwot Fana specialized university hospital, eastern Ethiopia, 2020: A cross-sectional study design. BMC Pediatrics 2021, 21, 125. [Google Scholar] [CrossRef]
- Collins, A.; Weitkamp, J.H.; Wynn, J.L. Why are preterm newborns at increased risk of infection? Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F391–F394. [Google Scholar] [CrossRef]
- Clark, R.H.; Bloom, B.T.; Spitzer, A.R.; Gerstmann, D.R. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics 2006, 117, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Schrag, S.J.; Farley, M.M.; Petit, S.; Reingold, A.; Weston, E.J.; Pondo, T.; Hudson Jain, J.; Lynfield, R. Epidemiology of Invasive Early-Onset Neonatal Sepsis, 2005 to 2014. Pediatrics 2016, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pammi, M.; Suresh, G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2020, 3, Cd007137. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.C.; Athalye-Jape, G.K.; Deshpande, G.C.; Simmer, K.N.; Patole, S.K. Probiotic Supplementation and Late-Onset Sepsis in Preterm Infants: A Meta-analysis. Pediatrics 2016, 137, e20153684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Frequency/Mean | Percentage |
|---|---|---|
| Age | 3 ± 5.2 | |
| <10 Days | 225 | 90.0 |
| 10–20 Days | 16 | 6.4 |
| 21–28 Days | 9 | 3.6 |
| Sex | ||
| Female | 107 | 42.8 |
| Male | 143 | 57.2 |
| Tribe | ||
| Sukuma | 93 | 37.2 |
| Haya | 25 | 10.0 |
| Chagga | 21 | 8.4 |
| Others | 111 | 44.4 |
| Place Of Delivery | ||
| Home | 12 | 4.8 |
| Hospital | 238 | 95.2 |
| Birth Weight | ||
| Low birth weight | 228 | 91.2 |
| Very low birth weight | 19 | 7.6 |
| Extreme low birth weight | 3 | 1.2 |
| Gestational age (weeks) | ||
| 28–32 | 53 | 212 |
| 32–34 | 79 | 31.6 |
| 34–36 | 115 | 46 |
| Less than 28 | 3 | 1.2 |
| Resuscitation at birth | ||
| No | 207 | 83.5 |
| Yes | 41 | 16.5 |
| Factor | Positive Culture n (%)/Mean/Median (IQR) | Neg Culture n (%)/Mean/Median (IQR) | Total n (%) | OR/Mean (95% CI) | p-Value |
|---|---|---|---|---|---|
