Current Perspectives on Management of Type 2 Diabetes in Youth
Abstract
1. Introduction
2. Lifestyle Modifications
3. Pharmacological Interventions
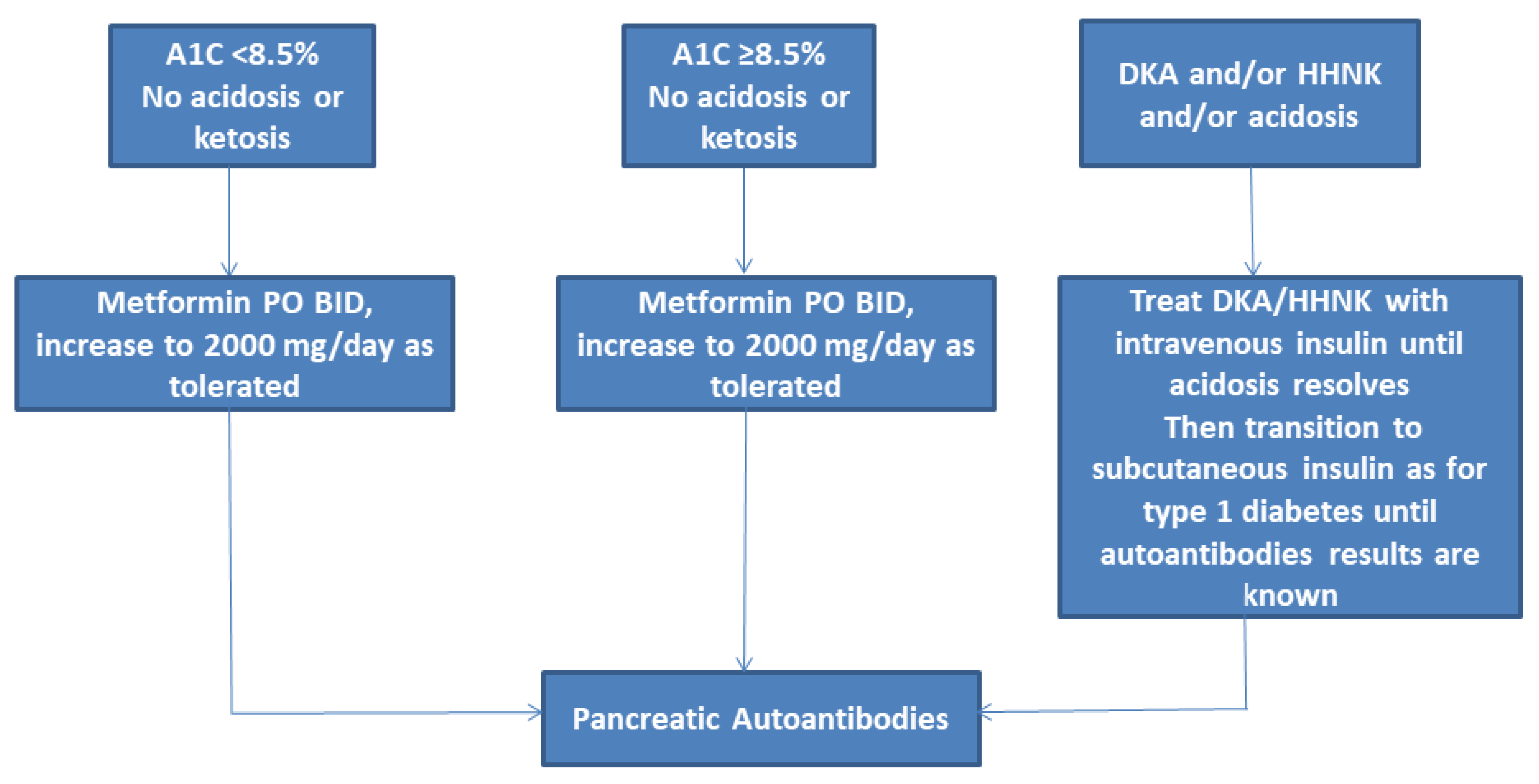
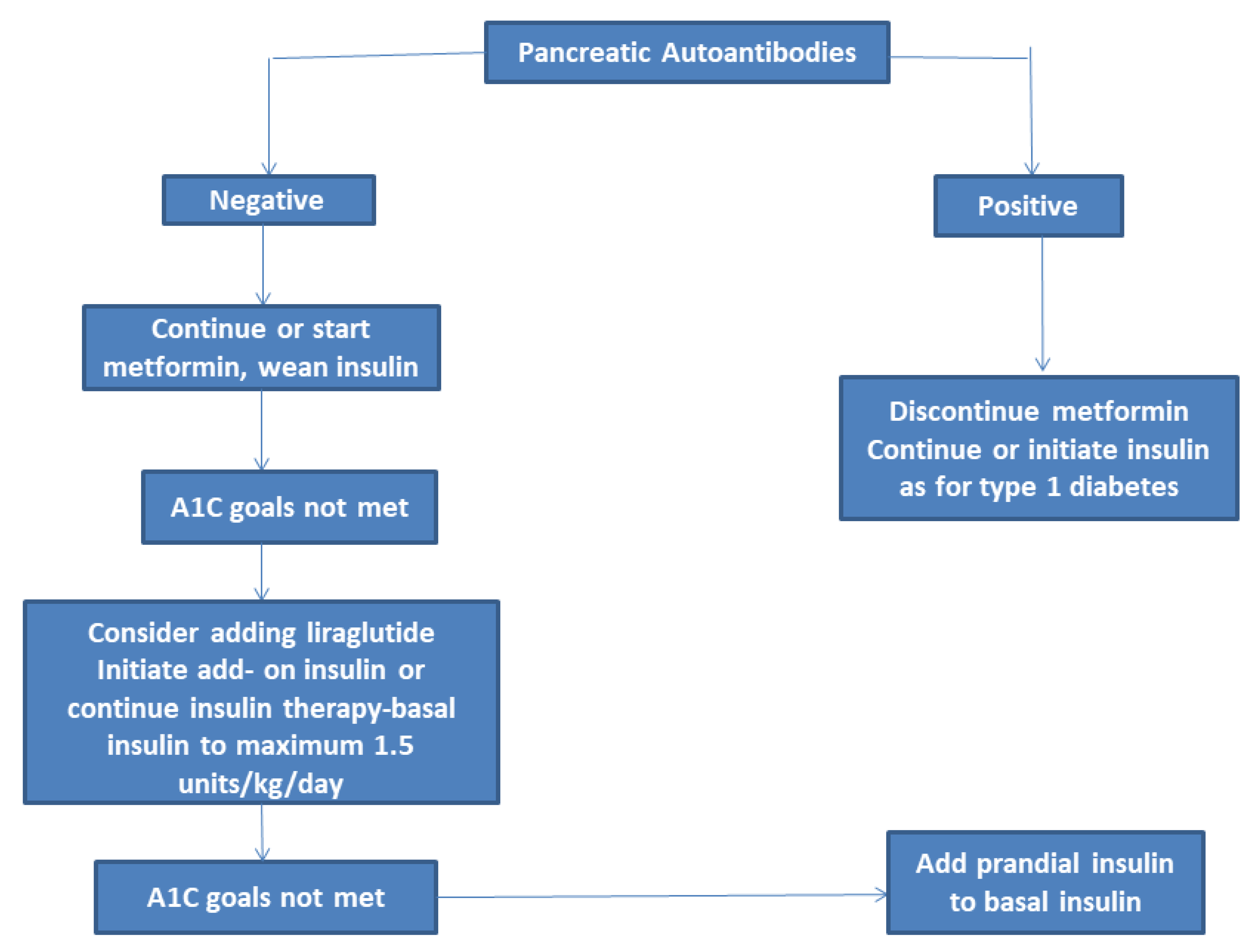
3.1. Metformin
3.2. Liraglutide
3.3. Thiazoledinediones
3.4. Sulfonylureas
3.5. Dipeptidyl Peptidase-4 (DPP-4) Inhibitors
3.6. Sodium-Glucose Co-Transporter 2 (SGLT2) Inhibitors
3.7. Insulin
| Drug Name | Mechanism of Action | FDA Indication | Dose | Side Effects | |
|---|---|---|---|---|---|
| Metformin | Increases insulin mediated glucose uptake in peripheral tissues and decreases hepatic glucose production | T2DM ≥ 10 years | Start at 500–1000 mg per day, Increase to 2000 mg/day in 1–2 divided doses | Diarrhea, nausea, epigastric discomfort, vomiting, flatulence, B12 deficiency, lactic acidosis | |
| Liraglutide | Increases glucose-dependent insulin release from the pancreatic beta cells and inhibits post-meal glucagon release, also slows gastric emptying | T2DM ≥ 10 years of age | Start at 0.6 mg subcutaneously once daily. Increase by 0.6 mg increments every 1–2 weeks or longer to a maximum of 1.8 mg daily | Nausea, vomiting, abdominal pain, diarrhea, potential hypoglycemia | |
| Rosiglitazone | Agonist of peroxisome proliferator-activated receptors (PPARs), mostly PPAR gamma that increases insulin sensitivity by its effect on adipose tissue and muscle to increase glucose utilization; also decreases hepatic glucose production | Not approved in adolescents | Start at 2 mg twice daily, then increase to 4 mg twice daily after 8 weeks | Weight gain, fluid retention, decreased bone density, heart failure, anemia | |
| Glimepiride | Stimulation of insulin secretion via inhibition of the adenosine triphosphate (ATP) sensitive potassium channel (K-ATP channel) in the pancreatic beta cells | Not approved in adolescents | Start at 1 mg daily, can increase in 1–2 mg increments to maximum of 8 mg daily | Weight gain, hypoglycemia | |
| Linagliptin | Prolonging the action of endogenous GLP-1 and GIP, translating in a glucose-appropriate increase in insulin secretion and suppression of glucagon release | Not approved in adolescents | 1 and 5 mg daily were used in one study [53] (5 mg was more effective in decreasing fasting blood glucose) | Upper respiratory infection, headache, and hypersensitivity reactions | |
| Empagliflozin | Decreases reabsorption of glucose and thereby increases urinary glucose excretion | Not approved in adolescents | Dose in adults: start at 10 mg daily, can increase to 25 mg once daily after 4–12 weeks | Vulvovaginal candidiasis, urinary tract infections, hypotension, risk of diabetic ketoacidosis | |
