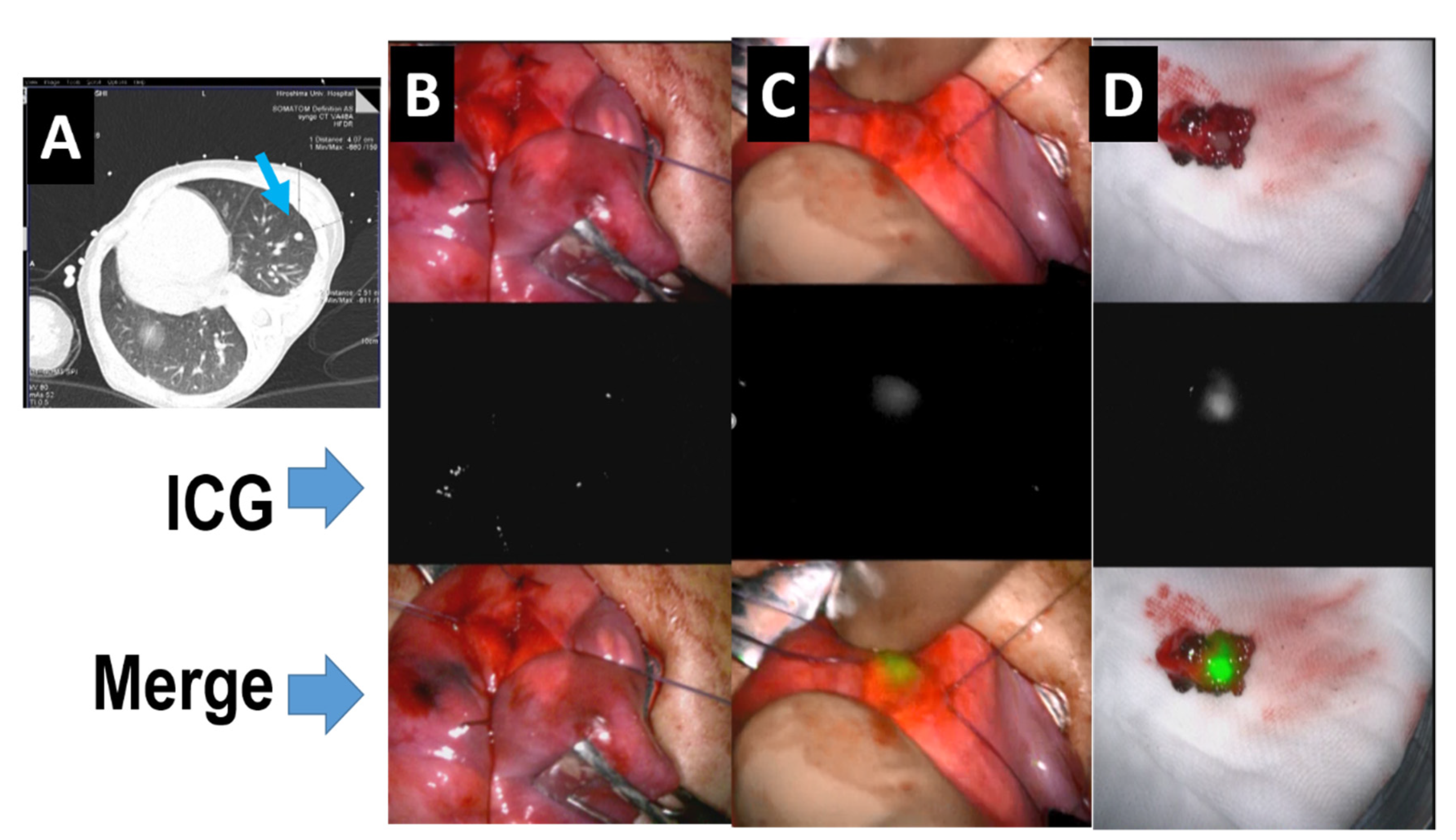
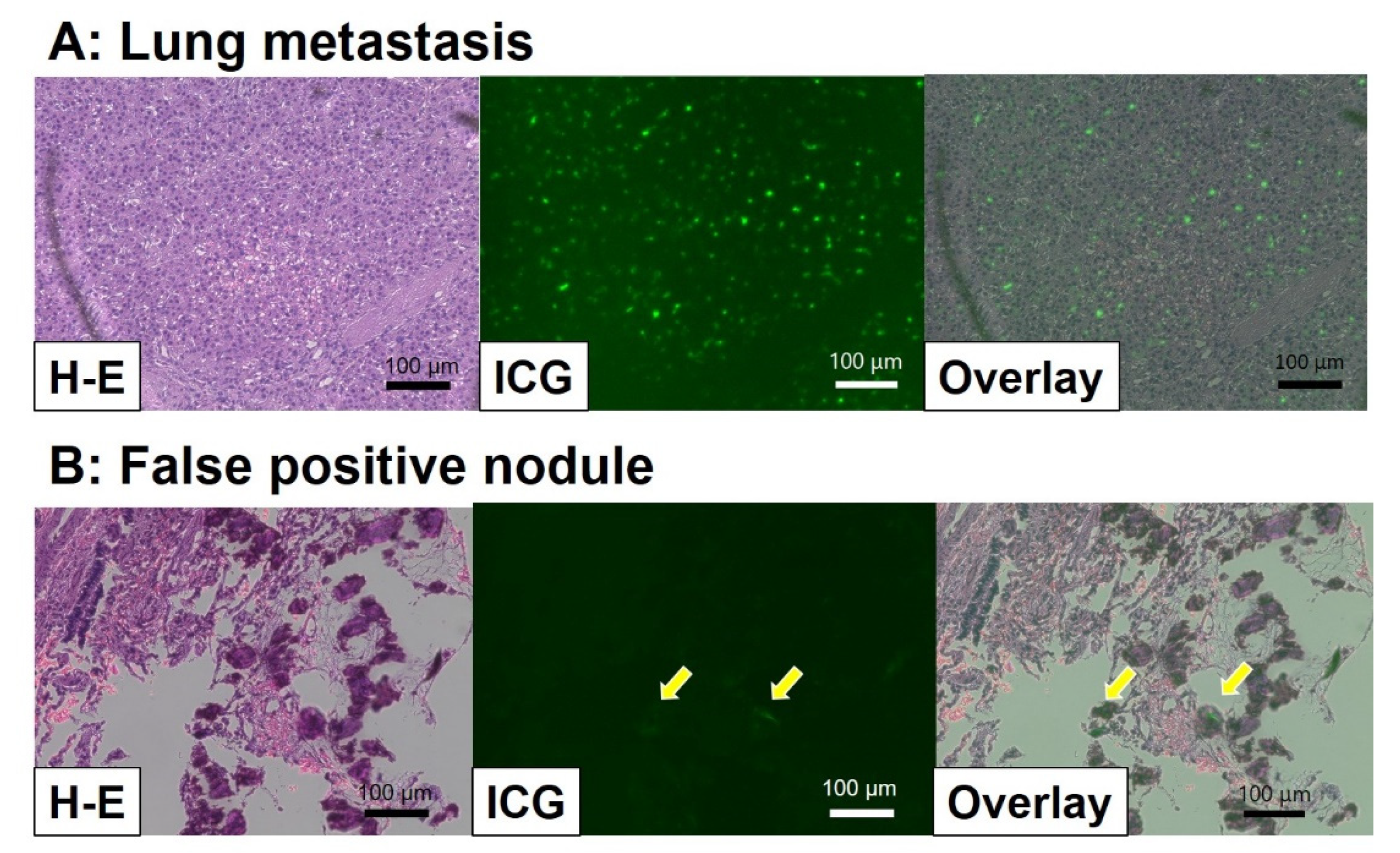
Abstract
In the past decade, navigation surgery using fluorescent indocyanine green (ICG) dye for hepatoblastoma (HB) has been developed for the resection of primary or metastatic tumors. Since HB cells can take up ICG but cannot excrete it to the bile duct, ICG remains in the HB cells, which can be used for navigation by fluorescent activation. The complete resection of the primary tumor as well as metastatic tumors, along with appropriate neoadjuvant and adjuvant chemotherapy, is essential for cure. ICG fluorescence can detect microscopic residual lesions in the primary lesion and identify micro-metastases in the lung or other lesions; consequently, ICG navigation surgery may improve outcomes for patients with HB. The basic technique and recent advances in ICG navigation for HB surgery are reviewed.
