Atopic Manifestations in Children Born Preterm: A Long-Term Observational Study
Abstract
:1. Introduction
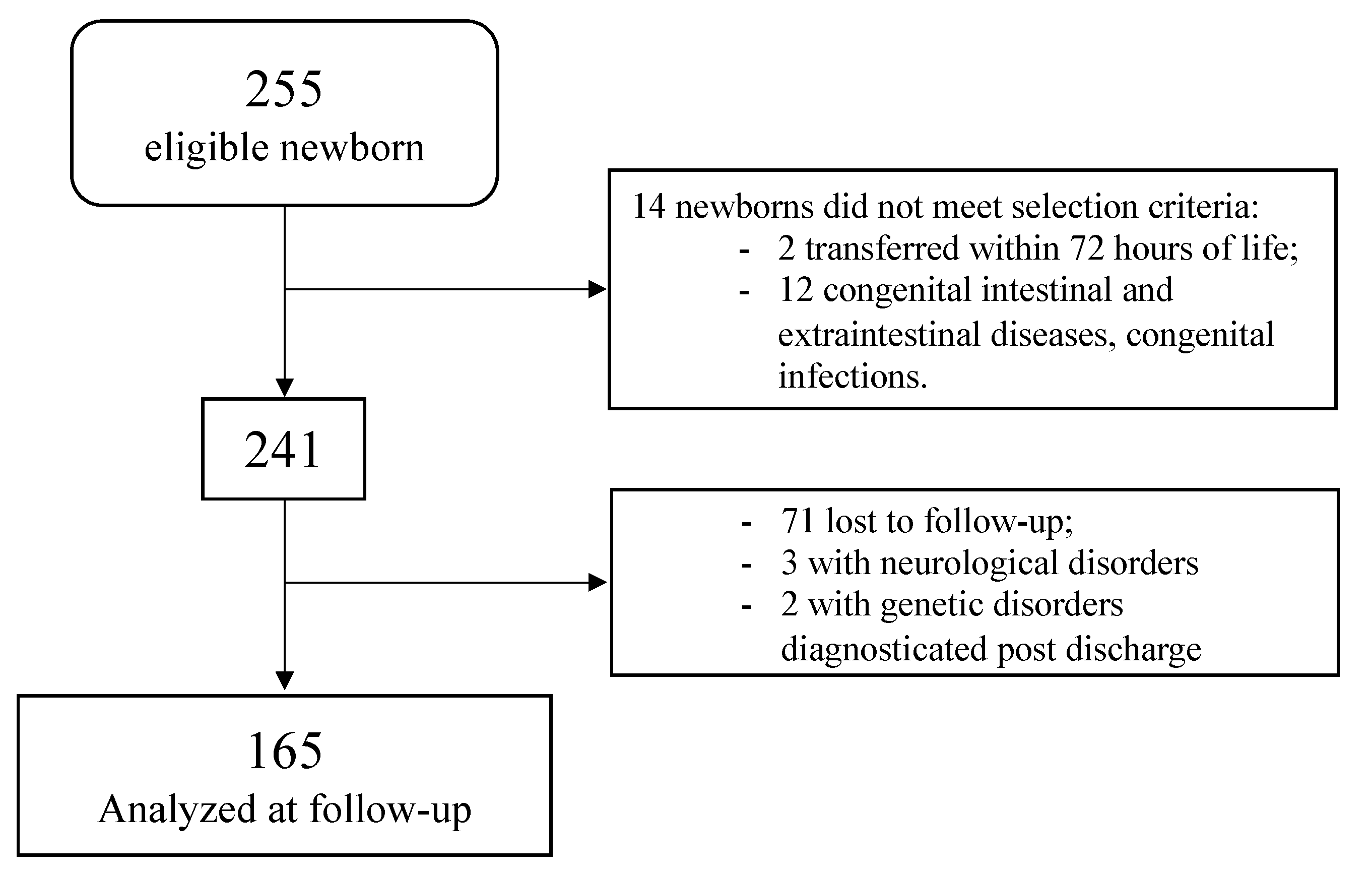
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aw, M.; Penn, J.; Gauvreau, G.M.; Lima, H.; Sehmi, R. Atopic March: Collegium Internationale Allergologicum Update 2020. Int. Arch. Allergy Immunol. 2020, 181, 1–10. [Google Scholar] [CrossRef]
- Sozańska, B.; Błaszczyk, M.; Pearce, N.; Cullinan, P. Atopy and Allergic Respiratory Disease in Rural Poland before and after Accession to the European Union. J. Allergy Clin. Immunol. 2014, 133, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Grammatikos, A.P. The Genetic and Environmental Basis of Atopic Diseases. Ann. Med. 2008, 40, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Groer, M.W.; Gregory, K.E.; Louis-Jacques, A.; Thibeau, S.; Walker, W.A. The Very Low Birth Weight Infant Microbiome and Childhood Health. Birth Defects Res. C Embryo Today 2015, 105, 252–264. [Google Scholar] [CrossRef]
- Goedicke-Fritz, S.; Härtel, C.; Krasteva-Christ, G.; Kopp, M.V.; Meyer, S.; Zemlin, M. Preterm Birth Affects the Risk of Developing Immune-Mediated Diseases. Front. Immunol. 2017, 8, 1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cinicola, B.; Conti, M.G.; Terrin, G.; Sgrulletti, M.; Elfeky, R.; Carsetti, R.; Fernandez Salinas, A.; Piano Mortari, E.; Brindisi, G.; De Curtis, M.; et al. The Protective Role of Maternal Immunization in Early Life. Front. Pediatr. 2021, 9, 638871. [Google Scholar] [CrossRef] [PubMed]
- Siltanen, M.; Kajosaari, M.; Pohjavuori, M.; Savilahti, E. Prematurity at Birth Reduces the Long-Term Risk of Atopy. J. Allergy Clin. Immunol. 2001, 107, 229–234. [Google Scholar] [CrossRef]
- Paller, A.S.; Spergel, J.M.; Mina-Osorio, P.; Irvine, A.D. The Atopic March and Atopic Multimorbidity: Many Trajectories, Many Pathways. J. Allergy Clin. Immunol. 2019, 143, 46–55. [Google Scholar] [CrossRef]
- Gonçalves, C.; Wandalsen, G.; Lanza, F.; Goulart, A.L.; Solé, D.; Dos Santos, A. Repercussions of Preterm Birth on Symptoms of Asthma, Allergic Diseases and Pulmonary Function, 6–14 Years Later. Allergol. Immunopathol. 2016, 44, 489–496. [Google Scholar] [CrossRef]
- Morata-Alba, J.; Romero-Rubio, M.T.; Castillo-Corullón, S.; Escribano-Montaner, A. Respiratory Morbidity, Atopy and Asthma at School Age in Preterm Infants Aged 32–35 Weeks. Eur. J. Pediatr. 2019, 178, 973–982. [Google Scholar] [CrossRef]