| Insulin: Basal (such as Glargine, Detemir, Degludec) and short acting (Aspart, Lispro) | Facilitates cellular uptake of glucose into myofibers and adipocytes and stimulates GLUT4 translocation | Approved for adolescents with T2DM | Basal insulin start at 0.25–0.5 units/kg/day and increase based on blood glucose. Dose of short acting is determined by blood glucose values | Hypoglycemia, weight gain, hypertrophy/lipoatrophy at injection site | |
4. Metabolic Surgery
- (1)
- Medically correctable cause of obesity;
- (2)
- An ongoing substance abuse problem (within the preceding year);
- (3)
- A medical, psychiatric, psychosocial, or cognitive condition that prevents adherence to postoperative dietary and medication regimens or impairs decisional capacity;
- (4)
- Current or planned pregnancy within 12 to 18 months of the procedure; and
- (5)
- Inability on the part of the patient or parent to comprehend the risks and benefits of the surgical procedure
5. Assessment of Comorbid Conditions
5.1. Dyslipidemia
5.2. Hypertension
5.3. Nonalcoholic Fatty Liver Disease
6. Assessment of Complications
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Murtagh, Elaine, and NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J. Type 2 Diabetes in Youth: Epidemiology and Current Research toward Prevention and Treatment. J. Am. Diet. Assoc. 2008, 108, S45–S51. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; Lawrence, J.M.; Dabelea, D.; Divers, J.; Isom, S.; Dolan, L.; Imperatore, G.; Linder, B.; Marcovina, S.; Pettitt, D.J.; et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N. Engl. J. Med. 2017, 376, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Dabelea, D.; Mayer-Davis, E.J.; Saydah, S.; Imperatore, G.; Linder, B.; Divers, J.; Bell, R.; Badaru, A.; Talton, J.W.; Crume, T.; et al. Prevalence of Type 1 and Type 2 Diabetes Among Children and Adolescents From 2001 to 2009. JAMA 2014, 311, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Pinhas-Hamiel, O.; Zeitler, P. The global spread of type 2 diabetes mellitus in children and adolescents. J. Pediatr. 2005, 146, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Likitmaskul, S.; Kiattisathavee, P.; Chaichanwatanakul, K.; Punnakanta, L.; Angsusingha, K.; Tuchinda, C. Increasing prevalence of type 2 diabetes mellitus in Thai children and adolescents associated with increasing prevalence of obesity. J. Pediatr. Endocrinol. Metab. 2003, 16, 71–78. [Google Scholar] [CrossRef]
- Urakami, T.; Kubota, S.; Nitadori, Y.; Harada, K.; Owada, M.; Kitagawa, T. Annual Incidence and Clinical Characteristics of Type 2 Diabetes in Children as Detected by Urine Glucose Screening in the Tokyo Metropolitan Area. Diabetes Care 2005, 28, 1876–1881. [Google Scholar] [CrossRef]
