Early Childhood Anemia in Ghana: Prevalence and Predictors Using Machine Learning Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset and Study Population
2.2. Study Variables and Measurements
2.3. Statistical Analysis
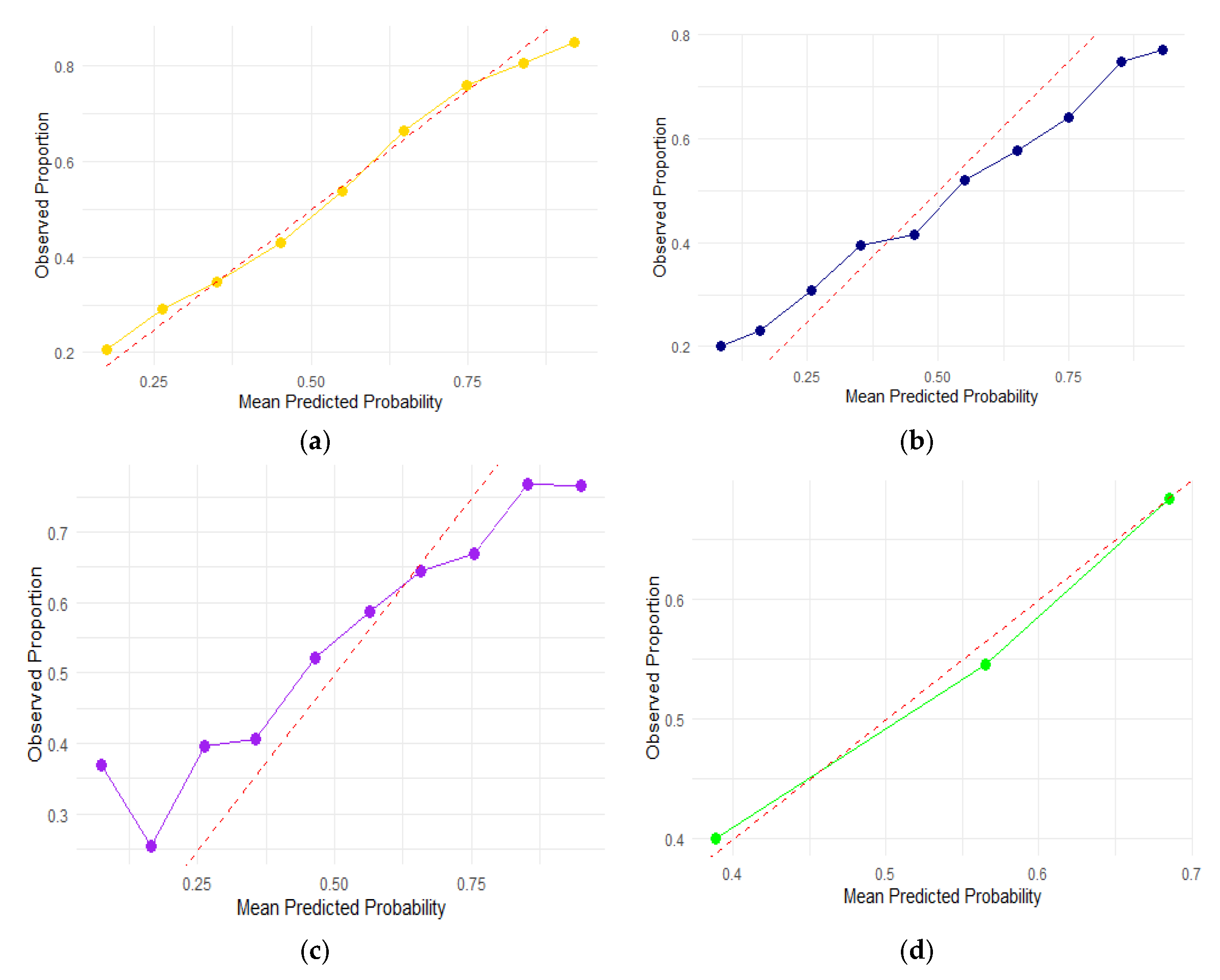
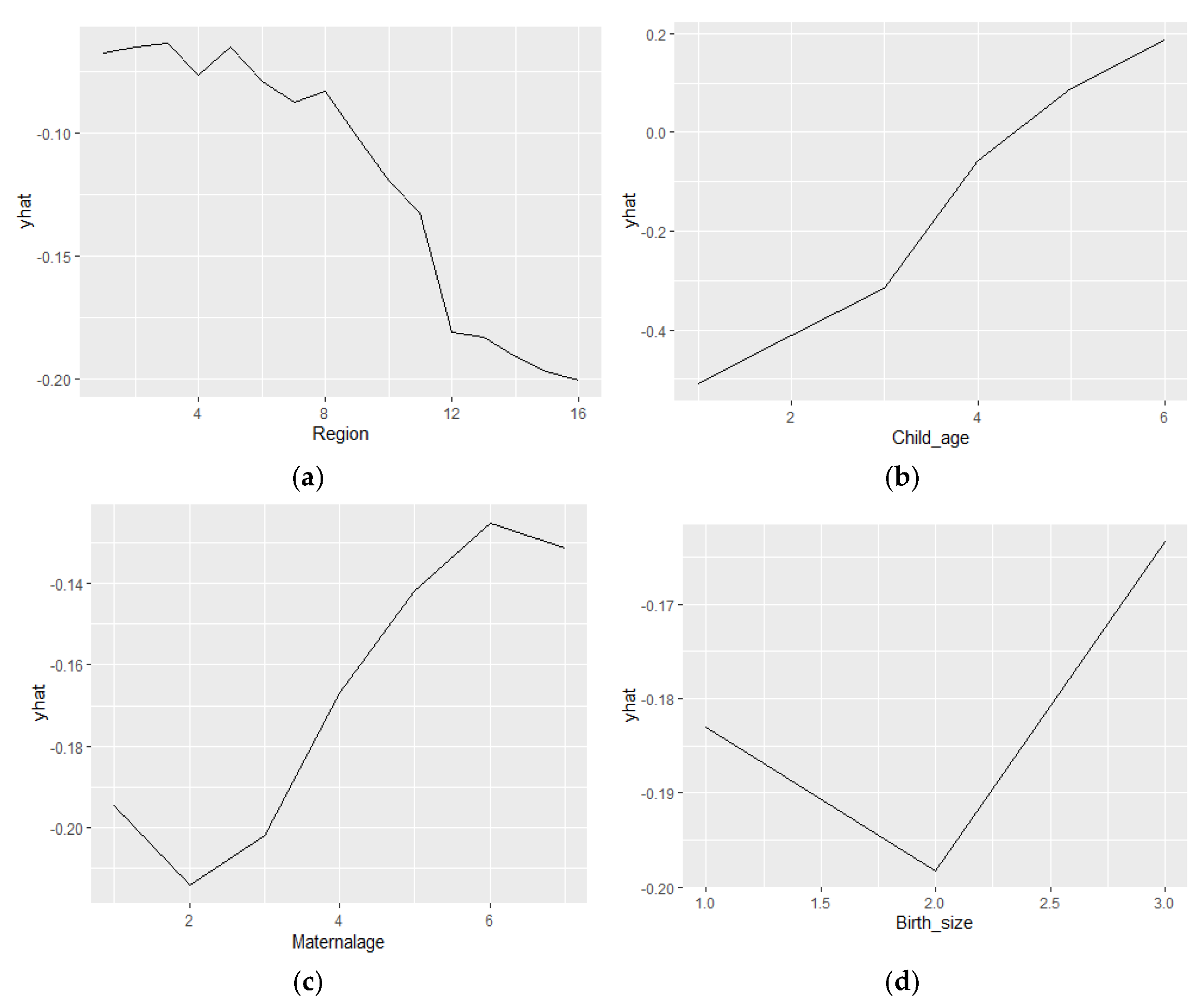
3. Results
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ML | Machine Learning |
| DT | Decision Tree |
| KNN | K-nearest Neighbor |
| RF | Random Forest |
| AUC | Area Under the Curve |
| ROC | Receiving Operating Curve |
References
- WHO. Anemia Policy Brief 2014. Available online: https://www.who.int/publications/i/item/WHO-NMH-NHD-14.4 (accessed on 9 February 2025).
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef] [PubMed]
- World Bank. Prevalence of Anemia Among Children (% of Children Ages 6–59 Months). 2021. Available online: https://data.worldbank.org/indicator/SH.ANM.CHLD.ZS (accessed on 4 July 2025).
- World Bank. Prevalence of Anemia Among Women of Reproductive Age (% of Women Ages 15–49). 2021. Available online: https://data.worldbank.org/indicator/SH.ANM.ALLW.ZS (accessed on 9 February 2025).