| Birth weight (g) | 1777.02 ± 429.24 | 1930.97 ± 490.25 | 153.95 (8.24, 299.66) | 0.04 | |
| Apgar score at 1 min | 7 (7, 8) | 8 (7, 8) | 0.56 (0.42, 0.76) | <0.001 | |
| Apgar score at 5 min | 9 (9, 10) | 10 (9, 10) | 0.64 (0.46, 0.90) | 0.01 | |
| Sex | |||||
| Female | 21 (39.62) | 86 (43.65) | 107 (42.80) | 1 | |
| Male | 32 (60.38) | 111 (56.35) | 143 (57.20) | 1.81 (0.64, 2.19) | 0.59 |
| Survival | |||||
| Dead | 17 (32.08) | 10 (5.08) | 27 (10.80) | 1 | |
| Alive | 36 (67.92) | 187 (94.92) | 223 (89.20) | 0.11 (0.05, 0.27) | <0.001 |
| Factor | Positive Culture n (%) | Negative Culture n (%) | Total n (%) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Gestation weeks | |||||
| 34–36 weeks | 14 (26.4) | 101 (51.3) | 115 (46.0) | 1 | |
| 32–34 weeks | 19 (35.9) | 60 (30.5) | 79 (31.6) | 2.28 (1.07, 4.90) | 0.034 |
| 28–32 weeks | 20 (37.7) | 36 (18.3) | 56 (22.4) | 4.01 (1.83, 8.77) | 0.001 |
| PROM | |||||
| No | 40 (75.5) | 184 (93.4) | 224 (89.6) | 1 | |
| Yes | 13 (24.5) | 13 (6.6) | 26 (10.4) | 4.60 (1.98, 10.69) | <0.001 |
| Maternal fever | |||||
| No | 48 (20.2) | 190 (79.8) | 238 (95.2) | 1 | |
| Yes | 5 (41.7) | 7 (58.3) | 12 (4.8) | 2.83 (0.86, 9.32) | 0.088 |
| Place of delivery | |||||
| Hospital | 46 (86.8) | 192 (97.5) | 238 (95.2) | 1 | |
| Home | 7 (13.2) | 5 (2.5) | 12 (4.8) | 5.84 (1.77, 19.29) | 0.004 |
| Meconium | |||||
| No | 46 (86.8) | 187 (94.9) | 233 (93.2) | 1 | |
| Yes | 7 (13.2) | 10 (5.1) | 12 (4.8) | 2.85 (1.03, 7.89) | 0.045 |
| Mode of delivery | |||||
| C/S | 19 (35.9) | 114 (57.9) | 133 (53.2) | 1 | |
| SVD | 34 (64.2) | 83 (42.1) | 117 (46.8) | 2.46 (1.31, 4.61) | 0.005 |
| Model 1: Maternal Associated Factors | ||||
|---|---|---|---|---|
| aOR | 95% CI | p Value | ||
| Gestational age (weeks) | 34–36 weeks | Ref | ||
| 32–34 weeks | 1.94 | 0.86, 4.36 | 0.109 | |
| 28–32 weeks | 2.73 | 1.20, 6.24 | 0.017 | |
| PROM | No | Ref | ||
| Yes | 4.28 | 1.71, 10.71 | 0.002 | |
| Place of delivery | Hospital | Ref | ||
| Home | 3.90 | 1.07, 14.19 | 0.039 | |
| Meconium | No | Ref | ||
| Yes | 1.56 | 0.45, 5.41 | 0.487 | |
