Mental Health Comorbidities in Pediatric Chronic Pain: A Narrative Review of Epidemiology, Models, Neurobiological Mechanisms and Treatment
Abstract
:1. Introduction
2. Epidemiology of Internalizing Mental Health Disorders in Pediatric Chronic Pain Population
2.1. Longitudinal Studies of Pediatric Chronic Pain and Internalizing Mental Health Issues in Adulthood
2.2. Co-Occurrence of Pediatric Chronic Pain and Internalizing Mental Health Issues
3. Models of Chronic Pain and Comorbid Anxiety, Depression and PTSD
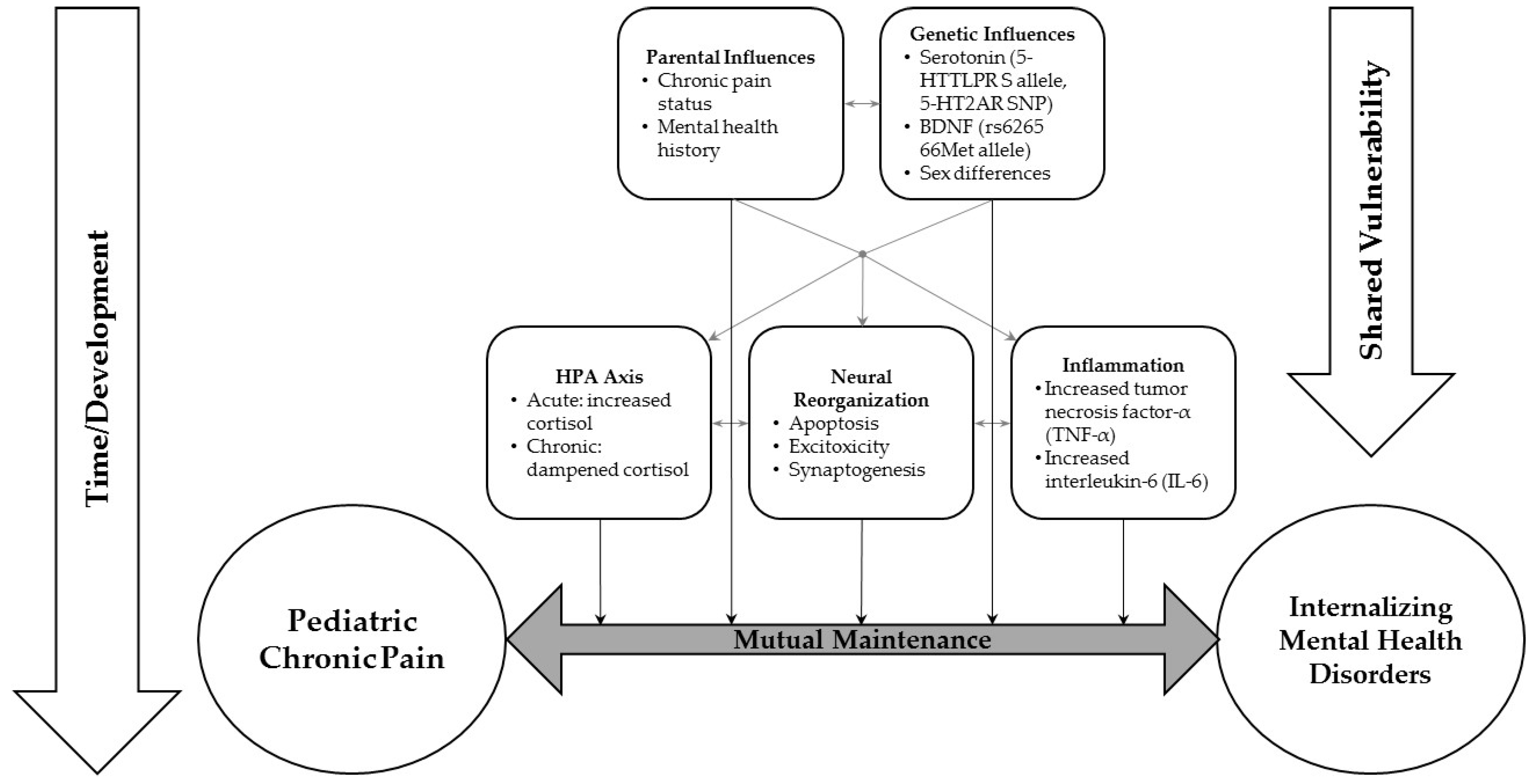
4. Potential Neurobiological Mechanisms Underlying Comorbid Chronic Pain and Anxiety, Depression or PTSD
4.1. The Hypothalamic–Pituitary–Adrenal Axis
4.2. Serotonin
4.3. Brain-Derived Neurotrophic Factor
4.4. Inflammation
4.5. Neuroimaging Chronic Pain and Psychopathology
5. Parental Mental Health Disorders in Pediatric Chronic Pain Population
6. Treatments for Comorbid Chronic Pain in Youth
7. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- King, S.; Chambers, C.T.; Huguet, A.; MacNevin, R.C.; McGrath, P.J.; Parker, L.; MacDonald, A.J. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain 2011, 152, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, C.B.; Essner, B.S.; Wright, D.; Fesinmeyer, M.D.; Palermo, T.M. The economic costs of chronic pain among a cohort of treatment-seeking adolescents in the united states. J. Pain 2014, 15, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Fearon, P.; Hotopf, M. Relation between headache in childhood and physical and psychiatric symptoms in adulthood: National birth cohort study. BMJ 2001, 322, 1145. [Google Scholar] [CrossRef] [PubMed]
- Shelby, G.D.; Shirkey, K.C.; Sherman, A.L.; Beck, J.E.; Haman, K.; Shears, A.R.; Horst, S.N.; Smith, C.A.; Garber, J.; Walker, L.S. Functional abdominal pain in childhood and long-term vulnerability to anxiety disorders. Pediatrics 2013, 132, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Hotopf, M.; Carr, S.; Mayou, R.; Wadsworth, M.; Wessely, S. Why do children have chronic abdominal pain, and what happens to them when they grow up? Population based cohort study. BMJ 1998, 316, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Asmundson, G.J.; Coons, M.J.; Taylor, S.; Katz, J. Ptsd and the experience of pain: Research and clinical implications of shared vulnerability and mutual maintenance models. Can. J. Psychiatry 2002, 47, 930–937. [Google Scholar] [PubMed]
- Asmundson, G.J.; Katz, J. Understanding the co-occurrence of anxiety disorders and chronic pain: State-of-the-art. Depress. Anxiety 2009, 26, 888–901. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.J.; Harvey, A.G. Chronic pain and posttraumatic stress disorder: Mutual maintenance? Clin. Psychol. Rev. 2001, 21, 857–877. [Google Scholar] [CrossRef]
- Asmundson, G.J. The emotional and physical pains of trauma: Contemporary and innovative approaches for treating co-occurring ptsd and chronic pain. Depress. Anxiety 2014, 31, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, C. Systematic review and meta-analysis of psychological therapies for children with chronic pain. Clin. Pract. Pediatr. Psychol. 2014, 39, 763–782. [Google Scholar]
- Shipton, E.A. The transition from acute to chronic post surgical pain. Anaesth. Intensive Care 2011, 39, 824–836. [Google Scholar] [PubMed]
- Groenewald, C.B.; Wright, D.R.; Palermo, T.M. Health care expenditures associated with pediatric pain-related conditions in the united states. Pain 2015, 156, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M. Impact of recurrent and chronic pain on child and family daily functioning: A critical review of the literature. J. Dev. Behav. Pediatr. 2000, 21, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Egger, H.L.; Angold, A.; Costello, E.J. Headaches and psychopathology in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry 1998, 37, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Egger, H.L.; Costello, E.J.; Erkanli, A.; Angold, A. Somatic complaints and psychopathology in children and adolescents: Stomach aches, musculoskeletal pains, and headaches. J. Am. Acad. Child Adolesc. Psychiatry 1999, 38, 852–860. [Google Scholar]
- Walker, L.S.; Sherman, A.L.; Bruehl, S.; Garber, J.; Smith, C.A. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain 2012, 153, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, L.; Zucker, N.; Copeland, W.E.; Bondy, C.L.; Egger, H.L.; Costello, E.J. Childhood somatic complaints predict generalized anxiety and depressive disorders during young adulthood in a community sample. Psychol. Med. 2015, 45, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Noel, M.; Groenewald, C.B.; Beals-Erickson, S.E.; Gebert, J.T.; Palermo, T.M. Chronic pain in adolescence and internalizing mental health disorders: A nationally representative study. Pain 2016, 157, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Balottin, U.; Fusar Poli, P.; Termine, C.; Molteni, S.; Galli, F. Psychopathological symptoms in child and adolescent migraine and tension-type headache: A meta-analysis. Cephalalgia 2013, 33, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.A.; Dyb, G.; Hagen, K.; Holmen, T.L.; Linde, M.; Wentzel-Larsen, T.; Zwart, J.A. Anxiety, depression and behavioral problems among adolescents with recurrent headache: The young-hunt study. J. Headache Pain 2014, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, T.A.; Bauer, B.D.; Carroll, A.E. Inpatient characteristics of the child admitted with chronic pain. Pediatrics 2013, 132, e422–e429. [Google Scholar] [CrossRef] [PubMed]
- Noel, M.; Beals-Erickson, S.E.; Law, E.F.; Alberts, N.; Palermo, T.M. Characterizing the pain narratives of parents of youth with chronic pain. Clin. J. Pain 2016, 32, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Simons, L.E.; Sieberg, C.B.; Claar, R.L. Anxiety and functional disability in a large sample of children and adolescents with chronic pain. Pain Res. Manag. 2012, 17, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Tegethoff, M.; Belardi, A.; Stalujanis, E.; Meinlschmidt, G. Comorbidity of mental disorders and chronic pain: Chronology of onset in adolescents of a national representative cohort. J. Pain 2015, 16, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Noel, M.; Wilson, A.C.; Holley, A.L.; Durkin, L.; Patton, M.; Palermo, T.M. Posttraumatic stress disorder symptoms in youth with versus without chronic pain. Pain 2016, 157, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Korterink, J.J.; Diederen, K.; Benninga, M.A.; Tabbers, M.M. Epidemiology of pediatric functional abdominal pain disorders: A meta-analysis. PLoS ONE 2015, 10, e0126982. [Google Scholar] [CrossRef] [PubMed]
- Egger, H.L.; Costello, E.J.; Erkanli, A.; Angold, A. Somatic complaints and psychopathology in children and adolescents: Stomach aches, musculoskeletal pains, and headaches. J. Am. Acad. Child Adolesc. Psychiatry 1999, 38, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Liedl, A.; Knaevelsrud, C. Chronic pain and PTSD: The perpetual avoidance model and its treatment implications. Torture 2008, 18, 69–76. [Google Scholar] [PubMed]