- Di Mauro, A.; Baldassarre, M.E.; Brindisi, G.; Zicari, A.M.; Tarantini, M.; Laera, N.; Capozza, M.; Panza, R.; Salvatore, S.; Pensabene, L.; et al. Hydrolyzed Protein Formula for Allergy Prevention in Preterm Infants: Follow-Up Analysis of a Randomized, Triple-Blind, Placebo-Controlled Study. Front. Pediatr. 2020, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Passariello, A. Diarrhea in Neonatal Intensive Care Unit. WJG 2010, 16, 2664. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Terrin, G. Recent Progress in Congenital Diarrheal Disorders. Curr. Gastroenterol. Rep. 2011, 13, 257–264. [Google Scholar] [CrossRef]
- Ferreira, C.R.; van Karnebeek, C.D.M. Inborn errors of metabolism. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 162, pp. 449–481. [Google Scholar]
- Nocerino, R.; Paparo, L.; Terrin, G.; Pezzella, V.; Amoroso, A.; Cosenza, L.; Cecere, G.; De Marco, G.; Micillo, M.; Albano, F.; et al. Cow’s Milk and Rice Fermented with Lactobacillus Paracasei CBA L74 Prevent Infectious Diseases in Children: A Randomized Controlled Trial. Clin. Nutr. 2017, 36, 118–125. [Google Scholar] [CrossRef]
- Salvia, G.; Cascioli, C.F.; Ciccimarra, F.; Terrin, G.; Cucchiara, S. A Case of Protein-Losing Enteropathy Caused by Intestinal Lymphangiectasia in a Preterm Infant. Pediatrics 2001, 107, 416–417. [Google Scholar] [CrossRef]
- Passariello, A.; Terrin, G.; Cecere, G.; Micillo, M.; Marco, G.; Di Costanzo, M.; Cosenza, L.; Leone, L.; Nocerino, R.; Berni Canani, R. Randomised Clinical Trial: Efficacy of a New Synbiotic Formulation Containing Lactobacillus Paracasei B21060 plus Arabinogalactan and Xilooligosaccharides in Children with Acute Diarrhoea. Aliment. Pharmacol. Ther. 2012, 35, 782–788. [Google Scholar] [CrossRef]
- Terrin, G.; Boscarino, G.; Gasparini, C.; Chiara, M.D.; Faccioli, F.; Onestà, E.; Parisi, P.; Spalice, A.; De Nardo, M.C.; Dito, L.; et al. Energy-Enhanced Parenteral Nutrition and Neurodevelopment of Preterm Newborns: A Cohort Study. Nutrition 2021, 111219. [Google Scholar] [CrossRef]
- Terrin, G.; Di Chiara, M.; Boscarino, G.; Versacci, P.; Di Donato, V.; Giancotti, A.; Pacelli, E.; Faccioli, F.; Onestà, E.; Corso, C.; et al. Echocardiography-Guided Management of Preterms With Patent Ductus Arteriosus Influences the Outcome: A Cohort Study. Front. Pediatr. 2020, 8, 582735. [Google Scholar] [CrossRef]
- Terrin, G.; Passariello, A.; Canani, R.B.; Manguso, F.; Paludetto, R.; Cascioli, C. Minimal Enteral Feeding Reduces the Risk of Sepsis in Feed-Intolerant Very Low Birth Weight Newborns. Acta Paediatr. 2009, 98, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Passariello, A.; Buccigrossi, V.; Terrin, G.; Guarino, A. The Nutritional Modulation of the Evolving Intestine. J. Clin. Gastroenterol. 2008, 42, S197–S200. [Google Scholar] [CrossRef] [PubMed]