- Nadeau, K.J.; Anderson, B.J.; Berg, E.G.; Chiang, J.L.; Chou, H.; Copeland, K.C.; Hannon, T.S.; Huang, T.T.-K.; Lynch, J.L.; Powell, J.; et al. Youth-Onset Type 2 Diabetes Consensus Report: Current Status, Challenges, and Priorities. Diabetes Care 2016, 39, 1635–1642. [Google Scholar] [CrossRef]
- Bacha, F.; Kim, J.Y.; Nasr, A.; Bacha, F.; Tfayli, H.; Lee, S.; Toledo, F.G.S. Insulin sensitivity across the lifespan from obese adolescents to obese adults with impaired glucose tolerance: Who is worse off? Pediatr. Diabetes 2017, 19, 205–211. [Google Scholar] [CrossRef]
- Zeitler, P.; Hirst, K.; Copeland, K.C.; El Ghormli, L.; Katz, L.L.; Levitsky, L.L.; Linder, B.; McGuigan, P.; White, N.H.; Wilfley, D. HbA1cAfter a Short Period of Monotherapy With Metformin Identifies Durable Glycemic Control Among Adolescents With Type 2 Diabetes. Diabetes Care 2015, 38, 2285–2292. [Google Scholar] [CrossRef]
- TODAY Study Group. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: The TODAY clinical trial. Diabetes Care 2013, 36, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.E.L.; Bacha, F.; Gidding, S.S.; Weinstock, R.S.; El Ghormli, L.; Libman, I.; Nadeau, K.J.; Porter, K.; Marcovina, S.; McKay, S.; et al. Lipid Profiles, Inflammatory Markers, and Insulin Therapy in Youth with Type 2 Diabetes. J. Pediatr. 2018, 196, 208–216.e2. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.J.; Sacre, J.W.; Harding, J.L.; Gregg, E.W.; Zimmet, P.Z.; Shaw, J.E. Young-onset type 2 diabetes mellitus—Implications for morbidity and mortality. Nat. Rev. Endocrinol. 2020, 16, 321–331. [Google Scholar] [CrossRef] [PubMed]
- TODAY Study Group. Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: The TODAY clinical trial. Diabetes Care 2013, 36, 1758–1764. [Google Scholar] [CrossRef]
- Bacha, F.; Bacha, F.; Grey, M.; Marcus, M.D.; White, N.H.; Zeitler, P. Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care 2018, 41, 2648–2668. [Google Scholar] [CrossRef]
- Rice Consortium; Rice Consortium Investigators. Effects of Treatment of Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes With Metformin Alone or in Combination With Insulin Glargine on beta-Cell Function: Comparison of Responses In Youth And Adults. Diabetes 2019, 68, 1670–1680. [Google Scholar]
- Zeitler, P.; Arslanian, S.; Fu, J.; Pinhas-Hamiel, O.; Reinehr, T.; Tandon, N.; Urakami, T.; Wong, J.; Maahs, D.M. ISPAD Clinical Practice Consensus Guidelines 2018: Type 2 diabetes mellitus in youth. Pediatr. Diabetes 2018, 19, 28–46. [Google Scholar] [CrossRef]
- TODAY Study Group. Safety and tolerability of the treatment of youth-onset type 2 diabetes: The TODAY experience. Diabetes Care 2013, 36, 1765–1771. [Google Scholar] [CrossRef]