- Norman, S.B.; Stein, M.B.; Dimsdale, J.E.; Hoyt, D.B. Pain in the aftermath of trauma is a risk factor for post-traumatic stress disorder. Psychol. Med. 2008, 38, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.A.; Kenardy, J.A.; Dow, B.L. Ptsd perpetuates pain in children with traumatic brain injury. J. Pediatr. Psychol. 2014, 39, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Holley, A.L.; Wilson, A.C.; Noel, M.; Palermo, T.M. Post-traumatic stress symptoms in children and adolescents with chronic pain: A topical review of the literature and a proposed framework for future research. Eur. J. Pain 2016, 20, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.D.; Champagne, F.A.; Liu, D.; Meaney, M.J. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann. N. Y. Acad. Sci. 1999, 896, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Gunnar, M.; Quevedo, K. The neurobiology of stress and development. Annu. Rev. Psychol. 2007, 58, 145–173. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Nemeroff, C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol. Psychiatry 2001, 49, 1023–1039. [Google Scholar] [CrossRef]
- Peters, K.L. Neonatal stress reactivity and cortisol. J. Perinat. Neonatal Nurs. 1998, 11, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Shanks, N.; Harbuz, M.S.; Jessop, D.S.; Perks, P.; Moore, P.M.; Lightman, S.L. Inflammatory disease as chronic stress. Ann. N. Y. Acad. Sci. 1998, 840, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Lentjes, E.G.; Griep, E.N.; Boersma, J.W.; Romijn, F.P.; de Kloet, E.R. Glucocorticoid receptors, fibromyalgia and low back pain. Psychoneuroendocrinology 1997, 22, 603–614. [Google Scholar] [CrossRef]
- Heim, C.; Ehlert, U.; Hanker, J.P.; Hellhammer, D.H. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosom. Med. 1998, 60, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Blumer, D.; Zorick, F.; Heilbronn, M.; Roth, T. Biological markers for depression in chronic pain. J. Nerv. Ment. Dis. 1982, 170, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Tennant, F.; Hermann, L. Normalization of serum cortisol concentration with opioid treatment of severe chronic pain. Pain Med. 2002, 3, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Geiss, A.; Varadi, E.; Steinbach, K.; Bauer, H.W.; Anton, F. Psychoneuroimmunological correlates of persisting sciatic pain in patients who underwent discectomy. Neurosci. Lett. 1997, 237, 65–68. [Google Scholar] [CrossRef]
- Griep, E.N.; Boersma, J.W.; Lentjes, E.G.; Prins, A.P.; van der Korst, J.K.; de Kloet, E.R. Function of the hypothalamic-pituitary-adrenal axis in patients with fibromyalgia and low back pain. J. Rheumatol. 1998, 25, 1374–1381. [Google Scholar] [PubMed]
- Korszun, A.; Young, E.A.; Singer, K.; Carlson, N.E.; Brown, M.B.; Crofford, L. Basal circadian cortisol secretion in women with temporomandibular disorders. J. Dent. Res. 2002, 81, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Blackburn-Munro, G.; Blackburn-Munro, R.E. Chronic pain, chronic stress and depression: Coincidence or consequence? J. Neuroendocrinol. 2001, 13, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R. Biology of posttraumatic stress disorder. J. Clin. Psychiatry 2001, 62 (Suppl. 17), 41–46. [Google Scholar] [PubMed]
- Boyer, P. Do anxiety and depression have a common pathophysiological mechanism? Acta Psychiatr. Scand. Suppl. 2000, 24–29. [Google Scholar] [CrossRef]
- Levitt, N.S.; Lindsay, R.S.; Holmes, M.C.; Seckl, J.R. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology 1996, 64, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Seckl, J. Glucocorticoids, prenatal stress and the programming of disease. Horm. Behav. 2011, 59, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Champagne, F.A.; Francis, D.D.; Mar, A.; Meaney, M.J. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 2003, 79, 359–371. [Google Scholar] [CrossRef]
- Caldji, C.; Tannenbaum, B.; Sharma, S.; Francis, D.; Plotsky, P.M.; Meaney, M.J. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. USA 1998, 95, 5335–5340. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.; Diorio, J.; Liu, D.; Meaney, M.J. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 1999, 286, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Diorio, J.; Tannenbaum, B.; Caldji, C.; Francis, D.; Freedman, A.; Sharma, S.; Pearson, D.; Plotsky, P.M.; Meaney, M.J. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 1997, 277, 1659–1662. [Google Scholar] [CrossRef] [PubMed]
- Menard, J.L.; Champagne, D.L.; Meaney, M.J. Variations of maternal care differentially influence ‘fear’ reactivity and regional patterns of cfos immunoreactivity in response to the shock-probe burying test. Neuroscience 2004, 129, 297–308. [Google Scholar] [CrossRef] [PubMed]
- van Hasselt, F.N.; Cornelisse, S.; Zhang, T.Y.; Meaney, M.J.; Velzing, E.H.; Krugers, H.J.; Joels, M. Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus 2012, 22, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Y.; Bagot, R.; Parent, C.; Nesbitt, C.; Bredy, T.W.; Caldji, C.; Fish, E.; Anisman, H.; Szyf, M.; Meaney, M.J. Maternal programming of defensive responses through sustained effects on gene expression. Biol. Psychol. 2006, 73, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Diorio, J.; Day, J.C.; Francis, D.D.; Meaney, M.J. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat. Neurosci. 2000, 3, 799–806. [Google Scholar] [PubMed]
- Pena, C.J.; Neugut, Y.D.; Calarco, C.A.; Champagne, F.A. Effects of maternal care on the development of midbrain dopamine pathways and reward-directed behavior in female offspring. Eur. J. Neurosci. 2014, 39, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Starr-Phillips, E.J.; Beery, A.K. Natural variation in maternal care shapes adult social behavior in rats. Dev. Psychobiol. 2014, 56, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Essex, M.J.; Klein, M.H.; Cho, E.; Kalin, N.H. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biol. Psychiatry 2002, 52, 776–784. [Google Scholar] [CrossRef]
- Barry, T.J.; Murray, L.; Fearon, R.M.; Moutsiana, C.; Cooper, P.; Goodyer, I.M.; Herbert, J.; Halligan, S.L. Maternal postnatal depression predicts altered offspring biological stress reactivity in adulthood. Psychoneuroendocrinology 2015, 52, 251–260. [Google Scholar] [CrossRef] [PubMed]
- McCauley, J.; Kern, D.E.; Kolodner, K.; Dill, L.; Schroeder, A.F.; DeChant, H.K.; Ryden, J.; Derogatis, L.R.; Bass, E.B. Clinical characteristics of women with a history of childhood abuse: Unhealed wounds. JAMA 1997, 277, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Yaari, A.; Eisenberg, E.; Adler, R.; Birkhan, J. Chronic pain in holocaust survivors. J. Pain Symptom Manag. 1999, 17, 181–187. [Google Scholar] [CrossRef]
- Young Casey, C.; Greenberg, M.A.; Nicassio, P.M.; Harpin, R.E.; Hubbard, D. Transition from acute to chronic pain and disability: A model including cognitive, affective, and trauma factors. Pain 2008, 134, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Young, E.A.; Haskett, R.F.; Murphy-Weinberg, V.; Watson, S.J.; Akil, H. Loss of glucocorticoid fast feedback in depression. Arch. Gen. Psychiatry 1991, 48, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R. Biology of posttraumatic stress disorder. J Clin Psychiatry 2000, 61 (Suppl. 7), 14–21. [Google Scholar] [PubMed]
