The Scarcity of Literature on the Psychological, Social, and Emotional Effects of Gastroparesis in Children
Abstract
1. Introduction
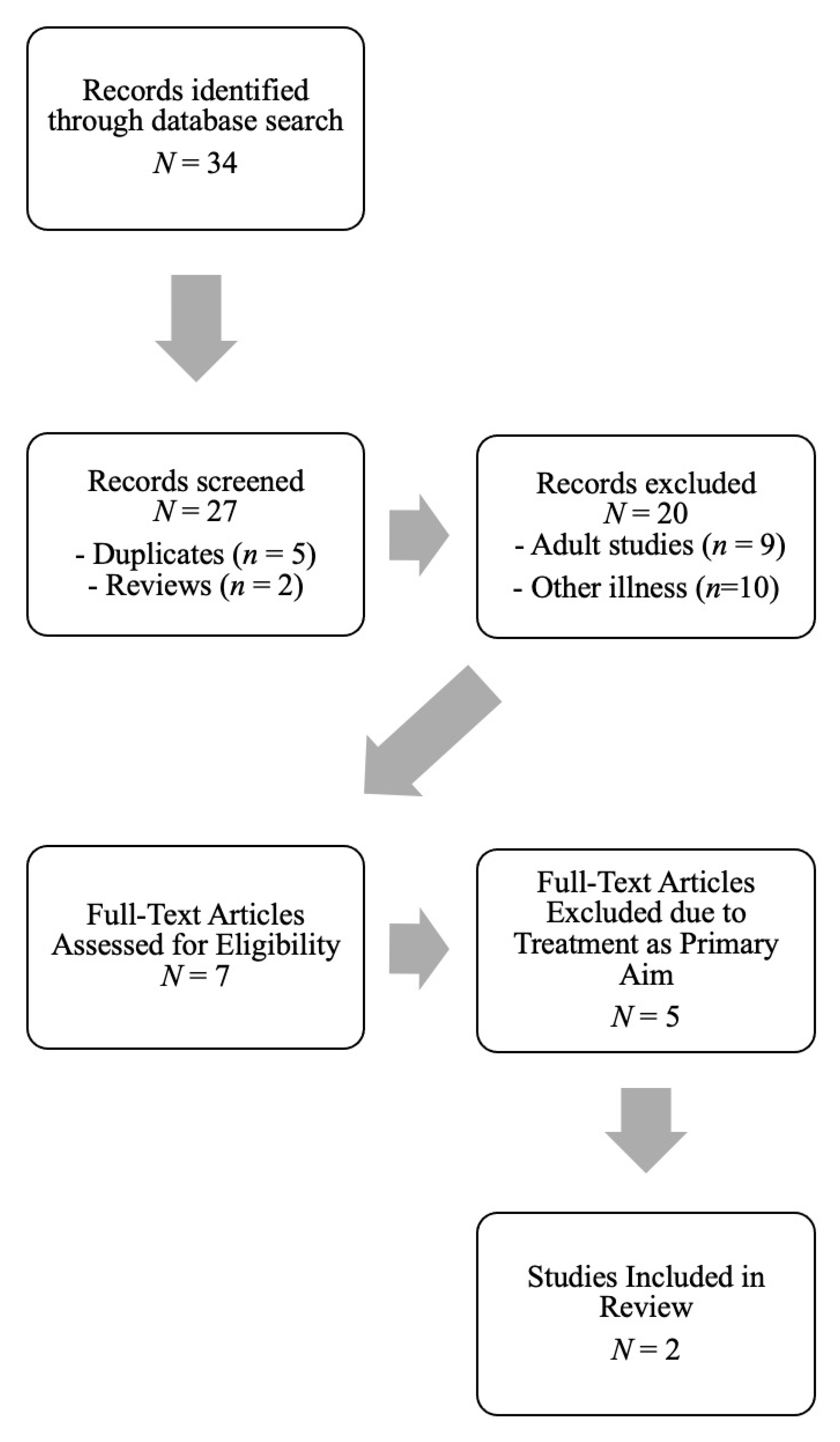
2. Methods
2.1. Inclusion Criteria
2.2. Procedures
- Concept 1
- “Gastroparesis” OR “idiopathic gastroparesis” OR “post-viral gastroparesis” OR “diabetic gastroparesis.”