- Reinehr, T.; Kiess, W.; Kapellen, T.; Andler, W. Insulin Sensitivity among Obese Children and Adolescents, According to Degree of Weight Loss. Pediatrics 2004, 114, 1569–1573. [Google Scholar] [CrossRef]
- Reinehr, T.; Lass, N.; Toschke, C.; Rothermel, J.; Lanzinger, S.; Holl, R.W. Which Amount of BMI-SDS Reduction Is Necessary to Improve Cardiovascular Risk Factors in Overweight Children? J. Clin. Endocrinol. Metab. 2016, 101, 3171–3179. [Google Scholar] [CrossRef]
- Barlow, S.E.; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120 (Suppl. 4), S164–S192. [Google Scholar] [CrossRef] [PubMed]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric Obesity—Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef] [PubMed]
- Willi, S.M.; Martin, K.; Datko, F.M.; Brant, B.P. Treatment of Type 2 Diabetes in Childhood Using a Very-Low-Calorie Diet. Diabetes Care 2004, 27, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- American Diabetes Association. Children and Adolescents: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. 1), S163–S182. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef]
- Shaibi, G.Q.; Cruz, M.L.; Ball, G.D.; Weigensberg, M.J.; Salem, G.J.; Crespo, N.C.; Goran, M.I. Effects of Resistance Training on Insulin Sensitivity in Overweight Latino Adolescent Males. Med. Sci. Sports Exerc. 2006, 38, 1208–1215. [Google Scholar] [CrossRef]
- Dias, I.; Farinatti, P.; Souza, M.D.G.C.D.; Manhanini, D.P.; Balthazar, E.; Dantas, D.L.S.; Pinto, E.H.D.A.; Bouskela, E.; Kraemer-Aguiar, L.G. Effects of Resistance Training on Obese Adolescents. Med. Sci. Sports Exerc. 2015, 47, 2636–2644. [Google Scholar] [CrossRef]
- Fedewa, M.V.; Gist, N.H.; Evans, E.M.; Dishman, R.K. Exercise and Insulin Resistance in Youth: A Meta-Analysis. Pediatr. 2013, 133, e163–e174. [Google Scholar] [CrossRef]
- American Diabetes Association. Lifestyle Management: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S38–S50. [Google Scholar] [CrossRef]
- Laffel, L.; Chang, N.; Grey, M.; Hale, D.; Higgins, L.; Hirst, K.; Izquierdo, R.; Larkin, M.; Macha, C.; Pham, T.; et al. Metformin monotherapy in youth with recent onset type 2 diabetes: Experience from the prerandomization run-in phase of the TODAY study. Pediatr. Diabetes 2012, 13, 369–375. [Google Scholar] [CrossRef] [PubMed]
- TODAY Study Group. Design of a family-based lifestyle intervention for youth with type 2 diabetes: The TODAY study. Int. J. Obes. (Lond.) 2010, 34, 217–226. [Google Scholar] [CrossRef] [PubMed]
- TODAY Study Group; Zeitler, P.; Hirst, K.; Pyle, L.; Linder, B.; Copeland, K.; Arslanian, S.; Cuttler, L.; Nathan, D.M.; Tollefsen, S.; et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N. Engl. J. Med. 2012, 366, 2247–2256. [Google Scholar] [PubMed]