| Mode of delivery | C/S | Ref | ||
| SVD | 1.87 | 0.94, 3.74 | 0.076 | |
| Model 2: Neonate Repeated Covariates Associated with Positive Blood Culture | ||||
| Temperature | At admission | 1.51 | 0.92, 2.47 | 0.102 |
| RBG | At admission | 0.81 | 0.65, 1.01 | 0.055 |
| At 8 h | 1.34 | 1.07, 1.67 | 0.010 | |
| At 24 h | 1.21 | 0.92, 1.58 | 0.173 | |
| Oxygen saturation | At admission | 0.94 | 0.88, 0.99 | 0.031 |
| At 8 h | 0.99 | 0.90, 1.09 | 0.719 | |
| At 24 h | 0.96 | 0.85, 1.09 | 0.505 | |
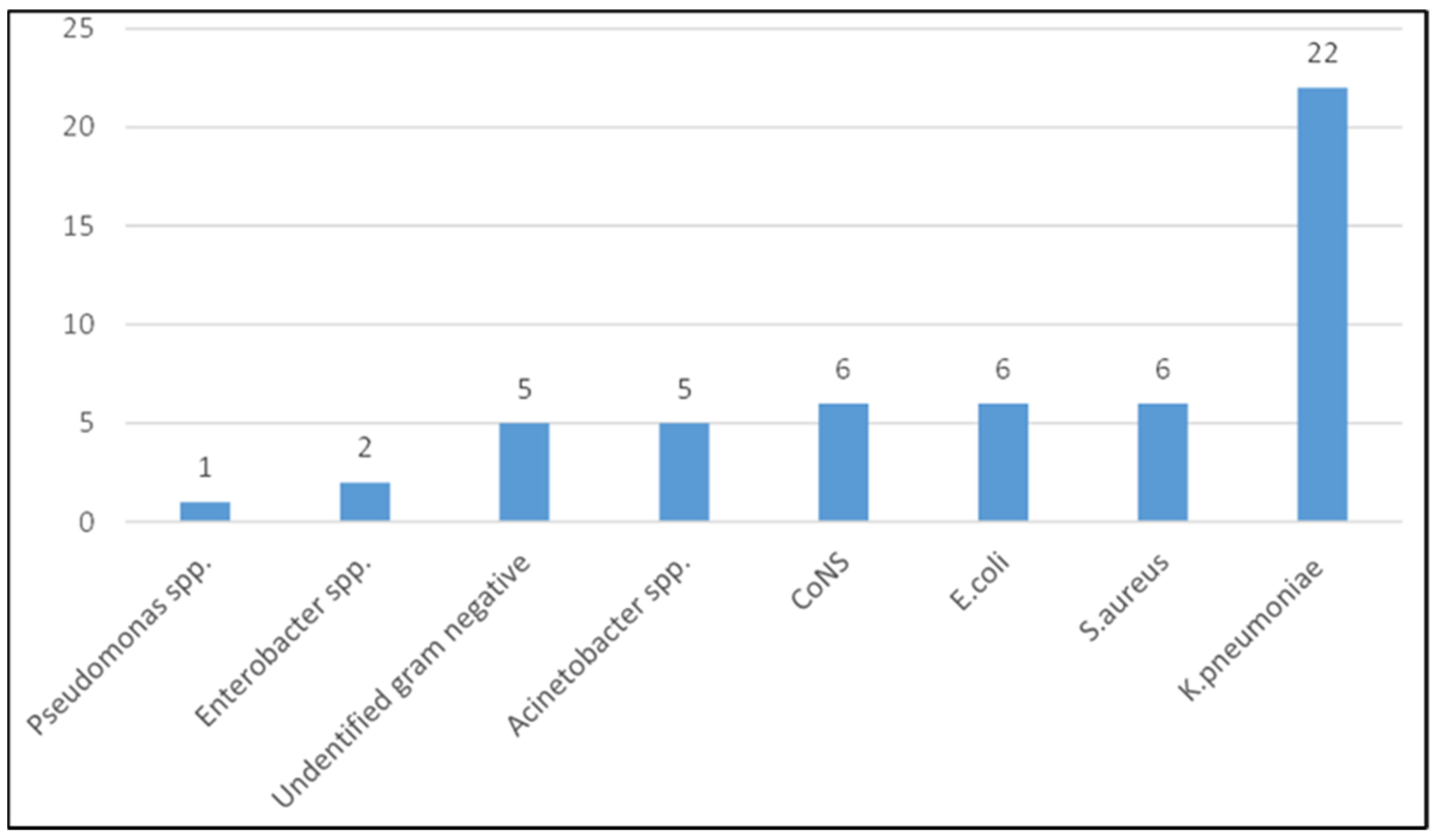
| Antibiotic | K. Pneumoniae (n = 22) | % Resistance | Other Gram Negative Bacteria (n = 19) | % Resistance | Total Gram Negative Bacteria (n = 41) | % Resistance |
|---|---|---|---|---|---|---|
| AMP | 22 | 100.0 | 18 | 94.7 | 40 | 97.6 |
| SXT | 19 | 86.4 | 18 | 94.7 | 37 | 90.2 |
| AMC | 19 | 86.4 | 12 | 63.2 | 31 | 75.6 |
| CN | 21 | 95.5 | 8 | 42.1 | 29 | 70.7 |
| CIP | 13 | 59.1 | 9 | 47.4 | 22 | 53.7 |
| CRO | 19 | 86.4 | 16 | 84.2 | 35 | 85.4 |
| CTX | 19 | 86.4 | 16 | 84.2 | 35 | 85.4 |
| TZP | 12 | 54.5 | 9 | 47.4 | 21 | 51.2 |
| MEM | 0 | 0.0 | 3 | 15.8 | 3 | 7.3 |
| AK | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Msanga, D.R.; Parpia, F.; Konje, E.T.; Hokororo, A.; Mshana, S.E. High Mortality among Premature Neonates with Positive Blood Culture Neonatal Sepsis in a Tertiary Hospital, Tanzania: A Call for Action. Children 2021, 8, 1037. https://doi.org/10.3390/children8111037
Msanga DR, Parpia F, Konje ET, Hokororo A, Mshana SE. High Mortality among Premature Neonates with Positive Blood Culture Neonatal Sepsis in a Tertiary Hospital, Tanzania: A Call for Action. Children. 2021; 8(11):1037. https://doi.org/10.3390/children8111037
Chicago/Turabian StyleMsanga, Delfina R., Fatema Parpia, Eveline T. Konje, Adolfine Hokororo, and Stephen E. Mshana. 2021. "High Mortality among Premature Neonates with Positive Blood Culture Neonatal Sepsis in a Tertiary Hospital, Tanzania: A Call for Action" Children 8, no. 11: 1037. https://doi.org/10.3390/children8111037