- Muhtz, C.; Rodriguez-Raecke, R.; Hinkelmann, K.; Moeller-Bertram, T.; Kiefer, F.; Wiedemann, K.; May, A.; Otte, C. Cortisol response to experimental pain in patients with chronic low back pain and patients with major depression. Pain Med. 2013, 14, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Grunau, R.E.; Cepeda, I.L.; Chau, C.M.; Brummelte, S.; Weinberg, J.; Lavoie, P.M.; Ladd, M.; Hirschfeld, A.F.; Russell, E.; Koren, G.; et al. Neonatal pain-related stress and nfkbia genotype are associated with altered cortisol levels in preterm boys at school age. PLoS ONE 2013, 8, e73926. [Google Scholar] [CrossRef] [PubMed]
- Brummelte, S.; Chau, C.M.; Cepeda, I.L.; Degenhardt, A.; Weinberg, J.; Synnes, A.R.; Grunau, R.E. Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology 2015, 51, 151–163. [Google Scholar] [CrossRef] [PubMed]
- McCormack, K.; Sanchez, M.M.; Bardi, M.; Maestripieri, D. Maternal care patterns and behavioral development of rhesus macaque abused infants in the first 6 months of life. Dev. Psychobiol. 2006, 48, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Birmaher, B.; Perel, J.; Dahl, R.E.; Moreci, P.; Nelson, B.; Wells, W.; Ryan, N.D. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biol. Psychiatry 1997, 42, 669–679. [Google Scholar] [CrossRef]
- De Bellis, M.D.; Chrousos, G.P.; Dorn, L.D.; Burke, L.; Helmers, K.; Kling, M.A.; Trickett, P.K.; Putnam, F.W. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. J. Clin. Endocrinol. Metab. 1994, 78, 249–255. [Google Scholar] [PubMed]
- Weems, C.F.; Carrion, V.G. The association between ptsd symptoms and salivary cortisol in youth: The role of time since the trauma. J. Trauma. Stress 2007, 20, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Laplante, P.; Diorio, J.; Meaney, M.J. Serotonin regulates hippocampal glucocorticoid receptor expression via a 5-ht7 receptor. Brain Res. Dev. Brain Res. 2002, 139, 199–203. [Google Scholar] [CrossRef]
- Charnay, Y.; Leger, L. Brain serotonergic circuitries. Dialogues Clin. Neurosci. 2010, 12, 471–487. [Google Scholar] [PubMed]
- Suzuki, R.; Rygh, L.J.; Dickenson, A.H. Bad news from the brain: Descending 5-ht pathways that control spinal pain processing. Trends Pharmacol. Sci. 2004, 25, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C. Is serotonin hyperalgesic or analgesic? Curr. Pain Headache Rep. 2006, 10, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Russell, I.J.; Vipraio, G.; Ross, K.; Anderson, J. Serotonin levels, pain threshold, and fibromyalgia symptoms in the general population. J. Rheumatol. 1997, 24, 555–559. [Google Scholar] [PubMed]
- Russell, I.J.; Vaeroy, H.; Javors, M.; Nyberg, F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheumatol. 1992, 35, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Aira, Z.; Buesa, I.; Salgueiro, M.; Bilbao, J.; Aguilera, L.; Zimmermann, M.; Azkue, J.J. Subtype-specific changes in 5-ht receptor-mediated modulation of c fibre-evoked spinal field potentials are triggered by peripheral nerve injury. Neuroscience 2010, 168, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Nicholl, B.I.; Holliday, K.L.; Macfarlane, G.J.; Thomson, W.; Davies, K.A.; O’Neill, T.W.; Bartfai, G.; Boonen, S.; Casanueva, F.F.; Finn, J.D.; et al. Association of htr2a polymorphisms with chronic widespread pain and the extent of musculoskeletal pain: Results from two population-based cohorts. Arthritis Rheumatol. 2011, 63, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Hanley, G.E.; Oberlander, T.F. Neurodevelopmental outcomes following prenatal exposure to serotonin reuptake inhibitor antidepressants: A "social teratogen" or moderator of developmental risk? Birth Defects Res. A Clin. Mol. Teratol. 2012, 94, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Heils, A.; Teufel, A.; Petri, S.; Stober, G.; Riederer, P.; Bengel, D.; Lesch, K.P. Allelic variation of human serotonin transporter gene expression. J. Neurochem. 1996, 66, 2621–2624. [Google Scholar] [CrossRef] [PubMed]
- Offenbaecher, M.; Bondy, B.; de Jonge, S.; Glatzeder, K.; Kruger, M.; Schoeps, P.; Ackenheil, M. Possible association of fibromyalgia with a polymorphism in the serotonin transporter gene regulatory region. Arthritis Rheumatol. 1999, 42, 2482–2488. [Google Scholar] [CrossRef]
- Cohen, H.; Buskila, D.; Neumann, L.; Ebstein, R.P. Confirmation of an association between fibromyalgia and serotonin transporter promoter region (5- httlpr) polymorphism, and relationship to anxiety-related personality traits. Arthritis Rheumatol. 2002, 46, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Homberg, J.R.; Lesch, K.P. Looking on the bright side of serotonin transporter gene variation. Biol. Psychiatry 2011, 69, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Wankerl, M.; Stalder, T.; Kirschbaum, C.; Alexander, N. The serotonin transporter gene-linked polymorphic region (5-httlpr) and cortisol stress reactivity: A meta-analysis. Mol. Psychiatry 2013, 18, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.R.; Mattay, V.S.; Tessitore, A.; Kolachana, B.; Fera, F.; Goldman, D.; Egan, M.F.; Weinberger, D.R. Serotonin transporter genetic variation and the response of the human amygdala. Science 2002, 297, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A.; Braus, D.F.; Smolka, M.N.; Wrase, J.; Puls, I.; Hermann, D.; Klein, S.; Grusser, S.M.; Flor, H.; Schumann, G.; et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat. Neurosci. 2005, 8, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.R.; Holmes, A. Genetics of emotional regulation: The role of the serotonin transporter in neural function. Trends Cogn. Sci. 2006, 10, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Canli, T.; Lesch, K.P. Long story short: The serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 2007, 10, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, A.M.; Shi, L.; Duman, R. Downregulation of bdnf mrna in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology 2002, 27, 133–142. [Google Scholar] [CrossRef]
- Govindarajan, A.; Rao, B.S.; Nair, D.; Trinh, M.; Mawjee, N.; Tonegawa, S.; Chattarji, S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc. Natl. Acad. Sci. USA 2006, 103, 13208–13213. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarasimhan, H.; Chattarji, S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS ONE 2012, 7, e30481. [Google Scholar] [CrossRef] [PubMed]