- Kumar, S.; King, E.C.; Christison, A.L.; Kelly, A.S.; Ariza, A.J.; Borzutzky, C.; Cuda, S.; Kirk, S.; Abraham-Pratt, I.; Ali, L.; et al. Health Outcomes of Youth in Clinical Pediatric Weight Management Programs in POWER. J. Pediatr. 2019, 208, 57–65.e4. [Google Scholar] [CrossRef] [PubMed]
- Savoye, M.; Shaw, M.; Dziura, J.; Tamborlane, W.V.; Rose, P.; Guandalini, C.; Goldberg-Gell, R.; Burgert, T.S.; Cali, A.M.; Weiss, R.; et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: A randomized controlled trial. JAMA 2007, 297, 2697–2704. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Barzilai, N.; Simonson, D.C. Mechanism of Metformin Action in Obese and Lean Noninsulin-Dependent Diabetic Subjects. J. Clin. Endocrinol. Metab. 1991, 73, 1294–1301. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Pernicova, I.; Korbonits, M. Metformin—Mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Jun, H.-S. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism 2014, 63, 9–19. [Google Scholar] [CrossRef]
- Tamborlane, W.V.; Barrientos-Pérez, M.; Fainberg, U.; Frimer-Larsen, H.; Hafez, M.; Hale, P.M.; Jalaludin, M.Y.; Kovarenko, M.; Libman, I.; Lynch, J.L.; et al. Liraglutide in Children and Adolescents with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 637–646. [Google Scholar] [CrossRef]
- Kelly, A.S.; Rudser, K.D.; Nathan, B.M.; Fox, C.K.; Metzig, A.M.; Coombes, B.J.; Fitch, A.K.; Bomberg, E.M.; Abuzzahab, M.J. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: A randomized, placebo-controlled, clinical trial. JAMA Pediatr. 2013, 167, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.S.; Auerbach, P.; Barrientos-Perez, M.; Gies, I.; Hale, P.M.; Marcus, C.; Mastrandrea, L.D.; Prabhu, N.; Arslanian, S. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N. Engl. J. Med. 2020, 382, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.F.; Ørsted, D.D.; Brown-Frandsen, K.; Marso, S.P.; Poulter, N.R.; Rasmussen, S.; Tornøe, K.; Zinman, B.; Buse, J.B. Liraglutide and Renal Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 839–848. [Google Scholar] [CrossRef]
- Hauner, H. The mode of action of thiazolidinediones. Diabetes Metab. Res. Rev. 2002, 18 (Suppl. 2), S10–S15. [Google Scholar] [CrossRef]
- Petersen, K.F.; Krssak, M.; Inzucchi, S.; Cline, G.W.; Dufour, S.; Shulman, G.I. Mechanism of troglitazone action in type 2 diabetes. Diabetes 2000, 49, 827–831. [Google Scholar] [CrossRef]
- Singh, S.; Loke, Y.K.; Furberg, C.D. Long-term risk of cardiovascular events with rosiglitazone: A meta-analysis. JAMA 2007, 298, 1189–1195. [Google Scholar] [CrossRef]