1. Introduction
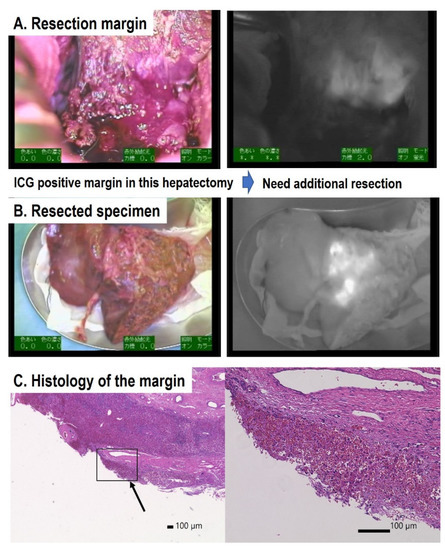
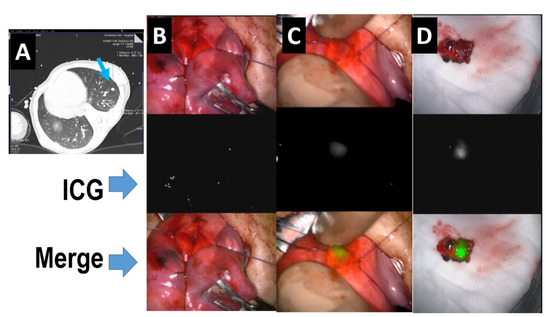
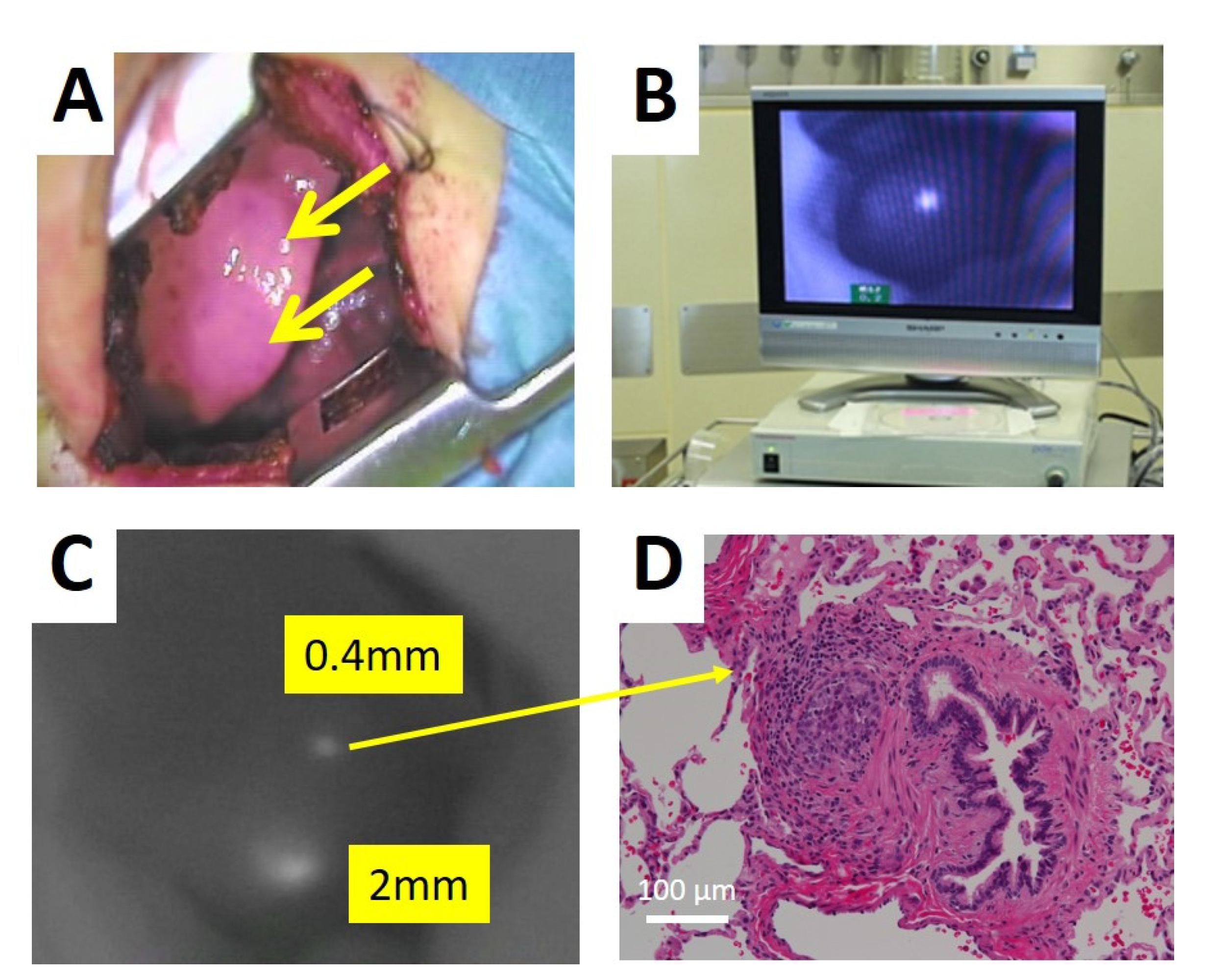
Hepatoblastoma (HB) is the most common pediatric malignant liver tumor, and is usually diagnosed in children under three years of age. HBs are classified according to international risk group by the Children’s Hepatic tumors International Consortium (CHIC) [1]. Since the outcomes for patients with HB depend on the complete resection of the tumor, patients with low-risk HBs, which are usually resectable at diagnosis, have more than 85% survival. In patients with intermediate-risk tumors, which are unresectable at diagnosis due to PRETEXT IV and/or positive annotation factors, such as portal vein invasion, hepatic vein invasion, and tumor rupture, the outcomes of those whose tumors become resectable with neoadjuvant chemotherapy are favorable [2,3]. In patients with high-risk tumors, who usually