Cerebral Palsy and Epilepsy in Children: Clinical Perspectives on a Common Comorbidity
Abstract
1. Introduction
2. Methods
3. Results
3.1. Types of Cerebral Palsy and Related Clinical Data
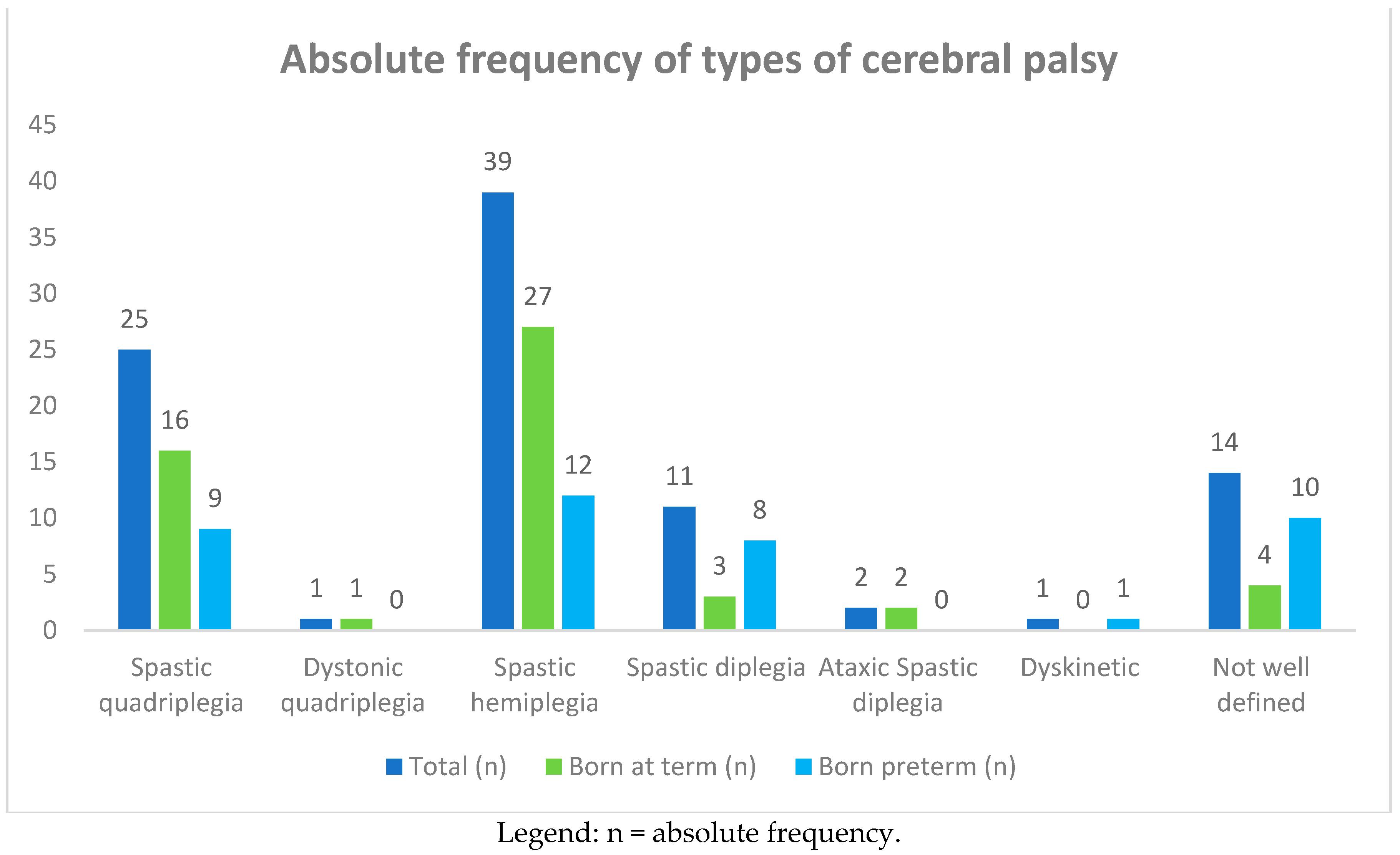
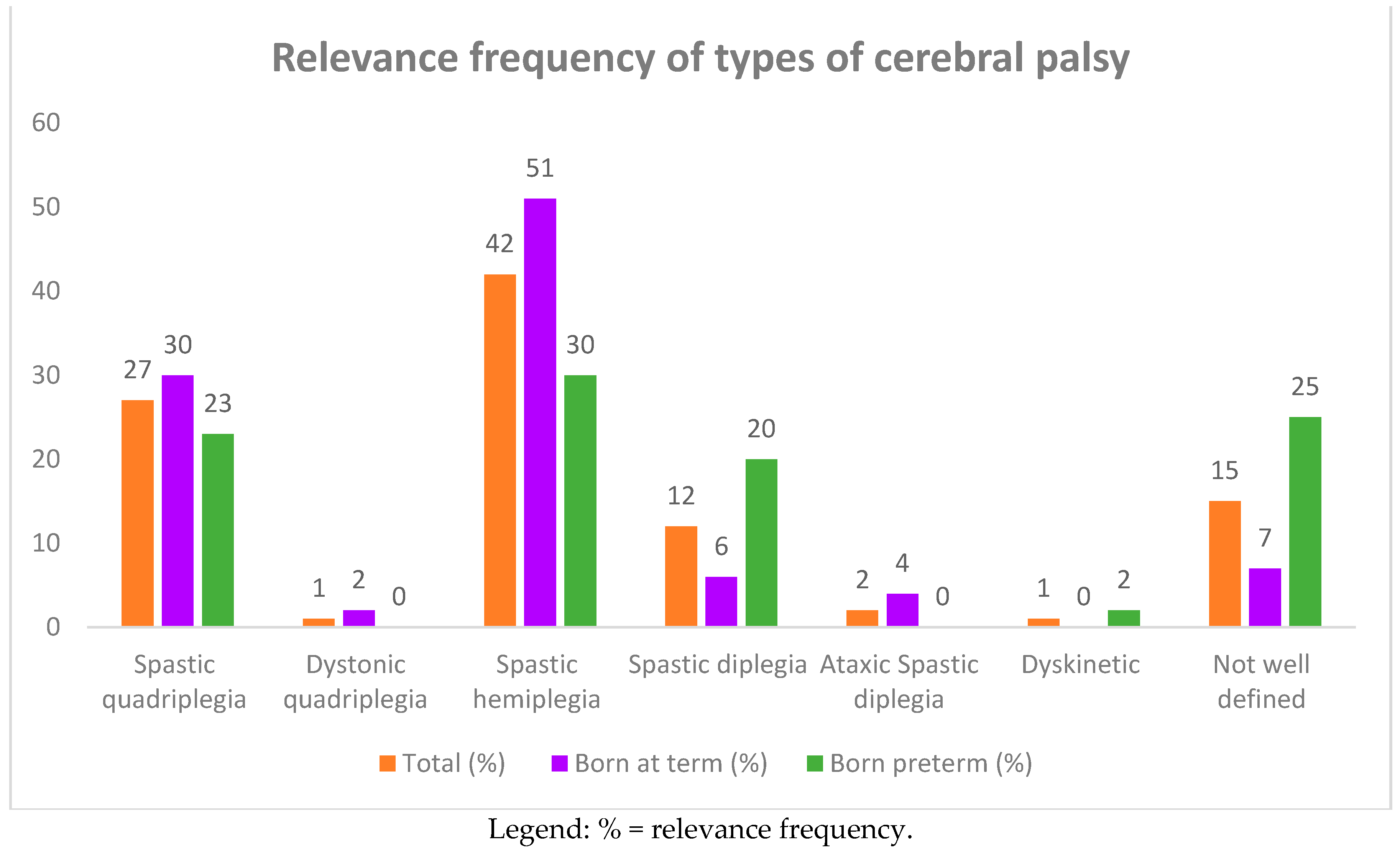
3.1.1. Types of Motor Dysfunction
3.1.2. Gestational Age and Birth Weight
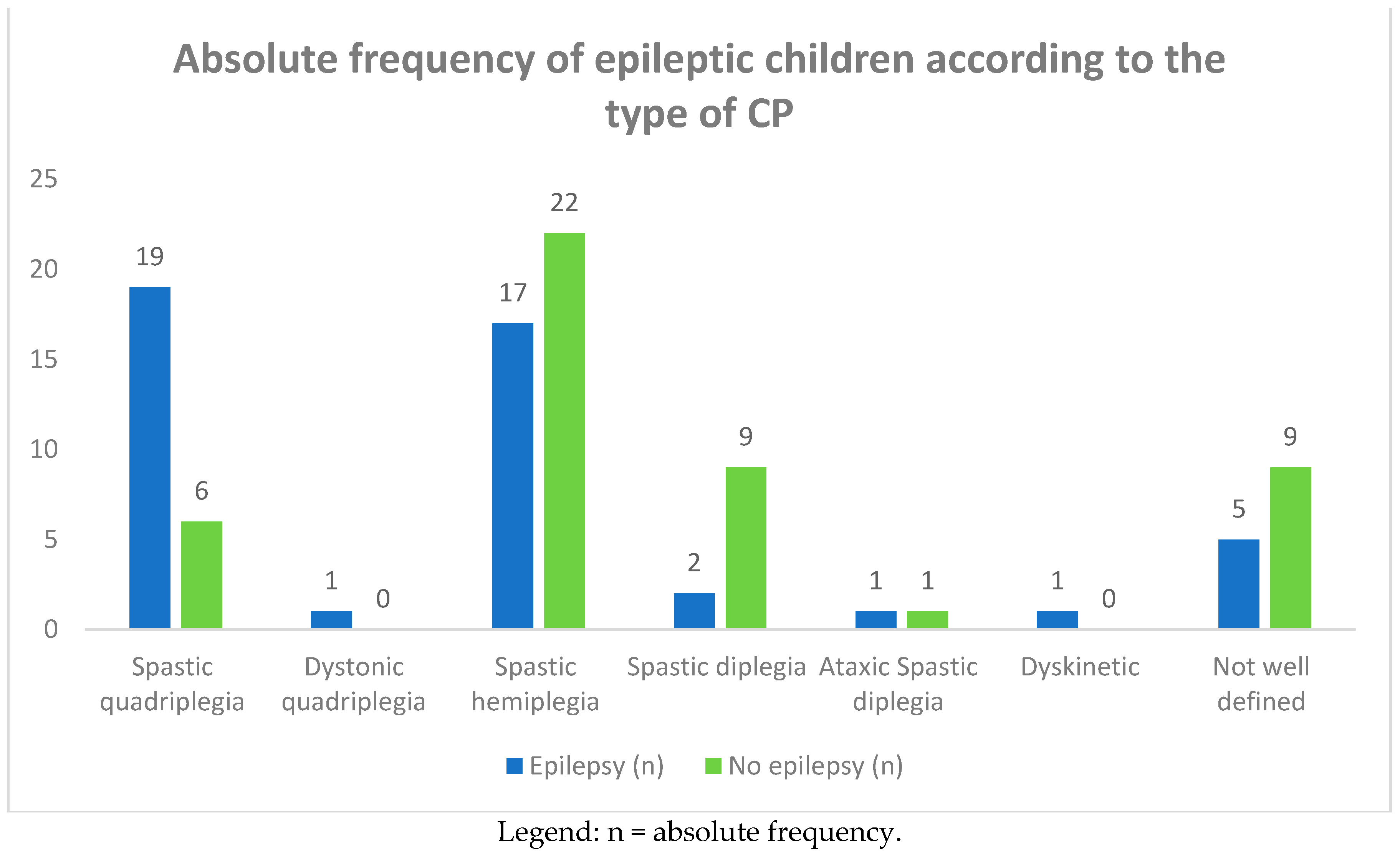
3.1.3. Type of CP and Frequency of Epilepsy
3.1.4. The GMFCS levels were performed in 28 CP children and, among these, in 12 CP epileptic children (Table 3). Comparison between the different levels of GMFCS and frequency of epilepsy was higher in children with IV–V levels of motor dysfunction.
3.1.5. Age of Onset of Epilepsy
3.1.6. Types of Epilepsy
3.1.7. Neuroimaging
3.1.8. Neuroimaging: Brain Malformations and Perinatal Damage
3.2. Clinical Data of CP Epileptic Children Compared to Epileptic Children without Other Cerebral Anomalies
3.2.1. Sex and Age of Onset of Seizures
3.2.2. Main Types of Epilepsy
3.2.3. Antiepileptic Treatment: Monotherapy vs. Polytherapy
3.2.4. Outcome of CP Epileptic Children
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. 2007, 49, 8–14. [Google Scholar] [CrossRef]
- Velde, A.T.; Morgan, C.; Novak, I.; Tantsis, E.; Badawi, N. Early Diagnosis and Classification of Cerebral Palsy: An Historical Perspective and Barriers to an Early Diagnosis. J. Clin. Med. 2019, 8, 1599. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.K.H.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.-P.J.-M.; Damiano, D.L.; Becher, J.G.J.; Gaebler-Spira, D.D.; Colver, A.A.; Reddihough, D.S.D.; et al. Cerebral palsy. Nat. Rev. Dis. Prim. 2016, 2, 15082. [Google Scholar] [CrossRef] [PubMed]