- Tuccori, M.; Filion, K.B.; Yin, H.; Yu, O.H.; Platt, R.W.; Azoulay, L. Pioglitazone use and risk of bladder cancer: Population based cohort study. BMJ 2016, 352, i1541. [Google Scholar] [CrossRef]
- Home, P.D.; Pocock, S.J.; Beck-Nielsen, H.; Curtis, P.S.; Gomis, R.; Hanefeld, M.; Jones, N.P.; Komajda, M.; McMurray, J.J.V. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): A multicentre, randomised, open-label trial. Lancet 2009, 373, 2125–2135. [Google Scholar] [CrossRef]
- Aguilar-Bryan, L.; Nichols, C.G.; Wechsler, S.W.; Clement, J.P.t.; Boyd, A.E., 3rd; Gonzalez, G.; Herrera-Sosa, H.; Nguy, K.; Bryan, J.; Nelson, D.A. Cloning of the beta cell high-affinity sulfonylurea receptor: A regulator of insulin secretion. Science 1995, 268, 423–426. [Google Scholar] [CrossRef]
- Gottschalk, M.; Danne, T.; Vlajnic, A.; Cara, J.F. Glimepiride versus Metformin as Monotherapy in Pediatric Patients with Type 2 Diabetes: A randomized, single-blind comparative study. Diabetes Care 2007, 30, 790–794. [Google Scholar] [CrossRef] [PubMed]
- DeMuth, H.-U.; McIntosh, C.H.; Pederson, R.A. Type 2 diabetes—Therapy with dipeptidyl peptidase IV inhibitors. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2005, 1751, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Tamborlane, W.V.; Laffel, L.M.; Weill, J.; Gordat, M.; Neubacher, D.; Retlich, S.; Hettema, W.; Hoesl, C.E.; Kaspers, S.; Marquard, J. Randomized, double-blind, placebo-controlled dose-finding study of the dipeptidyl peptidase-4 inhibitor linagliptin in pediatric patients with type 2 diabetes. Pediatr. Diabetes 2017, 19, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Gooßen, K.; Gräber, S. Longer term safety of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus: Systematic review and meta-analysis. Diabetes Obes. Metab. 2012, 14, 1061–1072. [Google Scholar] [CrossRef]
- Clar, C.; Gill, J.A.; Court, R.; Waugh, N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open 2012, 2, e001007. [Google Scholar] [CrossRef]
- Laffel, L.M.; Tamborlane, W.V.; Yver, A.; Simons, G.; Wu, J.; Nock, V.; Hobson, D.; Hughan, K.S.; Kaspers, S.; Marquard, J. Pharmacokinetic and pharmacodynamic profile of the sodium-glucose co-transporter-2 inhibitor empagliflozin in young people with Type 2 diabetes: A randomized trial. Diabet. Med. 2018, 35, 1096–1104. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. A novel approach to control hyperglycemia in type 2 diabetes: Sodium glucose co-transport (SGLT) inhibitors. Systematic review and meta-analysis of randomized trials. Ann. Med. 2011, 44, 375–393. [Google Scholar] [CrossRef]