- Boscarino, G.; Conti, M.G.; De Luca, F.; Di Chiara, M.; Deli, G.; Bianchi, M.; Favata, P.; Cardilli, V.; Di Nardo, G.; Parisi, P.; et al. Intravenous Lipid Emulsions Affect Respiratory Outcome in Preterm Newborn: A Case-Control Study. Nutrients 2021, 13, 1243. [Google Scholar] [CrossRef]
- Boscarino, G.; Conti, M.G.; Gasparini, C.; Onestà, E.; Faccioli, F.; Dito, L.; Regoli, D.; Spalice, A.; Parisi, P.; Terrin, G. Neonatal Hyperglycemia Related to Parenteral Nutrition Affects Long-Term Neurodevelopment in Preterm Newborn: A Prospective Cohort Study. Nutrients 2021, 13, 1930. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Tom, W.L.; Chamlin, S.L.; Feldman, S.R.; Hanifin, J.M.; Simpson, E.L.; Berger, T.G.; Bergman, J.N.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of Care for the Management of Atopic Dermatitis. J. Am. Acad. Dermatol. 2014, 70, 338–351. [Google Scholar] [CrossRef] [Green Version]
- Brand, P.L.P.; Baraldi, E.; Bisgaard, H.; Boner, A.L.; Castro-Rodriguez, J.A.; Custovic, A.; de Blic, J.; de Jongste, J.C.; Eber, E.; Everard, M.L.; et al. Definition, Assessment and Treatment of Wheezing Disorders in Preschool Children: An Evidence-Based Approach. Eur. Respir. J. 2008, 32, 1096–1110. [Google Scholar] [CrossRef]
- Berni Canani, R.; Nocerino, R.; Terrin, G.; Di Costanzo, M.; Cosenza, L.; Troncone, R. Food Allergy Diagnostic Practice in Italian Children. J. Allergy Clin. Immunol. 2012, 129, 1423–1424. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and Management of Food Allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Siltanen, M.; Wehkalampi, K.; Hovi, P.; Eriksson, J.G.; Strang-Karlsson, S.; Järvenpää, A.-L.; Andersson, S.; Kajantie, E. Preterm Birth Reduces the Incidence of Atopy in Adulthood. J. Allergy Clin. Immunol. 2011, 127, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A.; Lang, D.M.; Khan, D.A.; Craig, T.; Dreyfus, D.; Hsieh, F.; Sheikh, J.; Weldon, D.; Zuraw, B.; Bernstein, D.I.; et al. The Diagnosis and Management of Acute and Chronic Urticaria: 2014 Update. J. Allergy Clin. Immunol. 2014, 133, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Ünal, S.; Kaya, A.; Bilgin, L.; Misirlioğlu, E.; Kocabaş, C.N. Wheezing, Asthma, and Atopy in Premature Infants at 2 Years of Age. Turk. J. Med. Sci. 2017, 47, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Carstens, L.E.; Westerbeek, E.A.M.; van Zwol, A.; van Elburg, R.M. Neonatal Antibiotics in Preterm Infants and Allergic Disorders Later in Life. Pediatr. Allergy Immunol. 2016, 27, 759–764. [Google Scholar] [CrossRef]