have lung metastases, those whose metastases are diminished by chemotherapy or completely resected by thoracotomy have favorable outcomes [4]. Therefore, the complete resection of the primary liver tumor and the diminishment of metastases by chemotherapy and/or thoracotomy may be essential for the cure of patients with HB. Since HB primary tumors are usually diagnosed as large hepatic tumors, they are difficult to resect with a sufficient surgical safety margin. Therefore, more precise resection using navigation for the existence of tumor cells might be effective for improving outcomes for patients with HB.
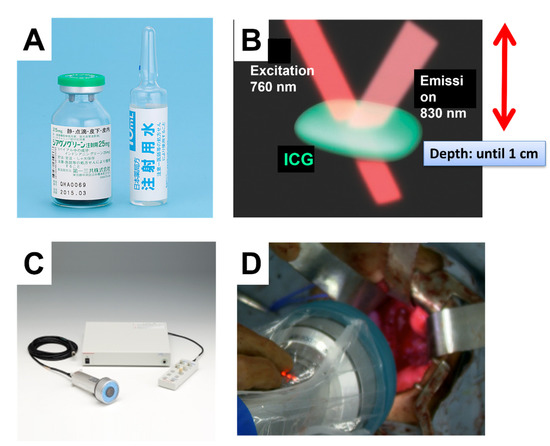
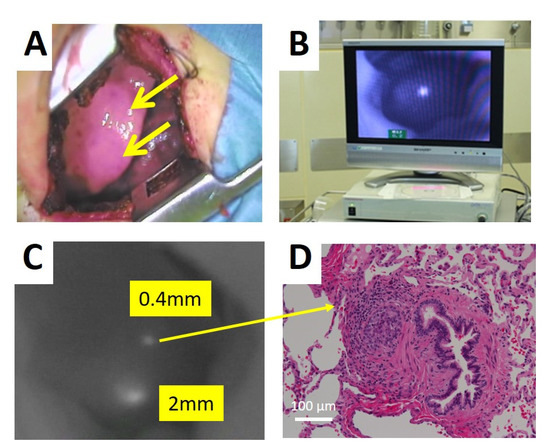
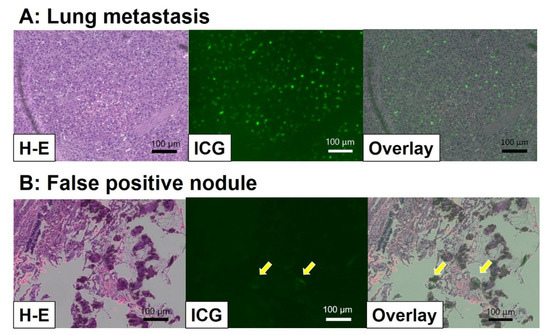
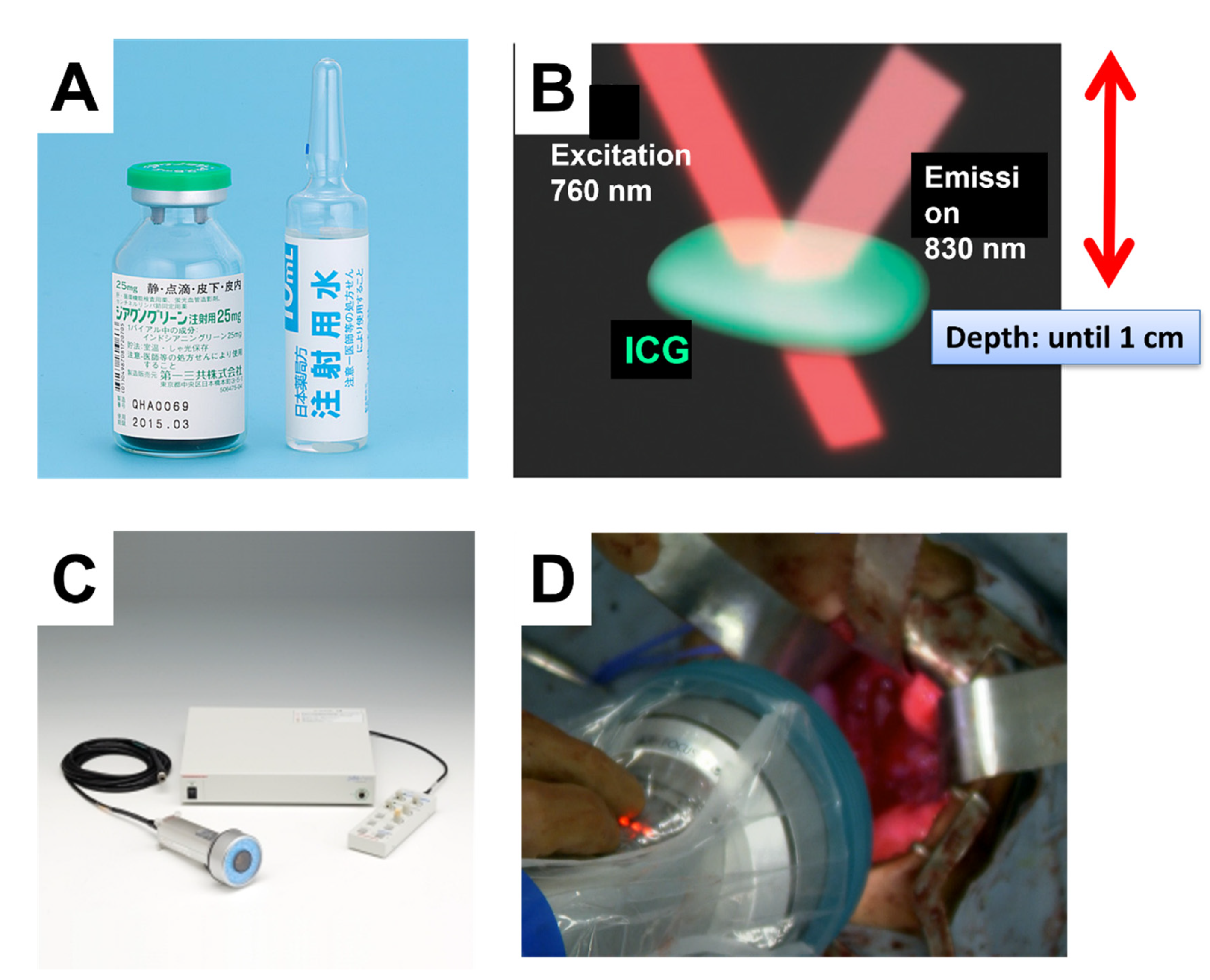
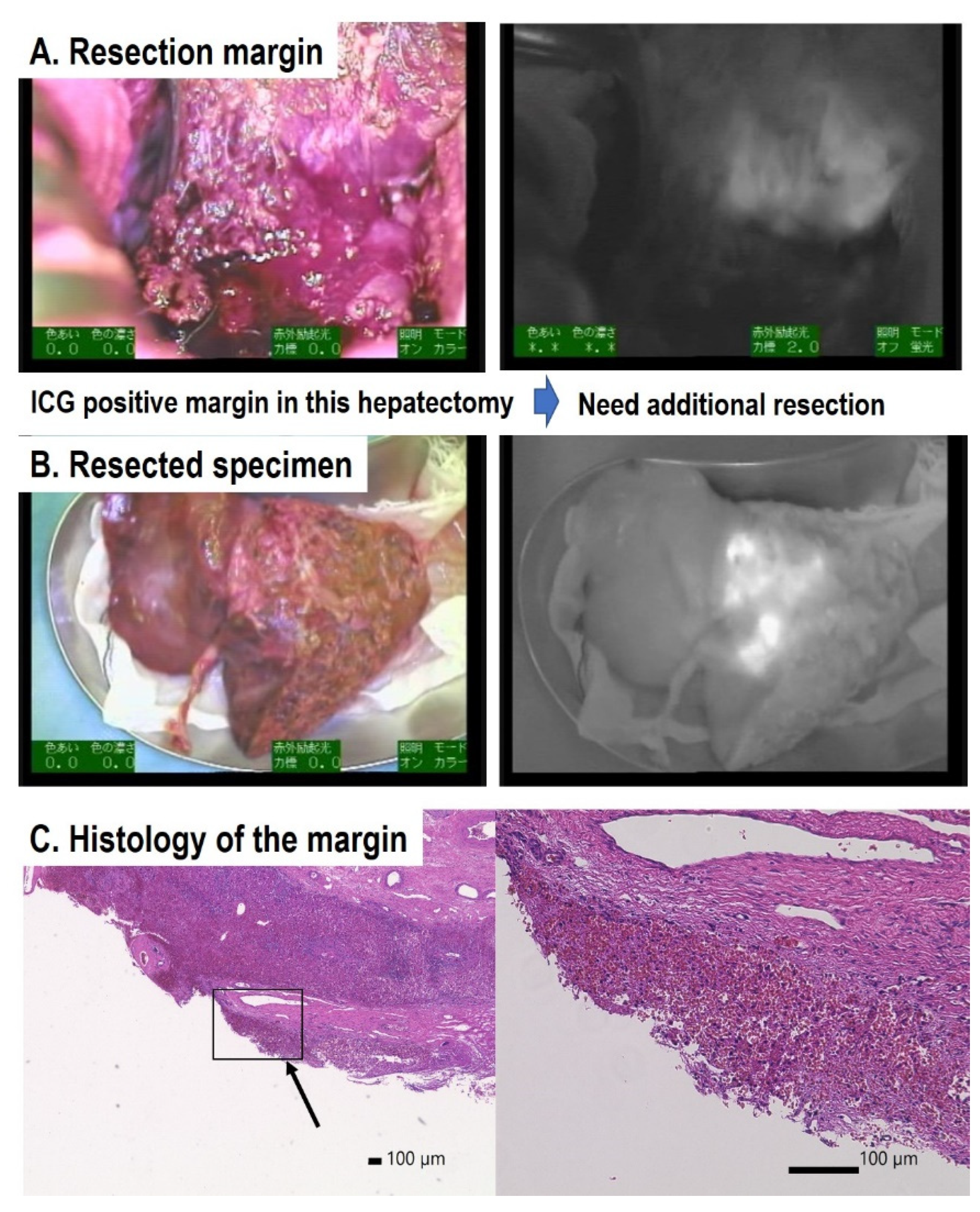