- Mutso, A.A.; Petre, B.; Huang, L.; Baliki, M.N.; Torbey, S.; Herrmann, K.M.; Schnitzer, T.J.; Apkarian, A.V. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J. Neurophysiol. 2014, 111, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Oathes, D.; Hush, J.; Darnall, B.; Charvat, M.; Mackey, S.; Etkin, A. Perturbed connectivity of the amygdala and its subregions with the central executive and default mode networks in chronic pain. Pain 2016, 157, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Duric, V.; McCarson, K.E. Effects of analgesic or antidepressant drugs on pain- or stress-evoked hippocampal and spinal neurokinin-1 receptor and brain-derived neurotrophic factor gene expression in the rat. J. Pharmacol. Exp. Ther. 2006, 319, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Heldt, S.A.; Stanek, L.; Chhatwal, J.P.; Ressler, K.J. Hippocampus-specific deletion of bdnf in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry 2007, 12, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, K.; Ishikawa, K.; Yasuda, S.; Kishishita, Y.; Kim, H.K.; Kakeda, T.; Yamamoto, M.; Norii, T.; Ishikawa, T. Intracerebroventricular 4-methylcatechol (4-mc) ameliorates chronic pain associated with depression-like behavior via induction of brain-derived neurotrophic factor (bdnf). Cell. Mol. Neurobiol. 2012, 32, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.F.; Goldberg, T.E.; Kolachana, B.S.; Callicott, J.H.; Mazzanti, C.M.; Straub, R.E.; Goldman, D.; Weinberger, D.R. Effect of comt val108/158 met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. USA 2001, 98, 6917–6922. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The bdnf val66met polymorphism affects activity-dependent secretion of bdnf and human memory and hippocampal function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef]
- Pezawas, L.; Verchinski, B.A.; Mattay, V.S.; Callicott, J.H.; Kolachana, B.S.; Straub, R.E.; Egan, M.F.; Meyer-Lindenberg, A.; Weinberger, D.R. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J. Neurosci. 2004, 24, 10099–10102. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.M.; Rodrigue, K.M.; Land, S.J.; Raz, N. Bdnf val66met polymorphism influences age differences in microstructure of the corpus callosum. Front. Hum. Neurosci. 2009, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Montag, C.; Weber, B.; Fliessbach, K.; Elger, C.; Reuter, M. The bdnf val66met polymorphism impacts parahippocampal and amygdala volume in healthy humans: Incremental support for a genetic risk factor for depression. Psychol. Med. 2009, 39, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Soliman, F.; Glatt, C.E.; Bath, K.G.; Levita, L.; Jones, R.M.; Pattwell, S.S.; Jing, D.; Tottenham, N.; Amso, D.; Somerville, L.H.; et al. A genetic variant bdnf polymorphism alters extinction learning in both mouse and human. Science 2010, 327, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Hajek, T.; Kopecek, M.; Hoschl, C. Reduced hippocampal volumes in healthy carriers of brain-derived neurotrophic factor val66met polymorphism: Meta-analysis. World J. Biol. Psychiatry 2012, 13, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L.; Bus, B.A.; Spinhoven, P.; Kaimatzoglou, A.; Oude Voshaar, R.C.; Penninx, B.W.; van, I.M.H.; Elzinga, B.M. A systematic review and meta-analysis on the association between bdnf val(66)met and hippocampal volume--a genuine effect or a winners curse? Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012, 159B, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Kang, D.H.; Yun, J.Y.; Lee, T.Y.; Jung, W.H.; Jang, J.H.; Kwon, J.S. Impact of the bdnf val66met polymorphism on regional brain gray matter volumes: Relevance to the stress response. Psychiatry Investig. 2013, 10, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Qing, H.; Lu, W.; Keegan, D.; Richardson, J.S.; Chlan-Fourney, J.; Li, X.M. Quetiapine attenuates the immobilization stress-induced decrease of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci. Lett. 2002, 321, 65–68. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Jing, D.; Bath, K.G.; Ieraci, A.; Khan, T.; Siao, C.J.; Herrera, D.G.; Toth, M.; Yang, C.; McEwen, B.S.; et al. Genetic variant bdnf (val66met) polymorphism alters anxiety-related behavior. Science 2006, 314, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.S.; Comim, C.M.; Valvassori, S.S.; Reus, G.Z.; Barbosa, L.M.; Andreazza, A.C.; Stertz, L.; Fries, G.R.; Gavioli, E.C.; Kapczinski, F.; et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases bdnf levels in the rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Pivac, N.; Kozaric-Kovacic, D.; Grubisic-Ilic, M.; Nedic, G.; Rakos, I.; Nikolac, M.; Blazev, M.; Muck-Seler, D. The association between brain-derived neurotrophic factor val66met variants and psychotic symptoms in posttraumatic stress disorder. World J. Biol. Psychiatry 2012, 13, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Cui, Y.L.; Yu, C.Q.; Wang, Q.S.; Zhang, Y. Tetrandrine exerts antidepressant-like effects in animal models: Role of brain-derived neurotrophic factor. Behav. Brain Res. 2013, 238, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.; Tu, C.H.; Chen, L.F.; Shen, H.D.; Chao, H.T.; Lin, M.W.; Hsieh, J.C. Association of brain-derived neurotrophic factor gene val66met polymorphism with primary dysmenorrhea. PLoS ONE 2014, 9, e112766. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.Y.; Rasmussen, N.A.; Fourie, N.H.; Berger, R.S.; Martino, A.C.; Gill, J.; Longchamps, R.; Wang, X.M.; Heitkemper, M.M.; Henderson, W.A. Sleep quality, bdnf genotype and gene expression in individuals with chronic abdominal pain. BMC Med. Genom. 2014, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Benedek, D.M.; Fullerton, C.S.; Forsten, R.D.; Naifeh, J.A.; Li, X.X.; Hu, X.Z.; Li, H.; Jia, M.; Xing, G.Q.; et al. Ptsd risk is associated with bdnf val66met and bdnf overexpression. Mol. Psychiatry 2014, 19, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Generaal, E.; Milaneschi, Y.; Jansen, R.; Elzinga, B.M.; Dekker, J.; Penninx, B.W. The brain-derived neurotrophic factor pathway, life stress, and chronic multi-site musculoskeletal pain. Mol. Pain 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Trang, T.; Beggs, S.; Salter, M.W. Brain-derived neurotrophic factor from microglia: A molecular substrate for neuropathic pain. Neuron. Glia Biol. 2011, 7, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.R.; Hutchinson, M.R.; Ledeboer, A.; Wieseler-Frank, J.; Milligan, E.D.; Maier, S.F. Norman cousins lecture. Glia as the “bad guys”: Implications for improving clinical pain control and the clinical utility of opioids. Brain Behav. Immun. 2007, 21, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Uceyler, N.; Sommer, C. Cytokine-related and histological biomarkers for neuropathic pain assessment. Pain Manag. 2012, 2, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Pankratz, S.; Bittner, S.; Kehrel, B.E.; Langer, H.F.; Kleinschnitz, C.; Meuth, S.G.; Gobel, K. The inflammatory role of platelets: Translational insights from experimental studies of autoimmune disorders. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Benson, S.; Engler, H.; Schedlowski, M.; Elsenbruch, S. Experimental endotoxemia as a model to study neuroimmune mechanisms in human visceral pain. Ann. N. Y. Acad. Sci. 2012, 1262, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.; Schwartzman, R.J.; Alexander, G. Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome. Brain Behav. Immun. 2009, 23, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Gelman, B.B.; Lisinicchia, J.G.; Tang, S.J. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J. Neurosci. 2012, 32, 10833–10840. [Google Scholar] [CrossRef] [PubMed]
- Brisby, H.; Olmarker, K.; Rosengren, L.; Cederlund, C.G.; Rydevik, B. Markers of nerve tissue injury in the cerebrospinal fluid in patients with lumbar disc herniation and sciatica. Spine 1999, 24, 742–746. [Google Scholar] [CrossRef]