- Morgan, J.; Williams, P.; Norris, F.; Williams, C.M.; Larkin, M.; Hampton, S. Eczema and Early Solid Feeding in Preterm Infants. Arch. Dis. Child. 2004, 89, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Kvenshagen, B.; Jacobsen, M.; Halvorsen, R. Atopic Dermatitis in Premature and Term Children. Arch. Dis. Child. 2009, 94, 202–205. [Google Scholar] [CrossRef]
- Haataja, P.; Korhonen, P.; Ojala, R.; Hirvonen, M.; Paassilta, M.; Gissler, M.; Luukkaala, T.; Tammela, O. Asthma and Atopic Dermatitis in Children Born Moderately and Late Preterm. Eur. J. Pediatr. 2016, 175, 799–808. [Google Scholar] [CrossRef]
- Barbarot, S.; Gras-Leguen, C.; Colas, H.; Garrot, E.; Darmaun, D.; Larroque, B.; Roze, J.C.; Ancel, P.Y. Lower Risk of Atopic Dermatitis among Infants Born Extremely Preterm Compared with Higher Gestational Age. Br. J. Dermatol. 2013, 169, 1257–1264. [Google Scholar] [CrossRef]
- Trønnes, H.; Wilcox, A.J.; Lie, R.T.; Markestad, T.; Moster, D. The Association of Preterm Birth with Severe Asthma and Atopic Dermatitis: A National Cohort Study. Pediatr. Allergy Immunol. 2013, 24, 782–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Yu, M.; Wang, P.; Qian, H.; Fan, Y.; Li, X.; Xu, Q.; Wang, X.; Wang, X.; Lu, C. Association between Maternal Diabetes Mellitus and Allergic Diseases in Children—a Systematic Review and Meta-Analysis. Pediatr. Allergy Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ouyang, F.; Story, R.E.; Pongracic, J.A.; Hong, X.; Wang, G.; Pearson, C.; Ortiz, K.; Bauchner, H.; Wang, X. Gestational Diabetes, Atopic Dermatitis, and Allergen Sensitization in Early Childhood. J. Allergy Clin. Immunol. 2009, 124, 1031–1038.e1-4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Plaut, M.; Bahnson, H.T.; Mitchell, H.; Radulovic, S.; Chan, S.; Fox, A.; Turcanu, V.; et al. Identifying Infants at High Risk of Peanut Allergy: The Learning Early About Peanut Allergy (LEAP) Screening Study. J. Allergy Clin. Immunol. 2013, 131, 135–143.e1-12. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.; Lou, W.; Tun, H.M.; Konya, T.B.; Morales-Lizcano, N.; Chari, R.S.; Field, C.J.; Guttman, D.S.; Mandal, R.; Wishart, D.S.; et al. From Birth to Overweight and Atopic Disease: Multiple and Common Pathways of the Infant Gut Microbiome. Gastroenterology 2021, 160, 128–144.e10. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Guttman, D.S.; Field, C.J.; Sears, M.R.; HayGlass, K.T.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Becker, A.B.; et al. Infant Gut Microbiota and Food Sensitization: Associations in the First Year of Life. Clin. Exp. Allergy 2015, 45, 632–643. [Google Scholar] [CrossRef]