- Griffin, T.P.; Dinneen, S.F. SGLT2 inhibitors increase risk for diabetic ketoacidosis in type 2 diabetes. Ann. Intern. Med. 2020, 173, JC40. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.H.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Kelsey, M.M.; Geffner, M.E.; Guandalini, C.; Pyle, L.; Tamborlane, W.V.; Zeitler, P.S.; White, N.H.; Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study Group. Presentation and effectiveness of early treatment of type 2 diabetes in youth: Lessons from the TODAY study. Pediatr. Diabetes 2016, 17, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L. Use of Continuous Glucose Monitoring in Youth-Onset Type 2 Diabetes. Curr. Diabetes Rep. 2017, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W.; Riddlesworth, T.D.; Ruedy, K.; Ahmann, A.; Haller, S.; Kruger, D.; McGill, J.B.; Polonsky, W.; Price, D.; Aronoff, S.; et al. Continuous Glucose Monitoring Versus Usual Care in Patients With Type 2 Diabetes Receiving Multiple Daily Insulin Injections: A Randomized Trial. Ann. Intern. Med. 2017, 167, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Inge, T.H.; Courcoulas, A.P.; Jenkins, T.M.; Michalsky, M.P.; Helmrath, M.A.; Brandt, M.L.; Harmon, C.M.; Zeller, M.H.; Chen, M.K.; Xanthakos, S.A.; et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N. Engl. J. Med. 2016, 374, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Inge, T.H.; Jenkins, T.M.; Xanthakos, S.A.; Dixon, J.B.; Daniels, S.R.; Zeller, M.H.; Helmrath, M.A. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): A prospective follow-up analysis. Lancet Diabetes Endocrinol. 2017, 5, 165–173. [Google Scholar] [CrossRef]
- Inge, T.H.; Miyano, G.; Bean, J.; Helmrath, M.; Courcoulas, A.; Harmon, C.M.; Chen, M.K.; Wilson, K.; Daniels, S.R.; Garcia, V.F.; et al. Reversal of Type 2 Diabetes Mellitus and Improvements in Cardiovascular Risk Factors After Surgical Weight Loss in Adolescents. Pediatrics 2009, 123, 214–222. [Google Scholar] [CrossRef]
- Khidir, N.; El-Matbouly, M.; Sargsyan, D.; Al-Kuwari, M.; Bashah, M.; Gagner, M. Five-year Outcomes of Laparoscopic Sleeve Gastrectomy: A Comparison Between Adults and Adolescents. Obes. Surg. 2018, 28, 2040–2045. [Google Scholar] [CrossRef]
- Inge, T.H.; Prigeon, R.L.; Elder, D.A.; Jenkins, T.M.; Cohen, R.M.; Xanthakos, S.A.; Benoit, S.C.; Dolan, L.M.; Daniels, S.R.; D’Alessio, D.A. Insulin Sensitivity and beta-Cell Function Improve after Gastric Bypass in Severely Obese Adolescents. J. Pediatr. 2015, 167, 1042–1048.e1. [Google Scholar] [CrossRef]
- Alqahtani, A.R.; Antonisamy, B.; Alamri, H.; Elahmedi, M.; Zimmerman, V.A. Laparoscopic Sleeve Gastrectomy in 108 Obese Children and Adolescents Aged 5 to 21 Years. Ann. Surg. 2012, 256, 266–273. [Google Scholar] [CrossRef]