- Concept 2
- “Psychosocial” OR “psychological” “anxiety” OR “depression” OR “mental health” OR “stigma” OR “health related quality of life” OR “bullying” OR “post-traumatic stress” OR “post-traumatic stress disorder.”
- Concept 3
- “Pediatric” OR “children” OR “adolescents.”
3. Search Results
4. Psychological Co-Morbidities
4.1. Anxiety and Depression
4.2. Health-Related Quality of Life
4.3. Eating Behaviors and Nutritional Assessment
4.4. Post-Traumatic Stress
4.5. Social Relationships and Stigma
5. Recommendations for Future Research
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fosso, C.L.; Quigley, E.M. A critical review of the current clinical landscape of gastroparesis. Gastroenterol. Hepatol. 2018, 14, 140–145. [Google Scholar]
- Ye, Y.; Bennett, D. The first national prevalence estimation of gastroparesis in the united states using a large-scale retrospective database. J. Clin. Gastroenterol. 2020, 54, 106. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.; Irani, K.; Jiang, H.; Goldstein, A.M. Clinical presentation, response to therapy, and outcome of gastroparesis in children. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, A.E. Epidemiology and natural history of gastroparesis. Gastroenterol. Clin. N. Am. 2015, 44, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.L.; Moore-Clingenpeel, M.; Yacob, D.; Di Lorenzo, C.; Mousa, H.M. The rising cost of hospital care for children with gastroparesis: 2004–2013. Neurogastroenterol. Motil. 2016, 28, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.L.; Di Lorenzo, C. Gastroparesis in the pediatric patient: Children are not little adults. Gastrointest. Disord. 2020, 2, 8. [Google Scholar] [CrossRef]
- Kovacic, K.; Elfar, W.; Rosen, J.M.; Yacob, D.; Raynor, J.; Mostamand, S.; Punati, J.; Fortunato, J.E.; Saps, M. Update on pediatric gastroparesis: A review of the published literature and recommendations for future research. Neurogastroenterol. Motil. 2019, 32. [Google Scholar] [CrossRef]
- Zoll, B.; Zhao, H.; Edwards, M.A.; Petrov, R.; Schey, R.; Parkman, H. Outcomes of surgical intervention for refractory gastroparesis: A systematic review. J. Surg. Res. 2018, 231, 263–269. [Google Scholar] [CrossRef]
- Abell, T.L.; Kedar, A.; Stocker, A.; Beatty, K.; McElmurray, L.; Hughes, M.; Rashed, H.; Kennedy, W.; Wendelschafer-Crabb, G.; Yang, X.; et al. Gastroparesis syndromes: Response to electrical stimulation. Neurogastroenterol. Motil. 2019, 31, e13534. [Google Scholar] [CrossRef]
- Thomas, A.; Ribeiro, B.D.S.; Malespin, M.; De Melo, S.W. Botulinum toxin as a treatment for refractory gastroparesis: A Literature review. Curr. Treat. Options Gastroenterol. 2018, 16, 479–488. [Google Scholar] [CrossRef]
- Setya, A.; Nair, P.; Cheng, S.X. Gastric electrical stimulation: An emerging therapy for children with intractable gastroparesis. World J. Gastroenterol. 2019, 25, 6880–6889. [Google Scholar] [CrossRef] [PubMed]
- Kassirer, J.P. Incorporating patients’ preferences into medical decisions. N. Engl. J. Med. 1994, 330, 1895–1896. [Google Scholar] [CrossRef] [PubMed]
- Islam, S. Gastroparesis in children. Curr. Opin. Pediatr. 2015, 27, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.C.; Stone, A.L.; Walker, L.S. Functional nausea in children: A review of the literature and need for diagnostic criteria. Children 2016, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Waseem, S.; Islam, S.; Kahn, G.; Moshiree, B.; Talley, N.J. Spectrum of gastroparesis in children. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 166–172. [Google Scholar] [CrossRef]
- Soykan, I.; Sivri, B.; Sarosiek, I.; Kiernan, B.; McCallum, R.W. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig. Dis. Sci. 1998, 43, 2398–2404. [Google Scholar] [CrossRef]