- Tillberg, E.; Isberg, B.; Persson, J.K.E. Hemiplegic (unilateral) cerebral palsy in northern Stockholm: Clinical assessment, brain imaging, EEG, epilepsy and aetiologic background factors. BMC Pediatr. 2020, 20, 116. [Google Scholar] [CrossRef]
- Hancı, F.; Türay, S.; Dilek, M.; Kabakuş, N. Epilepsy and drug-resistant epilepsy in children with cerebral palsy: A retrospective observational study. Epilepsy Behav. 2020, 112. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; De Vries, L.S. Early, accurate diagnosis and early intervention in cerebral palsy: Advances in diagnosis and treatment. JAMA Pediatr. 2017, 171, 897–907. [Google Scholar] [CrossRef]
- Patel, D.R.; Neelakantan, M.; Pandher, K.; Merrick, J. Cerebral palsy in children: A clinical overview. Transl. Pediatr. 2020, 9, S125–S135. [Google Scholar] [CrossRef]
- Michael-Asalu, A.; Taylor, G.; Cmpbell, H.; Lelea, L.L.; Kirby, R.S. Cerebral palsy: Diagnosis, epidemiology, genetics, and clinical update. Adv. Pediatr. 2019, 334, 189–208. [Google Scholar] [CrossRef]
- Korzeniewski, S.J.; Slaughter, J.; Lenski, M.; Haak, P.; Paneth, N. The complex aetiology of cerebral palsy. Nat. Rev. Neurol. 2018, 14, 528–543. [Google Scholar] [CrossRef]
- Hollung, S.J.; Bakken, I.J.; Vik, T.; Lydersen, S.; Wiik, R.; Aaberg, K.M.; Andersen, G.L. Comorbidities in cerebral palsy: A patient registry study. Dev. Med. Child Neurol. 2019, 62, 97–103. [Google Scholar] [CrossRef]
- Colver, A.; Fairhurst, C.; Pharoah, P. Cerebral palsy. Lancet 2014, 383, 1240–1249. [Google Scholar] [CrossRef]