- Inge, T.H.; Courcoulas, A.P.; Jenkins, T.M.; Michalsky, M.P.; Brandt, M.L.; Xanthakos, S.A.; Dixon, J.B.; Harmon, C.M.; Chen, M.K.; Xie, C.; et al. Five-Year Outcomes of Gastric Bypass in Adolescents as Compared with Adults. N. Engl. J. Med. 2019, 380, 2136–2145. [Google Scholar] [CrossRef]
- Inge, T.H.; Laffel, L.M.; Jenkins, T.M.; Marcus, M.D.; Leibel, N.I.; Brandt, M.L.; Haymond, M.; Urbina, E.M.; Dolan, L.M.; Zeitler, P.S.; et al. Comparison of Surgical and Medical Therapy for Type 2 Diabetes in Severely Obese Adolescents. JAMA Pediatr. 2018, 172, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Michalsky, M.P.; Inge, T.H.; Jenkins, T.M.; Xie, C.; Courcoulas, A.; Helmrath, M.; Brandt, M.L.; Harmon, C.M.; Chen, M.; Dixon, J.B.; et al. Cardiovascular Risk Factors After Adolescent Bariatric Surgery. Pediatrics 2018, 141, e20172485. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.C.; Bolling, C.F.; Michalsky, M.P.; Reichard, K.W.; Section on Obesity, Section on Surgery. Pediatric Metabolic and Bariatric Surgery: Evidence, Barriers, and Best Practices. Pediatrics 2019, 144, e20193223. [Google Scholar] [CrossRef]
- Pratt, J.S.A.; Browne, A.; Browne, N.T.; Bruzoni, M.; Cohen, M.; Desai, A.; Inge, T.; Linden, B.C.; Mattar, S.G.; Michalsky, M.; et al. ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg. Obes. Relat. Dis. 2018, 14, 882–901. [Google Scholar] [CrossRef]
- Pinhas-Hamiel, O.; Zeitler, P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet 2007, 369, 1823–1831. [Google Scholar] [CrossRef]
- McGrath, N.; Parker, G.; Dawson, P. Early presentation of type 2 diabetes mellitus in young New Zealand Maori. Diabetes Res. Clin. Pr. 1999, 43, 205–209. [Google Scholar] [CrossRef]
- Bjornstad, P.; Nehus, E.; El Ghormli, L.; Bacha, F.; Libman, I.M.; McKay, S.; Willi, S.M.; Laffel, L.; Arslanian, S.; Nadeau, K.J.; et al. Insulin Sensitivity and Diabetic Kidney Disease in Children and Adolescents With Type 2 Diabetes: An Observational Analysis of Data From the TODAY Clinical Trial. Am. J. Kidney Dis. 2018, 71, 65–74. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singhal, S.; Kumar, S. Current Perspectives on Management of Type 2 Diabetes in Youth. Children 2021, 8, 37. https://doi.org/10.3390/children8010037
Singhal S, Kumar S. Current Perspectives on Management of Type 2 Diabetes in Youth. Children. 2021; 8(1):37. https://doi.org/10.3390/children8010037
Chicago/Turabian StyleSinghal, Sachi, and Seema Kumar. 2021. "Current Perspectives on Management of Type 2 Diabetes in Youth" Children 8, no. 1: 37. https://doi.org/10.3390/children8010037
APA StyleSinghal, S., & Kumar, S. (2021). Current Perspectives on Management of Type 2 Diabetes in Youth. Children, 8(1), 37. https://doi.org/10.3390/children8010037