- Kadetoff, D.; Lampa, J.; Westman, M.; Andersson, M.; Kosek, E. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J. Neuroimmunol. 2012, 242, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Loggia, M.L.; Chonde, D.B.; Akeju, O.; Arabasz, G.; Catana, C.; Edwards, R.R.; Hill, E.; Hsu, S.; Izquierdo-Garcia, D.; Ji, R.R.; et al. Evidence for brain glial activation in chronic pain patients. Brain 2015, 138, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Fiore, N.T.; Austin, P.J. Are the emergence of affective disturbances in neuropathic pain states contingent on supraspinal neuroinflammation? Brain Behav. Immun. 2016, 56, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Goshen, I.; Kreisel, T.; Ben-Menachem-Zidon, O.; Licht, T.; Weidenfeld, J.; Ben-Hur, T.; Yirmiya, R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry 2008, 13, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Goshen, I.; Kreisel, T.; Ounallah-Saad, H.; Renbaum, P.; Zalzstein, Y.; Ben-Hur, T.; Levy-Lahad, E.; Yirmiya, R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 2007, 32, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, H.O.; del Rey, A. Immune-neuro-endocrine interactions: Facts and hypotheses. Endocr. Rev. 1996, 17, 64–102. [Google Scholar] [CrossRef] [PubMed]
- Pace, T.W.; Hu, F.; Miller, A.H. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav. Immun. 2007, 21, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Vogelzangs, N.; de Jonge, P.; Smit, J.H.; Bahn, S.; Penninx, B.W. Cytokine production capacity in depression and anxiety. Transl. Psychiatry 2016, 6, e825. [Google Scholar] [CrossRef] [PubMed]
- Lasselin, J.; Kemani, M.K.; Kanstrup, M.; Olsson, G.L.; Axelsson, J.; Andreasson, A.; Lekander, M.; Wicksell, R.K. Low-grade inflammation may moderate the effect of behavioral treatment for chronic pain in adults. J. Behav. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Beggs, S.; Currie, G.; Salter, M.W.; Fitzgerald, M.; Walker, S.M. Priming of adult pain responses by neonatal pain experience: Maintenance by central neuroimmune activity. Brain 2012, 135, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.H.; Goldstein, B.I. Inflammation in children and adolescents with neuropsychiatric disorders: A systematic review. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 274–296. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Brenhouse, H.C. Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Dev. Cogn. Neurosci. 2015, 11, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Luckenbaugh, D.; Charney, D.; Vythilingam, M. Sustained elevation of serum interleukin-6 and relative insensitivity to hydrocortisone differentiates posttraumatic stress disorder with and without depression. Biol. Psychiatry 2010, 68, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Lerman, I.; Davis, B.A.; Bertram, T.M.; Proudfoot, J.; Hauger, R.L.; Coe, C.L.; Patel, P.M.; Baker, D.G. Posttraumatic stress disorder influences the nociceptive and intrathecal cytokine response to a painful stimulus in combat veterans. Psychoneuroendocrinology 2016, 73, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Kopf, M.; Bachmann, M.F.; Marsland, B.J. Averting inflammation by targeting the cytokine environment. Nat. Rev. Drug Discov. 2010, 9, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.D.; Zubieta, J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 2005, 9, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Ceko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Duerden, E.G.; Albanese, M.C. Localization of pain-related brain activation: A meta-analysis of neuroimaging data. Hum. Brain Mapp. 2013, 34, 109–149. [Google Scholar] [CrossRef] [PubMed]
- Iadarola, M.J.; Coghill, R.C. Imaging of pain: Recent developments. Curr. Opin. Anaesthesiol. 1999, 12, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Willis, W.D.; Westlund, K.N. Neuroanatomy of the pain system and of the pathways that modulate pain. J. Clin. Neurophysiol. 1997, 14, 2–31. [Google Scholar] [CrossRef] [PubMed]
- Atlas, L.Y.; Lindquist, M.A.; Bolger, N.; Wager, T.D. Brain mediators of the effects of noxious heat on pain. Pain 2014, 155, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Coghill, R.C.; Sang, C.N.; Maisog, J.M.; Iadarola, M.J. Pain intensity processing within the human brain: A bilateral, distributed mechanism. J. Neurophysiol. 1999, 82, 1934–1943. [Google Scholar] [PubMed]
- Wager, T.D.; Atlas, L.Y.; Lindquist, M.A.; Roy, M.; Woo, C.W.; Kross, E. An fmri-based neurologic signature of physical pain. N. Engl. J. Med. 2013, 368, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, C.S.; Khan, S.A.; Xu, S.; Cha, M.; Masri, R.; Seminowicz, D.A. Behavioral, metabolic and functional brain changes in a rat model of chronic neuropathic pain: A longitudinal mri study. Neuroimage 2015, 107, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.B.; Regenbogen, C.; Ohse, M.C.; Frasnelli, J.; Freiherr, J.; Lundstrom, J.N. Brain activations during pain: A neuroimaging meta-analysis of patients with pain and healthy controls. Pain 2016, 157, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Baliki, M.N.; Petre, B.; Torbey, S.; Herrmann, K.M.; Huang, L.; Schnitzer, T.J.; Fields, H.L.; Apkarian, A.V. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012, 15, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, C.S.; Becerra, L.; Heinz, N.; Ludwick, A.; Rasooly, T.; Wu, R.; Johnson, A.; Schechter, N.L.; Borsook, D.; Nurko, S. Abdominal pain, the adolescent and altered brain structure and function. PLoS ONE 2016, 11, e0156545. [Google Scholar] [CrossRef] [PubMed]
- Simons, L.E.; Pielech, M.; Erpelding, N.; Linnman, C.; Moulton, E.; Sava, S.; Lebel, A.; Serrano, P.; Sethna, N.; Berde, C.; et al. The responsive amygdala: Treatment-induced alterations in functional connectivity in pediatric complex regional pain syndrome. Pain 2014, 155, 1727–1742. [Google Scholar] [CrossRef] [PubMed]
- Simons, L.E.; Erpelding, N.; Hernandez, J.; Serrano, P.; Zhang, K.; Lebel, A.; Sethna, N.; Berde, C.; Prabhu, S.; Becerra, L.; et al. Fear and reward circuit alterations in pediatric crps. Front. Hum. Neurosci. 2016, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lebel, A.; Becerra, L.; Wallin, D.; Moulton, E.A.; Morris, S.; Pendse, G.; Jasciewicz, J.; Stein, M.; Aiello-Lammens, M.; Grant, E.; et al. Fmri reveals distinct cns processing during symptomatic and recovered complex regional pain syndrome in children. Brain 2008, 131, 1854–1879. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Kalia, M. The role of corticosteroids and stress in chronic pain conditions. Metabolism 2010, 59 (Suppl. 1), S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Karatsoreos, I.N.; McEwen, B.S. Psychobiological allostasis: Resistance, resilience and vulnerability. Trends Cogn. Sci. 2011, 15, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Becerra, L.; Sava, S.; Simons, L.E.; Drosos, A.M.; Sethna, N.; Berde, C.; Lebel, A.A.; Borsook, D. Intrinsic brain networks normalize with treatment in pediatric complex regional pain syndrome. Neuroimage Clin. 2014, 6, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, J.F.; Fernandesl, S.V.; Soares, J.M.; Maia, L.; Goncalves, O.F.; Sampaio, A. Default mode network dissociation in depressive and anxiety states. Brain Imaging Behav. 2016, 10, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Li, X.; Chen, J.; Li, X.; Gu, R. Decreased intra- and inter-salience network functional connectivity is related to trait anxiety in adolescents. Front. Behav. Neurosci. 2015, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Li, L.; Li, L.; Suo, X.; Huang, X.; Lui, S.; Li, J.; Bi, F.; Kemp, G.J.; Gong, Q. Microstructural abnormalities in children with post-traumatic stress disorder: A diffusion tensor imaging study at 3.0t. Sci. Rep. 2015, 5, 8933. [Google Scholar] [CrossRef] [PubMed]