ICG is an organic anion that is almost exclusively taken up by the liver and rapidly excreted into the bile without undergoing biotransformation or enterohepatic circulation. Therefore, ICG clearance has been used as a valuable tool for identifying patients with impaired liver function and, in liver surgery, identifying patients at risk of developing postoperative liver dysfunction or surgical complications [5,6]. ICG is a safe and convenient tool for liver surgeons [7,8]. ICG is also taken up by malignant liver tumor cells, such as hepatocellular carcinoma, hepatoblastoma, and others. However, malignant cells do not excrete ICG because they do not have a connection to the bile duct. Therefore, ICG remains only in malignant hepatic cells after clearance from normal hepatic cells. Since ICG is effectively excited under far-infrared ray (FIR), ICG is useful for navigation to detect malignant hepatic cells.
In this review, which focuses on ICG fluorescence-guided navigation surgery for HB, we discuss the development, underlying ICG uptake mechanism, clinical applications, and future potential of this technology.
4. Conclusions
ICG fluorescence navigation surgery can be used safely and easily to identify the primary tumor and metastatic hepatoblastoma in real time during open and laparoscopic pneumonectomy and hepatobiliary surgery. With further developments in cancer-specific fluorophores and imaging systems, intraoperative fluorescence imaging will develop into an essential navigation tool and a standard surgical procedure for evaluating tumor cell spread, micrometastasis, and the risk of postoperative recurrence.
Funding
This work was supported by Grant name AMED (Academy for Medical Research and Development) (No. JP21lk0201066; No. JP21ck0106609) and Grant-in-Aid for Scientific Research (A) of JSPS (Japan Society for the Promotion of Science) (No. 16H02778).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Hiroshima University (No. E-2588, approved at 15 September 2021).