- Wong, G.K.; Shulman, R.J.; Malaty, H.M.; Czyzewski, D.; Seghers, V.J.; Thompson, D.; Chumpitazi, B.P. Relationship of gastrointestinal symptoms and psychosocial distress to gastric retention in children. J. Pediatr. 2014, 165, 85–91. [Google Scholar] [CrossRef]
- Kovacic, K.; Miranda, A.; Chelimsky, G.; Williams, S.; Simpson, P.; Li, B. Chronic idiopathic nausea of childhood. J. Pediatr. 2014, 164, 1104–1109. [Google Scholar] [CrossRef]
- Taft, T.H.; Guadagnoli, L.; Edlynn, E. Anxiety and depression in eosinophilic esophagitis: A scoping review and recommendations for future research. J. Asthma Allergy 2019, 12, 389–399. [Google Scholar] [CrossRef]
- Stapersma, L.; Brink, G.V.D.; Szigethy, E.M.; Escher, J.C.; Utens, E.M.W.J. Systematic review with meta-analysis: Anxiety and depression in children and adolescents with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2018, 48, 496–506. [Google Scholar] [CrossRef]
- Farup, C.E.; Leidy, N.K.; Murray, M.; Williams, G.R.; Helbers, L.; Quigley, E.M.M. Effect of domperidone on the health-related quality of life of patients with symptoms of diabetic gastroparesis. Diabetes Care 1998, 21, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Crowell, M.D.; Mathis, C. Gastroparesis: Quality of life and health care utilization. J. Clin. Gastroenterol. 2018, 52, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Warschburger, P.; Hänig, J.; Friedt, M.; Posovszky, C.; Schier, M.; Calvano, C. Health-related quality of life in children with abdominal pain due to functional or organic gastrointestinal disorders. J. Pediatr. Psychol. 2013, 39, 45–54. [Google Scholar] [CrossRef]
- Dolgin, M.J.; Katz, E.R.; McGinty, K.; Siegel, S.E. Anticipatory nausea and vomiting in pediatric cancer patients. Pediatrics 1985, 75, 547–552. [Google Scholar] [PubMed]
- Heruc, G.A.; Little, T.J.; Kohn, M.; Madden, S.; Clarke, S.; Horowitz, M.; Feinle-Bisset, C. Effects of starvation and short-term refeeding on gastric emptying and postprandial blood glucose regulation in adolescent girls with anorexia nervosa. Am. J. Physiol. Metab. 2018, 315, E565–E573. [Google Scholar] [CrossRef] [PubMed]
- Robson, J.; Laborda, T.; Fitzgerald, S.; Andersen, J.; Peterson, K.; O’Gorman, M.; Guthery, S.; Bennett-Murphy, L. Avoidant/restrictive food intake disorder in diet-treated children with eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 57–60. [Google Scholar] [CrossRef]
- Groetch, M.E.; Venter, C.; Skypala, I.; Vlieg-Boerstra, B.; Grimshaw, K.; Durban, R.; Cassin, A.; Henry, M.; Kliewer, K.; Kabbash, L.; et al. Dietary therapy and nutrition management of eosinophilic esophagitis: A work group report of the american academy of allergy, asthma, and immunology. J. Allergy Clin. Immunol. Pract. 2017, 5, 312–324. [Google Scholar] [CrossRef]
- Lund, J.I.; Toombs, E.; Radford, A.; Boles, K.; Mushquash, C. Adverse childhood experiences and executive function difficulties in children: A Systematic review. Child. Abus. Negl. 2020, 106, 104485. [Google Scholar] [CrossRef]
- Thompson, L.A.; Filipp, S.L.; Mack, J.A.; Mercado, R.E.; Barnes, A.; Bright, M.; Shenkman, E.A.; Gurka, M.J. Specific adverse childhood experiences and their association with other adverse childhood experiences, asthma and emotional, developmental and behavioral problems in childhood. Pediatr. Res. 2020, 1–10. [Google Scholar] [CrossRef]
- Rebicova, M.L.; Veselska, Z.D.; Husarova, D.; Gecková, A.M.; Van Dijk, J.P.; Reijneveld, S.A. The number of adverse childhood experiences is associated with emotional and behavioral problems among adolescents. Int. J. Environ. Res. Public Health 2019, 16, 2446. [Google Scholar] [CrossRef]