- Pannekoek, J.N.; van der Werff, S.J.; Meens, P.H.; van den Bulk, B.G.; Jolles, D.D.; Veer, I.M.; van Lang, N.D.; Rombouts, S.A.; van der Wee, N.J.; Vermeiren, R.R. Aberrant resting-state functional connectivity in limbic and salience networks in treatment--naive clinically depressed adolescents. J. Child Psychol. Psychiatry 2014, 55, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Schanberg, L.E.; Anthony, K.K.; Gil, K.M.; Lefebvre, J.C.; Kredich, D.W.; Macharoni, L.M. Family pain history predicts child health status in children with chronic rheumatic disease. Pediatrics 2001, 108, E47. [Google Scholar] [CrossRef] [PubMed]
- Garber, J.; Zeman, J.; Walker, L.S. Recurrent abdominal pain in children: Psychiatric diagnoses and parental psychopathology. J. Am. Acad. Child Adolesc. Psychiatry 1990, 29, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Hoftun, G.B.; Romundstad, P.R.; Rygg, M. Factors associated with adolescent chronic non-specific pain, chronic multisite pain, and chronic pain with high disability: The young-hunt study 2008. J. Pain 2012. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.E.; Hammen, C.; Lee, S.S. Parental serotonin transporter polymorphism (5-httlpr) moderates associations of stress and child behavior with parenting behavior. J. Clin. Child Adolesc. Psychol. 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.L.; Lubin, F.D.; Funk, A.J.; Sweatt, J.D. Lasting epigenetic influence of early-life adversity on the bdnf gene. Biol. Psychiatry 2009, 65, 760–769. [Google Scholar] [CrossRef] [PubMed]
- van der Doelen, R.H.; Arnoldussen, I.A.; Ghareh, H.; van Och, L.; Homberg, J.R.; Kozicz, T. Early life adversity and serotonin transporter gene variation interact to affect DNA methylation of the corticotropin-releasing factor gene promoter region in the adult rat brain. Dev. Psychopathol. 2015, 27, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S.; Greene, J.W. Children with recurrent abdominal pain and their parents: More somatic complaints, anxiety, and depression than other patient families? J. Pediatr. Psychol. 1989, 14, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Campo, J.V.; Bridge, J.; Lucas, A.; Savorelli, S.; Walker, L.; Di Lorenzo, C.; Iyengar, S.; Brent, D.A. Physical and emotional health of mothers of youth with functional abdominal pain. Arch. Pediatr. Adolesc. Med. 2007, 161, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.M.; Walters, A.S.; Shaffer, D.R. Caregiver models of self and others, coping, and depression: Predictors of depression in children with chronic pain. Health Psychol. 2002, 21, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Wolff, N.; Darlington, A.S.; Hunfeld, J.; Verhulst, F.; Jaddoe, V.; Hofman, A.; Passchier, J.; Tiemeier, H. Determinants of somatic complaints in 18-month-old children: The generation r study. J. Pediatr. Psychol. 2010, 35, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.S.; Birnie, K.A.; Chambers, C.T.; Wilson, A.C.; Caes, L.; Clark, A.J.; Lynch, M.; Stinson, J.; Campbell-Yeo, M. Offspring of parents with chronic pain: A systematic review and meta-analysis of pain, health, psychological, and family outcomes. Pain 2015, 156, 2256–2266. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.L.; Whitehead, W.E.; Von Korff, M.R.; Feld, A.D. Intergenerational transmission of gastrointestinal illness behavior. Am. J. Gastroenterol. 2000, 95, 451–456. [Google Scholar] [CrossRef] [PubMed]
- van Tilburg, M.A.; Levy, R.L.; Walker, L.S.; Von Korff, M.; Feld, L.D.; Garner, M.; Feld, A.D.; Whitehead, W.E. Psychosocial mechanisms for the transmission of somatic symptoms from parents to children. World J. Gastroenterol. 2015, 21, 5532–5541. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.L.; Wilson, A.C. Transmission of risk from parents with chronic pain to offspring: An integrative conceptual model. Pain 2016. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Law, E.F.; Fales, J.; Bromberg, M.H.; Jessen-Fiddick, T.; Tai, G. Internet-delivered cognitive-behavioral treatment for adolescents with chronic pain and their parents: A randomized controlled multicenter trial. Pain 2016, 157, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Valrie, C.R.; Karlson, C.W. Family and parent influences on pediatric chronic pain: A developmental perspective. Am. Psychol. 2014, 69, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.; Law, E.; Palermo, T.M.; Eccleston, C. Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst. Rev. 2014, 2014. [Google Scholar]
- Cunningham, N.R.; Jagpal, A.; Tran, S.T.; Kashikar-Zuck, S.; Goldschneider, K.R.; Coghill, R.C.; Lynch-Jordan, A.M. Anxiety adversely impacts response to cognitive behavioral therapy in children with chronic pain. J. Pediatr. 2016, 171, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Benore, E.; D'Auria, A.; Banez, G.A.; Worley, S.; Tang, A. The influence of anxiety reduction on clinical response to pediatric chronic pain rehabilitation. Clin. J. Pain 2015, 31, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.; Goodison-Farnsworth, E.; Jaaniste, T. Posttraumatic stress disorder in children with chronic pain. Pediatr. Pain Lett. 2015, 17, 35–39. [Google Scholar]
- Nelson, S.M.; Cunningham, N.R.; Kashikar-Zuck, S. A conceptual framework for understanding the role of adverse childhood experiences in pediatric chronic pain. Clin. J. Pain 2016. [Google Scholar] [CrossRef] [PubMed]
- Bosco, M.A.; Gallinati, J.L.; Clark, M.E. Conceptualizing and treating comorbid chronic pain and ptsd. Pain Res. Treat. 2013, 2013, 174728. [Google Scholar] [CrossRef] [PubMed]
- Plagge, J.M.; Lu, M.W.; Lovejoy, T.I.; Karl, A.I.; Dobscha, S.K. Treatment of comorbid pain and ptsd in returning veterans: A collaborative approach utilizing behavioral activation. Pain Med. 2013, 14, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Otis, J.D.; Keane, T.M.; Kerns, R.D.; Monson, C.; Scioli, E. The development of an integrated treatment for veterans with comorbid chronic pain and posttraumatic stress disorder. Pain Med. 2009, 10, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.B.; Tsao, J.C.I.; Seidman, L.C.; Ehrenreich-May, J.; Zeltzer, L.K. A unified, transdiagnositc treatment for adolescents with chronic pain and comorbid anxiety and depression. Cogn. Behav. Pract. 2012, 19, 56–67. [Google Scholar] [CrossRef]