- Lewandowski, K.C.; Stojanovic, N.; Press, M.; Tuck, S.M.; Szosland, K.; Bienkiewicz, M.; Vatish, M.; Lewinski, A.; Prelevic, G.M.; Randeva, H.S. Elevated Serum Levels of Visfatin in Gestational Diabetes: A Comparative Study across Various Degrees of Glucose Tolerance. Diabetologia 2007, 50, 1033–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Yang, H.; Zhao, Y. Variations of Tumor Necrosis Factor-Alpha, Leptin and Adiponectin in Mid-Trimester of Gestational Diabetes Mellitus. Chin. Med. J. 2008, 121, 701–705. [Google Scholar] [CrossRef]
- McGowan, E.C.; Bloomberg, G.R.; Gergen, P.J.; Visness, C.M.; Jaffee, K.F.; Sandel, M.; O’Connor, G.; Kattan, M.; Gern, J.; Wood, R.A. Influence of Early-Life Exposures on Food Sensitization and Food Allergy in an Inner-City Birth Cohort. J. Allergy Clin. Immunol. 2015, 135, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zutavern, A.; Brockow, I.; Schaaf, B.; Bolte, G.; von Berg, A.; Diez, U.; Borte, M.; Herbarth, O.; Wichmann, H.-E.; Heinrich, J.; et al. Timing of Solid Food Introduction in Relation to Atopic Dermatitis and Atopic Sensitization: Results from a Prospective Birth Cohort Study. Pediatrics 2006, 117, 401–411. [Google Scholar] [CrossRef]
- Prescott, S.L.; Smith, P.; Tang, M.; Palmer, D.J.; Sinn, J.; Huntley, S.J.; Cormack, B.; Heine, R.G.; Gibson, R.A.; Makrides, M. The Importance of Early Complementary Feeding in the Development of Oral Tolerance: Concerns and Controversies. Pediatr. Allergy Immunol. 2008, 19, 375–380. [Google Scholar] [CrossRef]
- Davidson, W.F.; Leung, D.Y.M.; Beck, L.A.; Berin, C.M.; Boguniewicz, M.; Busse, W.W.; Chatila, T.A.; Geha, R.S.; Gern, J.E.; Guttman-Yassky, E.; et al. Report from the National Institute of Allergy and Infectious Diseases Workshop on “Atopic Dermatitis and the Atopic March: Mechanisms and Interventions”. J. Allergy Clin. Immunol. 2019, 143, 894–913. [Google Scholar] [CrossRef]
- Tran, M.M.; Lefebvre, D.L.; Dharma, C.; Dai, D.; Lou, W.Y.W.; Subbarao, P.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Sears, M.R.; et al. Predicting the Atopic March: Results from the Canadian Healthy Infant Longitudinal Development Study. J. Allergy Clin. Immunol. 2018, 141, 601–607.e8. [Google Scholar] [CrossRef] [Green Version]