- Walsh, D.; McCartney, G.; Smith, M.; Armour, G. Relationship between childhood socioeconomic position and adverse childhood experiences (ACEs): A systematic review. J. Epidemiol. Community Health 2019, 73, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Petruccelli, K.; Davis, J.; Berman, T. Adverse childhood experiences and associated health outcomes: A systematic review and meta-analysis. Child. Abus. Negl. 2019, 97, 104127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nerwen, R.; Gabris, C. Addressing adverse childhood experiences in diverse racial and ethnic settings. Curr. Opin. Pediatr. 2019, 31, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Kassam-Addams, N.; Butler, L. What Do clinicians caring for children need to know about pediatric medical traumatic stress and the ethics of trauma-informed approaches? AMA J. Ethic. 2017, 19, 793–801. [Google Scholar] [CrossRef]
- Marsac, M.L.; Kassam-Adams, N.; Delahanty, U.L.; Widaman, K.F.; Barakat, L.P. Posttraumatic stress following acute medical trauma in children: A proposed model of bio-psycho-social processes during the peri-trauma period. Clin. Child. Fam. Psychol. Rev. 2014, 17, 399–411. [Google Scholar] [CrossRef]
- Perez, M.N.; Sharkey, C.M.; Tackett, A.P.; DeLozier, A.M.; Bakula, D.M.; Gamwell, K.L.; Mayes, S.; McNall, R.; Chaney, J.M.; Clawson, A.H.; et al. Post traumatic stress symptoms in parents of children with cancer: A mediation model. Pediatr. Hematol. Oncol. 2018, 35, 231–244. [Google Scholar] [CrossRef]
- Phipps, S.; Long, A.; Hudson, M.; Rai, S.N. Symptoms of post-traumatic stress in children with cancer and their parents: Effects of informant and time from diagnosis. Pediatr. Blood Cancer 2005, 45, 952–959. [Google Scholar] [CrossRef]
- Malarbi, S.T.; Muscara, F.; Barnett, P.L.J.; Palmer, C.S.; Stargatt, R. Post-traumatic stress symptoms and cognition in children exposed to motor vehicle accident trauma. Child. Neuropsychol. 2019, 26, 560–575. [Google Scholar] [CrossRef]
- Iljazi, A.; Ashina, H.; Al-Khazali, H.M.; Ashina, M.; Schytz, H.W.; Ashina, S. Post-traumatic stress disorder attributed to traumatic brain injury in children—A systematic review. Brain Inj. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Moser, D.A.; Aue, T.; Wang, Z.; Serpa, S.R.; Favez, N.; Peterson, B.S.; Schechter, D.S. Limbic brain responses in mothers with post-traumatic stress disorder and comorbid dissociation to video clips of their children. Stress 2013, 16, 493–502. [Google Scholar] [CrossRef]
- Mather, F.J.; Tate, R.L.; Hannan, T.J. Post-traumatic stress disorder in children following road traffic accidents: A comparison of those with and without mild traumatic brain injury. Brain Inj. 2003, 17, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Aftyka, A.; Rybojad, B.; Rozalska-Walaszek, I.; Rzoñca, P.; Humeniuk, E. Post-traumatic stress disorder in parents of children hospitalized in the neonatal intensive care unit (NICU): Medical and demographic risk factors. Psychiatr. Danub. 2014, 26, 347–352. [Google Scholar] [PubMed]
- Norberg, A.L.; Pöder, U.; Ljungman, G.; Von Essen, L. Objective and subjective factors as predictors of post-traumatic stress symptoms in parents of children with cancer—A longitudinal study. PLoS ONE 2012, 7, e36218. [Google Scholar] [CrossRef]