- Umezaki, Y.; Badran, B.W.; DeVries, W.H.; Moss, J.; Gonzales, T.; George, M.S. The efficacy of daily prefrontal repetitive transcranial magnetic stimulation (rtms) for burning mouth syndrome (bms): A randomized controlled single-blind study. Brain Stimul. 2016, 9, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Picarelli, H.; Teixeira, M.J.; de Andrade, D.C.; Myczkowski, M.L.; Luvisotto, T.B.; Yeng, L.T.; Fonoff, E.T.; Pridmore, S.; Marcolin, M.A. Repetitive transcranial magnetic stimulation is efficacious as an add-on to pharmacological therapy in complex regional pain syndrome (crps) type i. J. Pain 2010, 11, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, M.E.; Simis, M.; Grecco, L.C.; Battistella, L.R.; Baptista, A.F.; Fregni, F. Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: A randomized placebo-controlled clinical trial. Front. Hum. Neurosci. 2016, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Short, E.B.; Borckardt, J.J.; Anderson, B.S.; Frohman, H.; Beam, W.; Reeves, S.T.; George, M.S. Ten sessions of adjunctive left prefrontal rtms significantly reduces fibromyalgia pain: A randomized, controlled pilot study. Pain 2011, 152, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Ayache, S.S.; Sorel, M.; Farhat, W.H.; Zouari, H.G.; Ciampi de Andrade, D.; Ahdab, R.; Menard-Lefaucheur, I.; Brugieres, P.; Goujon, C. Analgesic effects of repetitive transcranial magnetic stimulation of the motor cortex in neuropathic pain: Influence of theta burst stimulation priming. Eur. J. Pain 2012, 16, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.S.; Lima, M.C.; Ferreira, M.J.; Wagner, T.; Rigonatti, S.P.; Castro, A.W.; Souza, D.R.; Riberto, M.; Freedman, S.D.; et al. A sham-controlled, phase ii trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 2006, 122, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Defrin, R.; Grunhaus, L.; Zamir, D.; Zeilig, G. The effect of a series of repetitive transcranial magnetic stimulations of the motor cortex on central pain after spinal cord injury. Arch. Phys. Med. Rehabil. 2007, 88, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.; George, M.S.; Grammer, G.; Janicak, P.G.; Pascual-Leone, A.; Wirecki, T.S. The clinical tms society consensus review and treatment recommendations for tms therapy for major depressive disorder. Brain Stimul. 2016, 9, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Canadian Agency for Drugs and Technologies in Health. Cadth rapid response reports. In Transcranial Magnetic Stimulation for the Treatment of Adults with Ptsd, Gad, or Depression: A Review of Clinical Effectiveness and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2014. [Google Scholar]
- Paes, F.; Machado, S.; Arias-Carrion, O.; Velasques, B.; Teixeira, S.; Budde, H.; Cagy, M.; Piedade, R.; Ribeiro, P.; Huston, J.P.; et al. The value of repetitive transcranial magnetic stimulation (rtms) for the treatment of anxiety disorders: An integrative review. CNS Neurol. Disord. Drug Targets 2011, 10, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Milardi, D.; Russo, M.; Terranova, C.; Rizzo, V.; Cacciola, A.; Marino, S.; Calabro, R.S.; Quartarone, A. Non-invasive brain stimulation, a tool to revert maladaptive plasticity in neuropathic pain. Front. Hum. Neurosci. 2016, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- DosSantos, M.F.; Ferreira, N.; Toback, R.L.; Carvalho, A.C.; DaSilva, A.F. Potential mechanisms supporting the value of motor cortex stimulation to treat chronic pain syndromes. Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 1995, 332, 1351–1362. [Google Scholar] [PubMed]
- Elenkov, I.J.; Webster, E.L.; Torpy, D.J.; Chrousos, G.P. Stress, corticotropin-releasing hormone, glucocorticoids, and the immune/inflammatory response: Acute and chronic effects. Ann. N. Y. Acad. Sci. 1999, 876, 1–11; discussion 11-13. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.S. Early adrenalectomy stimulates subsequent growth and development of the rat brain. Exp. Neurol. 1983, 82, 432–446. [Google Scholar] [CrossRef]
- Meaney, M.J.; Diorio, J.; Francis, D.; Widdowson, J.; LaPlante, P.; Caldji, C.; Sharma, S.; Seckl, J.R.; Plotsky, P.M. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Dev. Neurosci. 1996, 18, 49–72. [Google Scholar] [CrossRef] [PubMed]
- Korte, S.M. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci. Biobehav. Rev. 2001, 25, 117–142. [Google Scholar] [CrossRef]
- Knook, L.M.; Lijmer, J.G.; Konijnenberg, A.Y.; Taminiau, B.; van Engeland, H. The course of chronic pain with and without psychiatric disorders: A 6-year follow-up study from childhood to adolescence and young adulthood. J. Clin. Psychiatry 2012, 73, e134–e139. [Google Scholar] [CrossRef] [PubMed]
- Mulak, A.; Tache, Y.; Larauche, M. Sex hormones in the modulation of irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 2433–2448. [Google Scholar] [CrossRef] [PubMed]
- Karshikoff, B.; Lekander, M.; Soop, A.; Lindstedt, F.; Ingvar, M.; Kosek, E.; Olgart Hoglund, C.; Axelsson, J. Modality and sex differences in pain sensitivity during human endotoxemia. Brain Behav. Immun. 2015, 46, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, A.M.; Bachiocco, V.; Costantino, A.; Stefani, R.; Ceccarelli, I.; Bertaccini, A.; Meriggiola, M.C. Cross-sex hormone administration changes pain in transsexual women and men. Pain 2007, 132 (Suppl. 1), S60–S67. [Google Scholar] [CrossRef] [PubMed]
| Study | n | Design | Age (Year) | Assessment Time Points | Pain assessment | Internalizing Disorders/Symptoms Assessment | Findings |
|---|---|---|---|---|---|---|---|
| Longitudinal studies of pediatric chronic pain and internalizing mental health issues into adulthood | |||||||
| Egger et al, 1998 [14] | 1013 | Longitudinal cohort study | 5–15 | Assessed annually over 3 years | CAPA somatization section | CAPA | 40.8% of girls with depression reported headaches as compared to girls without depression (10.5%). 34.1% of girls with an anxiety disorder reported having headaches as compared with girls without an anxiety disorder (10%). 19.2% of boys with CD and 10% of boys with ODD reported having headaches as compared to boys without externalizing disorders (9.6%). |
| Egger et al, 1999 [15] | 3733 | Longitudinal cohort study | 9–16 | Assessed annually over 3 years | CAPA somatization section | CAPA | Girls with stomach aches (OR 7.2, CI 2.8–18.5) and musculoskeletal pain (OR 3.4, CI 1.5–8.0) were more likely to have anxiety as compared to pain-free girls. Boys with stomach aches were likely to have ODD (OR 3.6, CI 1.6–8.1) and ADHD (OR 3.5, CI 1.8–7.1) as compared to boys without stomach aches. Both girls (OR 12.9, CI 4.5–37.0) and boys (OR 10.5, CI 2.3–48.0) with musculoskeletal pain were more likely to report depression compared to children without musculoskeletal pain. |
| Hotopf et al, 1998 [5] | 3637 | Longitudinal cohort study | 7–36 | 7, 11, 15 years old | Parent report of abdominal pain at ages 7, 11 and 15 years | N/A | Youth who had abdominal pain were more likely to develop a psychiatric disorder by Time 2 (OR 2.72, CI 1.65–4.49). Pain in childhood was not associated with heightened risk of physical symptoms in adulthood (OR 1.39, CI 0.83–2.36). |
| 36 years old | Self-report of physical symptoms (back pain, headache, abdominal pain, chest pain, dizziness, and rheumatism) | Semi–structured psychiatric interview (Present State Examination) | |||||
| Fearon et al, 2001 [3] | 11,407 | Longitudinal cohort study | 7–33 | 7, 11, 16, 23 years old | Parent report of headache at ages 7 and 11 (binary variable) | Bristol Social Adjustment Guide | Youth suffering from frequent headaches were more likely to have recurrent headaches in adulthood (OR 2.22, CI 1.62–3.06), physical symptoms (OR 1.75, CI 1.46–2.10), and psychiatric disorders (OR 1.41, CI 1.20–1.66). |