Informed Consent Statement
Written informed consent was obtained from the patients in Hiroshima University Hospital to publish this paper. The data of other patients was cited from published papers.
Data Availability Statement
Since this is a review article, no new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The author would like to thank N. Kitagawa, R. Souzaki, T. Taguchi, M. Kano, and T. Kuroda for their contributions to applying fluorescence imaging to hepatoblastoma surgery in JPLT study. I also thank all the members of the liver tumor committee of the Japan Children’s Cancer Group (JCCG) for their discussions of ICG navigation surgery in the treatment of hepatoblastoma.
Conflicts of Interest
The author declares that there are no conflict of interest.
References
- Meyers, R.L.; Maibach, R.; Hiyama, E.; Haberle, B.; Krailo, M.; Rangaswami, A.; Aronson, D.C.; Malogolowkin, M.H.; Perilongo, G.; von Schweinitz, D.; et al. Risk-stratified staging in paediatric hepatoblastoma: A unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol. 2017, 18, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Hiyama, E. Current therapeutic strategies for childhood hepatic malignant tumors. Int. J. Clin. Oncol. 2013, 18, 943–945. [Google Scholar] [CrossRef]
- Hiyama, E.; Hishiki, T.; Watanabe, K.; Ida, K.; Ueda, Y.; Kurihara, S.; Yano, M.; Hoshino, K.; Yokoi, A.; Yakama, Y.; et al. Outcome and late complications of hepatoblastomas treated using the Japanese Study Group for Pediatric Liver Tumor (JPLT)-2 protocol. J. Clin. Oncol. 2020, 38, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Hishiki, T.; Watanabe, K.; Ida, K.; Hoshino, K.; Iehara, T.; Aoki, Y.; Kazama, T.; Kihira, K.; Takama, Y.; Taguchi, T.; et al. The role of pulmonary metastasectomy for hepatoblastoma in children with metastasis at diagnosis: Results from the JPLT-2 study. J. Pediatr. Surg. 2017, 52, 2051–2055. [Google Scholar] [CrossRef]
- Narasaki, H.; Noji, T.; Wada, H.; Ebihara, Y.; Tsuchikawa, T.; Okamura, K.; Tanaka, E.; Shichinohe, T.; Hirano, S. Intraoperative Real-Time Assessment of Liver Function with Near-Infrared Fluorescence Imaging. Eur. Surg. Res. 2017, 58, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Nakaseko, Y.; Ishizawa, T.; Saiura, A. Fluorescence-guided surgery for liver tumors. J. Surg. Oncol. 2018, 118, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, T.; Saiura, A.; Kokudo, N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg. Nutr. 2016, 5, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Kono, Y.; Ishizawa, T.; Tani, K.; Harada, N.; Kaneko, J.; Saiura, A.; Bandai, Y.; Kokudo, N. Techniques of Fluorescence Cholangiography During Laparoscopic Cholecystectomy for Better Delineation of the Bile Duct Anatomy. Medicine 2015, 94, e1005. [Google Scholar] [CrossRef]
- Tanaka, M.; Inoue, Y.; Mise, Y.; Ishizawa, T.; Arita, J.; Takahashi, Y.; Saiura, A. Laparoscopic deroofing for polycystic liver disease using laparoscopic fusion indocyanine green fluorescence imaging. Surg. Endosc. 2016, 30, 2620–2623. [Google Scholar] [CrossRef]
- Verbeek, F.P.; Schaafsma, B.E.; Tummers, Q.R.; van der Vorst, J.R.; van der Made, W.J.; Baeten, C.I.; Bonsing, B.A.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L.; et al. Optimization of near-infrared fluorescence cholangiography for open and laparoscopic surgery. Surg. Endosc. 2014, 28, 1076–1082. [Google Scholar] [CrossRef]
- Lieto, E.; Galizia, G.; Cardella, F.; Mabilia, A.; Basile, N.; Castellano, P.; Orditura, M.; Auricchio, A. Indocyanine Green Fluorescence Imaging-Guided Surgery in Primary and Metastatic Liver Tumors. Surg. Innov. 2018, 25, 62–68. [Google Scholar] [CrossRef]