- Reid, S.M.; Dagia, C.D.; Ditchfield, M.R.; Reddihough, D.S. Grey matter injury patterns in cerebral palsy: Associations between structural involvement on MRI and clinical outcomes. Dev. Med. Child Neurol. 2015, 57, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.S.; Mackay, M.T.; Fahey, M.; Reddihough, D.; Reid, S.M.; Williams, K.; Harvey, A.S. Seizures in Children with Cerebral Palsy and White Matter Injury. Pediatrics 2017, 139, e20162975. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.; Meletti, S.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Himmelmann, K. Putting prevention into practice for the benefit of children and young people with cerebral palsy. Arch. Dis. Child. 2018, 103, 1100. [Google Scholar] [CrossRef] [PubMed]
- Zelnik, N.; Konopnicki, M.; Bennett-Back, O.; Castel-Deutsch, T.; Tirosh, E. Risk factors for epilepsy in children with cerebral palsy. Eur. J. Paediatr. Neurol. 2010, 14, 67–72. [Google Scholar] [CrossRef]
- Sellier, E.; Uldall, P.; Calado, E.; Sigurdardóttir, S.; Torrioli, M.G.; Platt, M.J.; Cans, C. Epilepsy and cerebral palsy: Characteristics and trends in children born in 1976–1998. Eur. J. Paediatr. Neurol. 2012, 16, 48–55. [Google Scholar] [CrossRef]
- Shehata, G.A.; El-Tallawy, H.N.; Farghaly, W.M.; Badary, R.; Rageh, T.A. Epileptic and cognitive changes in children with cerebral palsy: An Egyptian study. Neuropsychiatr. Dis. Treat. 2014, 10, 971. [Google Scholar] [CrossRef]
- Sadowska, M.; Sarecka-Hujar, B.; Kopyta, I. Evaluation of Risk Factors for Epilepsy in Pediatric Patients with Cerebral Palsy. Brain Sci. 2020, 10, 481. [Google Scholar] [CrossRef]
- Verrotti, A.; Agostinelli, S.; Coppola, G.; Parisi, P.; Chiarelli, F. A 12-month longitudinal study of calcium metabolism and bone turnover during valproate monotherapy. Eur. J. Neurol. 2009, 17, 232–237. [Google Scholar] [CrossRef]