| 33 years old | Self-report of physical symptoms (back pain, headache, twitches, rheumatism, indigestion, heart racing, worries about health) | Presence of four or more symptoms on a psychiatric morbidity self-report scale | |||||
| Walker et al, 2012 [16] | 843 | Longitudinal cohort study | 12–21 | 12 years old | API, CSI | N/A | At 21 years, participants with High Pain Dysfunctional profile were at a higher risk of having a pain-related FGID (OR 3.45, CI 1.95–6.11), FGID and non-abdominal chronic pain (OR 2.6, CI 1.45–4.66), FGID and anxiety or depressive disorder (OR 2.84, CI 1.35–6.00) as compared with Low Pain Adaptive profile participants. |
| 21 years old | Rome III, PPQ | ADIS | |||||
| Shelby et al, 2013 [4] | 491 | Longitudinal cohort study | 8–21 | 8–17 years old | Vanderbilt Pediatric Gastroenterology Service evaluation of FAP | N/A | At follow-up, participants with FAP were more likely to meet criteria for lifetime (OR 4.9, CI 2.83–7.43) and current (OR 3.57, CI 2.00–6.36) anxiety disorder and lifetime depressive disorder (OR 2.62, CI 1.56–4.40) as compared to controls. Participants with FAP, who developed FGID by follow-up, were more likely to meet criteria for any lifetime (OR 7.31, CI 4.17–12.81) or current (OR 5.09, CI 2.70–9.59) anxiety disorder and any lifetime depressive disorder (OR 4.14, CI 2.31–7.40) as compared to controls. Participants with FAP, who have not met criteria for FGID by follow-up, were still more likely to have any lifetime (OR 3.36, CI 2.01–5.63) or current (OR 2.68, CI 1.44–4.99) anxiety disorder as compared to controls. |
| 4 years after initial assessment | Rome III | ADIS | |||||
| Shanahan et al, 2015 [17] | 1420 | Longitudinal cohort study | 9–26 | 9–16 years old, assessed 4–7 times | Self- and parent-report of recurrent (at least one one-hour episode at least once a week in the past three months) pain (headache, abdominal or muscle pain) | CAPA | 34.4% of children reported somatic complaints. Participants with somatic complaints were more likely to have depressive (OR 6.90, CI 3.57–13.34) or anxiety (OR2.75, CI 1.55–4.89) disorders in childhood versus pain-free peers. Children with somatic complaints in childhood were more likely to develop depressive (OR 3.21, CI 1.54–6.70) or any anxiety disorders (CI 2.32, CI 1.30–4.14) by young adulthood as compared to pain-free participants. Sex differences in the likelihood of developing psychiatric disorders in adulthood following somatic complaints in childhood were not revealed. |
| 19, 21, 24–26 | Recurrent headache binomial variable within the YAPA | YAPA | |||||
| Noel et al, 2016 [18] | 14,790 | Longitudinal cohort study | 12–32 | Wave I and II: 12–18 | Self-report general health survey—frequency of headache, stomach ache, muscle/joints pain. Chronic pain was defined as pain at wave I and/or wave II | N/A | 21.9% of participants reported having chronic pain during adolescence. Youth with chronic pain reported higher rates of lifetime depressive (24.5%) and anxiety (21.1%) disorders versus youth without chronic pain. Chronic pain in youth was associated with a greater likelihood of having lifetime anxiety (OR 1.33, CI 1.09–1.63) and depressive (OR 1.38, CI 1.16–1.64) disorders. |
| Wave IV: ages 24–32 | N/A | Diagnosis of PTSD, anxiety, and/or depression by a health care provider | |||||
| Studies of co-occurring pediatric chronic pain and internalizing mental health issues | |||||||
| Balottin et al, 2013 [19] | 1124 | Meta-analysis | Mean ages: 11.6 (migraine), 12.3 (tension-type headache), 11.75 controls | N/A | ICHD I or II | CBCL | Having tension-type headaches was associated with higher internalizing symptoms (Hedge’s g = 2.344). |
| Blaauw et al, 2014 [20] | 4872 | Cross-sectional study | 12–17 | N/A | Headache interview assessing frequency of migraine, tension-type headache or unclassifiable headache over the last year | SCL-5 | Recurrent headache of any type (migraine, tension-type) was associated with anxiety and depression symptoms (at the age of 12–14 years, OR 2.50, CI 1.61–2.61; at the age of 15–17 years, OR 1.64, CI 1.39–1.93). |
| Coffelt et al, 2013 [21] | 3752 | Retrospective cohort study | Mean age 13.54 | N/A | Hospital record of chronic pain diagnoses (e.g., psychogenic pain not otherwise specified, chronic pain syndrome, complex regional pain syndrome) | Hospital record of a psychiatric diagnosis. | 44% of youth with chronic pain have been diagnosed with a psychiatric condition, specifically, an affective (28%), anxiety (18%), somatization (6%) disorder or PTSD (2.4%). |
| Noel et al, 2016 [22] | 195 | Cross-sectional study | 10–17 | N/A | Self-report of pain characteristics (i.e., pain intensity, frequency, location, unpleasantness, and duration over the previous seven days); pain interference sub-scale of the PROMIS-25 Pediatric Profile | CPSS-5 | 32% of youth with chronic pain reported clinically significant PTSD symptoms as compared to 8% of pain-free peers. Parents of youth with chronic pain had higher levels of clinically significant PTSD symptoms (8%) as compared with parents’ of youth without chronic pain (1%). Among the chronic pain group, PTSD symptoms were significantly associated with pain intensity, unpleasantness, interference, and quality of life. |
| Simons et al, 2012 [23] | 655 | Retrospective chart review | 8–17 | N/A | 11-point NRS | RCMAS | 11% of youth with chronic pain reported clinically significant levels of anxiety, 31% underreported their anxiety levels. |
| Tegethoff et al, 2015 [24] | 6483 | Cross-sectional study | 13–18 years | N/A | Self-report chronic pain conditions checklist | CIDI; parent-report SAQ | 25.93% of youth reported having chronic pain and mental health disorder in their lifetime. Any type or chronic pain increased the risk of developing eating (OR 2.63, CI 1.63–4.24), anxiety (OR 2.42, CI 2.03–2.88), affective (OR 2.32, CI 1.85–2.91), or any mental (OR 2.51, CI 2.12–2.98) disorder. The onset of any mental health disorder preceded any chronic pain (OR 1.64, CI 1.44–1.86). |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinall, J.; Pavlova, M.; Asmundson, G.J.G.; Rasic, N.; Noel, M. Mental Health Comorbidities in Pediatric Chronic Pain: A Narrative Review of Epidemiology, Models, Neurobiological Mechanisms and Treatment. Children 2016, 3, 40. https://doi.org/10.3390/children3040040
Vinall J, Pavlova M, Asmundson GJG, Rasic N, Noel M. Mental Health Comorbidities in Pediatric Chronic Pain: A Narrative Review of Epidemiology, Models, Neurobiological Mechanisms and Treatment. Children. 2016; 3(4):40. https://doi.org/10.3390/children3040040
Chicago/Turabian StyleVinall, Jillian, Maria Pavlova, Gordon J. G. Asmundson, Nivez Rasic, and Melanie Noel. 2016. "Mental Health Comorbidities in Pediatric Chronic Pain: A Narrative Review of Epidemiology, Models, Neurobiological Mechanisms and Treatment" Children 3, no. 4: 40. https://doi.org/10.3390/children3040040
APA StyleVinall, J., Pavlova, M., Asmundson, G. J. G., Rasic, N., & Noel, M. (2016). Mental Health Comorbidities in Pediatric Chronic Pain: A Narrative Review of Epidemiology, Models, Neurobiological Mechanisms and Treatment. Children, 3(4), 40. https://doi.org/10.3390/children3040040