- Ishizawa, T.; Masuda, K.; Urano, Y.; Kawaguchi, Y.; Satou, S.; Kaneko, J.; Hasegawa, K.; Shibahara, J.; Fukayama, M.; Tsuji, S.; et al. Mechanistic background and clinical applications of indocyanine green fluorescence imaging of hepatocellular carcinoma. Ann. Surg. Oncol. 2014, 21, 440–448. [Google Scholar] [CrossRef]
- Ishizawa, T.; Fukushima, N.; Shibahara, J.; Masuda, K.; Tamura, S.; Aoki, T.; Hasegawa, K.; Beck, Y.; Fukayama, M.; Kokudo, N. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009, 115, 2491–2504. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, N.; Otani, T.; Hashidate, H.; Maeda, C.; Katada, T.; Sudo, N.; Manabe, S.; Ikeno, Y.; Toyoda, A.; Katayanagi, N. Real-time detection of hepatic micrometastases from pancreatic cancer by intraoperative fluorescence imaging: Preliminary results of a prospective study. Cancer 2012, 118, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Shi, R.; Hou, J.C.; Liu, Z.R.; Cui, Z.L.; Li, Y.; Wu, D.; Shi, Y.; Shen, Z.Y. Liver tumor boundaries identified intraoperatively using real-time indocyanine green fluorescence imaging. J. Cancer Res. Clin. Oncol. 2017, 143, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souzaki, R.; Kawakubo, N.; Matsuura, T.; Yoshimaru, K.; Koga, Y.; Takemoto, J.; Shibui, Y.; Kohashi, K.; Hayashida, M.; Oda, Y.; et al. Navigation surgery using indocyanine green fluorescent imaging for hepatoblastoma patients. Pediatr. Surg. Int. 2019, 35, 551–557. [Google Scholar] [CrossRef]
- Yamada, Y.; Ohno, M.; Fujino, A.; Kanamori, Y.; Irie, R.; Yoshioka, T.; Miyazaki, O.; Uchida, H.; Fukuda, A.; Sakamoto, S.; et al. Fluorescence-Guided Surgery for Hepatoblastoma with Indocyanine Green. Cancers 2019, 11, 1215. [Google Scholar] [CrossRef] [Green Version]
- Chen-Yoshikawa, T.F.; Hatano, E.; Yoshizawa, A.; Date, H. Clinical application of projection mapping technology for surgical resection of lung metastasis. Interact Cardiovasc. Thorac. Surg. 2017, 25, 1010–1011. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, N.; Yamada, Y.; Hoshino, K.; Kawaida, M.; Mori, T.; Abe, K.; Fujimura, T.; Matsubara, K.; Hibi, T.; Shinoda, M.; et al. Living Donor Liver Re-Transplantation for Recurrent Hepatoblastoma in the Liver Graft following Complete Eradication of Peritoneal Metastases under Indocyanine Green Fluorescence Imaging. Cancers 2019, 11, 730. [Google Scholar] [CrossRef] [Green Version]
- Yamamichi, T.; Oue, T.; Yonekura, T.; Owari, M.; Nakahata, K.; Umeda, S.; Nara, K.; Ueno, T.; Uehara, S.; Usui, N. Clinical application of indocyanine green (ICG) fluorescent imaging of hepatoblastoma. J. Pediatr. Surg. 2015, 50, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, N.; Shinkai, M.; Mochizuki, K.; Usui, H.; Miyagi, H.; Nakamura, K.; Tanaka, M.; Tanaka, Y.; Kusano, M.; Ohtsubo, S. Navigation using indocyanine green fluorescence imaging for hepatoblastoma pulmonary metastases surgery. Pediatr. Surg. Int. 2015, 31, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, E. Fluorescence- Guided Surgery in Pediatric Solid Tumors. In Proceedings of the SIOP2020, KeyNote Lecture, Ottawa, ON, Canada, 16 October 2020. [Google Scholar]
- Yamada, Y.; Hoshino, K.; Mori, T.; Kawaida, M.; Abe, K.; Takahashi, N.; Fujimura, T.; Kameyama, K.; Kuroda, T. Metastasectomy of Hepatoblastoma Utilizing a Novel Overlay Fluorescence Imaging System. J. Laparoendosc Adv. Surg. Tech. A 2018, 28, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Takase, S.; Takada, A.; Matsuda, Y. Studies on the pathogenesis of the constitutional excretory defect of indocyanine green. Gastroenterol. Jpn. 1982, 17, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Onda, N.; Kimura, M.; Yoshida, T.; Shibutani, M. Preferential tumor cellular uptake and retention of indocyanine green for in vivo tumor imaging. Int. J. Cancer 2016, 139, 673–682. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).