- World Bank. Prevalence of Anemia Among Pregnant Women (%). 2021. Available online: https://data.worldbank.org/indicator/SH.PRG.ANEM (accessed on 9 February 2025).
- Tesema, G.A.; Worku, M.G.; Tessema, Z.T.; Teshale, A.B.; Alem, A.Z.; Yeshaw, Y.; Alamneh, T.S.; Liyew, A.M. Prevalence and determinants of severity levels of anemia among children aged 6–59 months in sub-Saharan Africa: A multilevel ordinal logistic regression analysis. PLoS ONE 2021, 16, e0249978. [Google Scholar] [CrossRef]
- Aheto, J.M.K.; Alhassan, Y.; Puplampu, A.E.; Boglo, J.K.; Sedzro, K.M. Anemia prevalence and its predictors among children under-five years in Ghana. A multilevel analysis of the cross-sectional 2019 Ghana Malaria Indicator Survey. Health Sci. Rep. 2023, 6, e1643. [Google Scholar] [CrossRef] [PubMed]
- Asmare, A.A.; Tegegne, A.S.; Belay, D.B.; Agmas, Y.A. Coexisting predictors for undernutrition indices among under-five children in West Africa: Application of a multilevel multivariate ordinal logistic regression model. BMC Nutr. 2025, 11, 112. [Google Scholar]
- American Society of Hematology. The Role of Red Blood Cells in Anemia. Available online: https://www.hematology.org/education/patients/anemia (accessed on 9 February 2025).
- Horton, S.; Ross, J. The economics of iron deficiency. Food Policy 2003, 28, 51–75. [Google Scholar] [CrossRef]
- Shekar, M.; Kakietek, J.; Eberwein, J.D.; Walters, D. An Investment Framework for Nutrition; World Bank: Washington, DC, USA, 2016. [Google Scholar]
- Hasan, M.; Tahosin, M.S.; Farjana, A.; Sheakh, M.A.; Hasan, M.M. A Harmful Disorder: Predictive and Comparative Analysis for fetal Anemia Disease by Using Different Machine Learning Approaches. In Proceedings of the 2023 11th International Symposium on Digital Forensics and Security (ISDFS), Chattanooga, TN, USA, 11–12 May 2023; pp. 1–6. [Google Scholar] [CrossRef]
- El-Shafie, A.M.; Kasemy, Z.A.; Omar, Z.A.; Alkalash, S.H.; Salama, A.A.; Mahrous, K.S.; Hewedy, S.M.; Kotb, N.M.; Abd El-Hady, H.S.; Eladawy, E.S.; et al. Prevalence of short stature and malnutrition among Egyptian primary school children and their coexistence with Anemia. Ital. J. Pediatr. 2020, 46, 91. [Google Scholar] [CrossRef]
- Roberts, D.J.; Matthews, G.; Snow, R.W.; Zewotir, T.; Sartorius, B. Investigating the spatial variation and risk factors of childhood anaemia in four sub-Saharan African countries. BMC Public Health 2020, 20, 126. [Google Scholar] [CrossRef]
- Kassebaum, N.J. The Global Burden of Anemia. Hematol. Oncol. Clin. N. Am. 2016, 30, 247–308. [Google Scholar] [CrossRef]
- Gardner, W.M.; Razo, C.; McHugh, T.A.; Hagins, H.; Vilchis-Tella, V.M.; Hennessy, C.; Taylor, H.J.; Perumal, N.; Fuller, K.; Cercy, K.M.; et al. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990–2021: Findings from the Global Burden of Disease Study 2021. Lancet Haematol. 2023, 10, e713–e734. [Google Scholar] [CrossRef]
- Sarna, A.; Porwal, A.; Ramesh, S.; Agrawal, P.K.; Acharya, R.; Johnston, R.; Khan, N.; Sachdev, H.P.S.; Nair, K.M.; Ramakrishnan, L.; et al. Characterisation of the types of anaemia prevalent among children and adolescents aged 1–19 years in India: A population-based study. Lancet Child. Adolesc. Health 2020, 4, 515–525. [Google Scholar] [CrossRef]
- Mou, J.; Zhou, H.; Feng, Z.; Huang, S.; Wang, Z.; Zhang, C.; Wang, Y. A Case-Control Study of the Factors Associated with Anemia in Chinese Children Aged 3–7 years Old. Anemia 2023, 2023, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Institute for Health Metrics and Evaluation. The Lancet: New Study Reveals Global Anemia Cases Remain Persistently High Among Women and Children. Anemia Rates Decline for Men. 31 July 2023. Available online: https://www.healthdata.org/news-events/newsroom/news-releases/lancet-new-study-reveals-global-anemia-cases-remain-persistently (accessed on 4 July 2025).