- Gough, H.; Grabenhenrich, L.; Reich, A.; Eckers, N.; Nitsche, O.; Schramm, D.; Beschorner, J.; Hoffmann, U.; Schuster, A.; Bauer, C.; et al. Allergic Multimorbidity of Asthma, Rhinitis and Eczema over 20 Years in the German Birth Cohort MAS. Pediatr. Allergy Immunol. 2015, 26, 431–437. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Depner, M.; Karvonen, A.M.; Renz, H.; Braun-Fahrländer, C.; Schmausser-Hechfellner, E.; Pekkanen, J.; Riedler, J.; Dalphin, J.-C.; et al. Phenotypes of Atopic Dermatitis Depending on the Timing of Onset and Progression in Childhood. JAMA Pediatr. 2017, 171, 655. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.D.; Wright, A.L.; Taussig, L.M.; Holberg, C.J.; Halonen, M.; Morgan, W.J. Asthma and Wheezing in the First Six Years of Life. N. Engl. J. Med. 1995, 332, 133–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, A.J.; Angelica, B.; Su, J.; Lodge, C.J.; Hill, D.J.; Erbas, B.; Bennett, C.M.; Gurrin, L.C.; Axelrad, C.; Abramson, M.J.; et al. Age at Onset and Persistence of Eczema Are Related to Subsequent Risk of Asthma and Hay Fever from Birth to 18 Years of Age. Pediatr. Allergy Immunol. 2017, 28, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Barberio, G.; Pajno, G.B.; Vita, D.; Caminiti, L.; Canonica, G.W.; Passalacqua, G. Does a ‘Reverse’ Atopic March Exist? Allergy 2008, 63, 1630–1632. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Kuo, H.-F.; Huang, C.-H.; Yang, S.-N.; Lee, M.-S.; Hung, C.-H. Early Life Exposure to Antibiotics and the Risk of Childhood Allergic Diseases: An Update from the Perspective of the Hygiene Hypothesis. J. Microbiol. Immunol. Infect. 2013, 46, 320–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| All Newborns (n = 165) | At Least One Atopic Manifestation | Atopic Dermatitis | Wheeze | Food Allergy | |||||
|---|---|---|---|---|---|---|---|---|---|
| Case (n = 72) | Control (n = 93) | Case (n = 32) | Control (n = 133) | Case (n = 37) | Control (n = 128) | Case (n = 12) | Control (n = 153) | ||
| Antenatal corticosteroids a | 112 (67.9) | 46 (63.9) | 66 (71.0) | 20 (62.5) | 92 (69.2) | 24 (64.9) | 88 (68.8) | 7 (58.3) | 105 (68.6) |
| Prepartum antibiotic prophylaxis b | 45 (27.4) | 22 (31.0) | 23 (24.7) | 5 (15.6) | 40 (30.1) | 12 (32.4) | 33 (26.0) | 2 (16.7) | 40 (26.1) |
| Gestational diabetes | 16 (9.8) | 11 (15.5) * | 5 (5.4) | 8 (25.0) ** | 8 (6.0) | 4 (10.8) | 12 (9.5) | 1 (8.3) | 15 (9.8) |
| Intrauterine growth restriction | 17 (10.4) | 7 (9.9) | 10 (10.9) | 5 (15.6) | 12 (9.0) | 1 (2.7) | 16 (12.5) | 2 (16.7) | 15 (9.8) |