- Fan, H.-C.; Lee, H.-S.; Chang, K.-P.; Lee, Y.-Y.; Lai, H.-C.; Hung, P.-L.; Lee, H.-F.; Chi, C.-S. The Impact of Anti-Epileptic Drugs on Growth and Bone Metabolism. Int. J. Mol. Sci. 2016, 17, 1242. [Google Scholar] [CrossRef] [PubMed]

| Type of Cerebral Palsy | Total | Born at Term | Born Preterm | |||
|---|---|---|---|---|---|---|
| Absolute Frequency | Relative frequency % | Absolute Frequency | Relative Frequency % | Absolute Frequency | Relative Frequency % | |
| Spastic quadriplegia | 25 | 27 | 16 | 30 | 9 | 23 |
| Dystonic quadriplegia | 1 | 1 | 1 | 2 | - | - |
| Spastic hemiplegia | 39 | 42 | 27 | 51 | 12 | 30 |
| Spastic diplegia | 11 | 12 | 3 | 6 | 8 | 20 |
| Ataxic spastic diplegia | 2 | 2 | 2 | 4 | - | - |
| Dyskinetic | 1 | 1 | - | - | 1 | 2 |
| Poorly defined | 14 | 15 | 4 | 7 | 10 | 25 |
| Total | 93 | 100 | 53 | 57 | 40 | 43 |
| Type of Cerebral Palsy | Epilepsy | No Epilepsy | ||
|---|---|---|---|---|
| Absolute Frequency | Relative Frequency % | Absolute Frequency | Relative Frequency % | |
| Spastic quadriplegia | 19 | 46 | 6 | 13 |
| Dystonic quadriplegia | 1 | 2 | - | - |
| Spastic hemiplegia | 17 | 39 | 22 | 47 |
| Spastic diplegia | 2 | 4 | 9 | 19 |
| Ataxic spastic diplegia | 1 | 2 | 1 | 2 |
| Dyskinetic | 1 | 2 | - | - |
| Poorly defined | 5 | 10 | 9 | 19 |
| Total | 46 | 49 | 47 | 51 |
| Epilepsy | No Epilepsy | ||||
|---|---|---|---|---|---|
| GMFCS Levels | Children | Absolute Freq. Relative Freq % | Absolute Freq. Relative Freq % | ||
| GMFCS I | 2 | - | - | 2 | 7.14% |
| GMFCS II | 4 | 1 | 3.57% | 3 | 10.71% |
| GMFCS III | 2 | 1 | 3.57% | 1 | 3.57% |
| GMFCS IV | 13 | 5 | 17.86% | 8 | 28.57% |
| GMFCS V | 7 | 5 | 17.86% | 2 | 7.14% |
| Total | 28 | 12 | 42.86 % | 16 | 57.13% |