- Norberg, A.L.; Pöder, U.; Von Essen, L. Early avoidance of disease- and treatment-related distress predicts post-traumatic stress in parents of children with cancer. Eur. J. Oncol. Nurs. 2011, 15, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Pinquart, M. Systematic review: Bullying involvement of children with and without chronic physical illness and/or physical/sensory disability—A meta-analytic comparison with healthy/nondisabled Peers. J. Pediatr. Psychol. 2016, 42, 245–259. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Miller, V.; Firth, D.; Suresh-Babu, M.V.; Mir, P.; Thomas, A.G. Quality of life of parents and siblings of children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 1999, 28, S40–S42. [Google Scholar] [CrossRef]
- Rabbett, H.; Elbadri, A.; Thwaites, R. Quality of life in children with Crohn’s disease. J. Pediatr. Gastroenterol. Nutr. 1996, 23, 528–533. [Google Scholar] [CrossRef]
- Fales, J.; Rice, S.; Aaron, R.; Palermo, T.M. Traditional and cyber-victimization among adolescents with and without chronic pain. Health Psychol. 2018, 37, 291–300. [Google Scholar] [CrossRef]
- Herbert, L.J.; Shemesh, E.; Bender, B. Clinical management of psychosocial concerns related to food allergy. J. Allergy Clin. Immunol. Pract. 2016, 4, 205–213. [Google Scholar] [CrossRef]
- Yang, L.H.; Kleinman, A.; Link, B.G.; Phelan, J.C.; Lee, S.; Good, B. Culture and stigma: Adding moral experience to stigma theory. Soc. Sci. Med. 2007, 64, 1524–1535. [Google Scholar] [CrossRef]
- Joachim, G.; Acorn, S. Living with chronic illness: The interface of stigma and normalization. Can. J. Nurs. Res. 2000, 32, 37–48. [Google Scholar] [PubMed]
- Hearn, M.; Whorwell, P.J.; Vasant, D.H. Stigma and irritable bowel syndrome: A taboo subject? Lancet Gastroenterol. Hepatol. 2020, 5, 607–615. [Google Scholar] [CrossRef]
- Puhl, R.M.; Suh, Y. Stigma and eating and weight disorders. Curr. Psychiatry Rep. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Gulewitsch, M.D.; Jusyte, A.; Mazurak, N.; Weimer, K.; Schönenberg, M. Preliminary evidence for increased parasympathetic activity during social inclusion and exclusion in adolescents with functional abdominal pain. J. Psychosom. Res. 2017, 98, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Friesen, C.A.; Rosen, J.M.; Schurman, J.V. Prevalence of overlap syndromes and symptoms in pediatric functional dyspepsia. BMC Gastroenterol. 2016, 16, 75. [Google Scholar] [CrossRef]
- Schurman, J.V.; Singh, M.; Singh, V.; Neilan, N.; Friesen, C.A. Symptoms and subtypes in pediatric functional dyspepsia: Relation to mucosal inflammation and psychological functioning. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 298–303. [Google Scholar] [CrossRef]
| Author Group | Year | Study Design | Sample Size | Constructs Evaluated | |
|---|---|---|---|---|---|
| 1 | Wong et al. | 2014 | Cross-Sectional | 100 (25 with GP) | Anxiety |
| 2 | Waseem et al. | 2012 | Retrospective | 239 | Anxiety, Depression |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taft, T.H.; Doerfler, B.; Edlynn, E.; Nguyen, L. The Scarcity of Literature on the Psychological, Social, and Emotional Effects of Gastroparesis in Children. Children 2020, 7, 115. https://doi.org/10.3390/children7090115
Taft TH, Doerfler B, Edlynn E, Nguyen L. The Scarcity of Literature on the Psychological, Social, and Emotional Effects of Gastroparesis in Children. Children. 2020; 7(9):115. https://doi.org/10.3390/children7090115
Chicago/Turabian StyleTaft, Tiffany H., Bethany Doerfler, Emily Edlynn, and Linda Nguyen. 2020. "The Scarcity of Literature on the Psychological, Social, and Emotional Effects of Gastroparesis in Children" Children 7, no. 9: 115. https://doi.org/10.3390/children7090115
APA StyleTaft, T. H., Doerfler, B., Edlynn, E., & Nguyen, L. (2020). The Scarcity of Literature on the Psychological, Social, and Emotional Effects of Gastroparesis in Children. Children, 7(9), 115. https://doi.org/10.3390/children7090115