| Cesarean section | 147 (89.1) | 63 (87.5) | 84 (90.3) | 27 (84.4) | 120 (90.2) | 34 (91.9) | 113 (88.3) | 10 (83.3) | 137 (89.5) |
| Maternal smoke during pregnancy | 52 (31.5) | 19 (26.4) | 33 (35.5) | 9 (28.1) | 43 (32.3) | 8 (21.6) | 44 (34.4) | 4 (33.3) | 48 (31.4) |
| Familiarity for atopy | 44 (26.7) | 15 (20.8) | 29 (31.2) | 7 (21.9) | 37 (27.8) | 7 (18.9) | 37 (28.9) | 4 (33.3) | 40 (26.1) |
| Familiarity for asthma | 13 (7.9) | 7 (9.7) | 6 (6.5) | 3 (9.4) | 10 (7.5) | 6 (16.2) * | 7 (5.5) | 0 (0) | 13 (8.5) |
| All Newborns (n = 165) | At Least One Atopic Manifestation | Atopic Dermatitis | Wheeze | Food Allergy | |||||
|---|---|---|---|---|---|---|---|---|---|
| Case (n = 72) | Control (n = 93) | Case (n = 32) | Control (n = 133) | Case (n = 37) | Control (n = 128) | Case (n = 12) | Control (n = 153) | ||
| Born in autumn or winter, No. (%) | 66 (40.0) | 42 (58.3) | 57 (61.3) | 13 (40.6) | 53 (39.8) | 18 (48.6) | 48 (37.5) | 3 (25.0) | 63 (41.2) |
| Gestational age, weeks | 30 (29 to 30) | 30 (29 to 30) | 30 (29 to 30) | 31 (30 to 31) * | 30 (29 to 30) | 29 (29 to 30) | 30 (29 to 30) | 30 (28 to 32) | 30 (29 to 30) |
| <29 weeks, No. (%) | 46 (27.9) | 16 (22.2) | 30 (32.3) | 2 (6.3) ** | 44 (33.1) | 12 (32.4) | 34 (26.6) | 4 (33.3) | 42 (27.5) |
| Birth weight, grams | 1283 (1232 to 1333) | 1325 (1255 to 1394) | 1250 (1178 to 1322) | 1359 (1258 to 1461) | 1264 (1207 to 1322) | 1289 (1188 to 1391) | 1281 (1222 to 1340) | 1280 (1089 to 1471) | 1283 (1230 to 1336) |
| ELBW, No. (%) | 37 (22.4) | 14 (19.4) | 23 (24.7) | 6 (18.8) | 31 (23.3) | 7 (18.9) | 30 (23.4) | 3 (25.0) | 34 (22.2) |
| SGA at birth, No. (%) | 30 (18.2) | 13 (18.1) | 17 (18.3) | 8 (25.0) | 22 (16.5) | 5 (13.5) | 25 (19.5) | 3 (25.0) | 27 (17.6) |
| Male, No. (%) | 86 (52.1) | 43 (59.7) | 43 (46.2) | 22 (68.8) * | 64 (48.1) | 22 (59.5) | 64 (50.0) | 6 (50.0) | 80 (52.3) |
| pH at birth | 7.3 (7.2 to 7.3) | 7.3 (7.2 to 7.3) | 7.3 (7.2 to 7.3) | 7.3 (7.2 to 7.3) | 7.3 (7.2 to 7.3) | 7.3 (7.2 to 7.3) | 7.3 (7.2 to 7.3) | 7.3 (7.2 to 7.3) | 7.3 (7.2 to 7.3) |
| CRIB II score a | 6 (5 to 6) | 6 (5 to 6) | 6 (5 to 7) | 5 (4 to 6) | 6 (5 to 7) | 6 (5 to 7) | 6 (5 to 6) | 5 (3 to 7) | 6 (5 to 6) |
| All Newborns (n = 165) | At Least One Atopic Manifestation | Atopic Dermatitis | Wheeze | Food Allergy | |||||
|---|---|---|---|---|---|---|---|---|---|
| Case (n = 72) | Control (n = 93) | Case (n = 32) | Control (n = 133) | Case (n = 37) | Control (n = 128) | Case (n = 12) | Control (n = 153) | ||