| Types of Seizures | Spastic Quadriplegia | Distonic Quadriplegia | Hemiplegia | Spastic Diplegia | Spastic Ataxic Diplegia | Dyskinetic Cerebral Palsy | Poorly Defined Cerebral Palsy |
|---|---|---|---|---|---|---|---|
| Focal then Generalized | 9 | 0 | 5 | 1 | 0 | 0 | 2 |
| Focal | 0 | 0 | 6 | 1 | 1 | 0 | 0 |
| West syndrome | 2 | 0 | 4 | 0 | 0 | 0 | 0 |
| Neonatal seizures | 5 | 1 | 4 | 0 | 0 | 1 | 4 |
| Epileptic encephalopathy | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavone, P.; Gulizia, C.; Le Pira, A.; Greco, F.; Parisi, P.; Di Cara, G.; Falsaperla, R.; Lubrano, R.; Minardi, C.; Spalice, A.; et al. Cerebral Palsy and Epilepsy in Children: Clinical Perspectives on a Common Comorbidity. Children 2021, 8, 16. https://doi.org/10.3390/children8010016
Pavone P, Gulizia C, Le Pira A, Greco F, Parisi P, Di Cara G, Falsaperla R, Lubrano R, Minardi C, Spalice A, et al. Cerebral Palsy and Epilepsy in Children: Clinical Perspectives on a Common Comorbidity. Children. 2021; 8(1):16. https://doi.org/10.3390/children8010016
Chicago/Turabian StylePavone, Piero, Carmela Gulizia, Alice Le Pira, Filippo Greco, Pasquale Parisi, Giuseppe Di Cara, Raffaele Falsaperla, Riccardo Lubrano, Carmelo Minardi, Alberto Spalice, and et al. 2021. "Cerebral Palsy and Epilepsy in Children: Clinical Perspectives on a Common Comorbidity" Children 8, no. 1: 16. https://doi.org/10.3390/children8010016
APA StylePavone, P., Gulizia, C., Le Pira, A., Greco, F., Parisi, P., Di Cara, G., Falsaperla, R., Lubrano, R., Minardi, C., Spalice, A., & Ruggieri, M. (2021). Cerebral Palsy and Epilepsy in Children: Clinical Perspectives on a Common Comorbidity. Children, 8(1), 16. https://doi.org/10.3390/children8010016