| Necrotizing enterocolitis | 6 (3.6) | 5 (6.9) | 1 (1.1) | 3 (9.4) | 3 (2.3) | 1 (2.7) | 5 (3.9) | 1 (8.3) | 5 (3.3) |
| Intraventricular hemorrhage | 6 (3.7) | 1 (1.4) | 5 (5.4) | 1 (3.1) | 5 (3.8) | 1 (2.7) | 5 (4.1) | 0 (0) | 6 (3.9) |
| Bronchopulmonary dysplasia | 7 (4.3) | 2 (2.8) | 5 (5.4) | 1 (3.1) | 6 (4.5) | 2 (5.4) | 5 (4.1) | 0 (0) | 7 (4.6) |
| Retinopathy of prematurity | 21 (12.7) | 11 (15.3) | 20 (21.5) | 2 (6.3) | 19 (14.3) | 4 (10.8) | 17 (13.3) | 1 (8.3) | 20 (13.1) |
| Sepsis proven by positive culture | 8 (4.8) | 3 (4.2) | 5 (5.4) | 1 (3.1) | 7 (5.3) | 2 (5.4) | 6 (4.7) | 0 (0) | 8 (5.2) |
| Noninvasive mechanical ventilation | 126 (76.4) | 57 (79.2) | 69 (74.2) | 26 (81.3) | 100 (75.2) | 27 (73.0) | 99 (77.3) | 7 (58.3) | 119 (77.8) |
| Invasive mechanical ventilation | 43 (26.1) | 16 (22.2) | 27 (29.0) | 5 (15.6) | 38 (28.6) | 10 (27.0) | 33 (25.8) | 2 (16.7) | 41 (26.8) |
| Prolonged use of antibiotic a | 34 (20.6) | 21 (29.2) * | 13 (14.0) | 10 (31.3) | 24 (18.0) | 12 (32.4) * | 22 (17.2) | 2 (16.7) | 32 (20.9) |
| Antibiotic treatment, mean days (95% CI) | 13 (12 to 14) | 14 (12 to 16) | 12 (11 to 13) | 13 (10 to 16) | 13 (12 to 14) | 14 (12 to 17) | 13 (11 to 14) | 14 (9 to 19) | 13 (12 to 14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, F.; Conti, M.G.; Boscarino, G.; Pannucci, C.; Dito, L.; Regoli, D.; Di Chiara, M.; Battaglia, G.; Prota, R.; Cinicola, B.; et al. Atopic Manifestations in Children Born Preterm: A Long-Term Observational Study. Children 2021, 8, 843. https://doi.org/10.3390/children8100843
Pagano F, Conti MG, Boscarino G, Pannucci C, Dito L, Regoli D, Di Chiara M, Battaglia G, Prota R, Cinicola B, et al. Atopic Manifestations in Children Born Preterm: A Long-Term Observational Study. Children. 2021; 8(10):843. https://doi.org/10.3390/children8100843
Chicago/Turabian StylePagano, Federica, Maria Giulia Conti, Giovanni Boscarino, Chiara Pannucci, Lucia Dito, Daniela Regoli, Maria Di Chiara, Giuseppe Battaglia, Rita Prota, Bianca Cinicola, and et al. 2021. "Atopic Manifestations in Children Born Preterm: A Long-Term Observational Study" Children 8, no. 10: 843. https://doi.org/10.3390/children8100843
APA StylePagano, F., Conti, M. G., Boscarino, G., Pannucci, C., Dito, L., Regoli, D., Di Chiara, M., Battaglia, G., Prota, R., Cinicola, B., Zicari, A. M., Aloi, M., Oliva, S., & Terrin, G. (2021). Atopic Manifestations in Children Born Preterm: A Long-Term Observational Study. Children, 8(10), 843. https://doi.org/10.3390/children8100843