- Gaston, R.T.; Habyarimana, F.; Ramroop, S. Joint modelling of anaemia and stunting in children less than five years of age in Lesotho: A cross-sectional case study. BMC Public Health 2022, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- Aliyo, A.; Jibril, A. Anemia and Associated Factors Among Under Five Year Old Children Who Attended Bule Hora General Hospital in West Guji zone, Southern Ethiopia. J. Blood Med. 2022, 13, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Athman, L.P.; Jonathan, A.; Musa, F.; Kipasika, H.J.; Mahawi, I.; Urio, F.; Ally, M.; Mutagonda, R.; Chirande, L.; Makani, J.; et al. Clinical depression prevalence and associated factors among adolescents with sickle cell anemia in dar es salaam, tanzania: A cross-sectional study. BMC Pediatr. 2025, 25, 10. [Google Scholar] [CrossRef]
- Asgedom, Y.S.; Habte, A.; Woldegeorgis, B.Z.; Koyira, M.M.; Kedida, B.D.; Fente, B.M.; Gebrekidan, A.Y.; Kassie, G.A. The prevalence of anemia and the factors associated with its severity among children aged 6–59 months in Ghana: A multi-level ordinal logistic regression. PLoS ONE 2024, 19, e0315232. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.; Kim, C.; Seo, E.; Kang, Y. Risk factors of anemia among children aged 6-59 months in Madagascar. Afr. J. Food Agric. Nutr. Dev. 2024, 24, 24611–24655. [Google Scholar] [CrossRef]
- Tesfaye, S.H.; Seboka, B.T.; Sisay, D. Application of machine learning methods for predicting childhood anaemia: Analysis of Ethiopian Demographic Health Survey of 2016. PLoS ONE 2024, 19, e0300172. [Google Scholar] [CrossRef]
- Sunuwar, D.R.; Singh, D.R.; Pradhan, P.M.S.; Shrestha, V.; Rai, P.; Shah, S.K.; Adhikari, B. Factors associated with anemia among children in South and Southeast Asia: A multilevel analysis. BMC Public Health 2023, 23, 343. [Google Scholar] [CrossRef]
- Gebreegziabher, T.; Sidibe, S. Prevalence and contributing factors of anaemia among children aged 6–24 months and 25–59 months in Mali. J. Nutr. Sci. 2023, 12, e112. [Google Scholar] [CrossRef]
- Ampofo, G.D.; Osarfo, J.; Okyere, D.D.; Kouevidjin, E.; Aberese-Ako, M.; Tagbor, H. Malaria and anaemia prevalence and associated factors among pregnant women initiating antenatal care in two regions in Ghana: An analytical cross-sectional study. BMC Pregnancy Childbirth 2025, 25, 617. [Google Scholar]
- Zimlich, R. Understanding Anemia in Kids. Healthline, 27 July 2022. [Google Scholar]
- Liu, Y.; Ren, W.; Wang, S.; Xiang, M.; Zhang, S.; Zhang, F. Global burden of anemia and cause among children under five years 1990–2019: Findings from the global burden of disease study 2019. Front. Nutr. 2024, 11, 1474664. [Google Scholar] [CrossRef]
- Souza, J.P.; Day, L.T.; Rezende-Gomes, A.C.; Zhang, J.; Mori, R.; Baguiya, A.; Jayaratne, K.; Osoti, A.; Vogel, J.P.; Campbell, O.; et al. A global analysis of the determinants of maternal health and transitions in maternal mortality. Lancet Glob. Health 2024, 12, e306–e316. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Torres, V.; Torres, N.; Davis, J.A.; Corrales-Medina, F.F. Anemia and Associated Risk Factors in Pediatric Patients. Pediatr. Health Med. Ther. 2023, 14, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.M.; Luna, S.A.; Siddique, Z. Machine-Learning-Based Disease Diagnosis: A Comprehensive Review. Healthcare 2022, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Sidey-Gibbons, J.A.M.; Sidey-Gibbons, C.J. Machine learning in medicine: A practical introduction. BMC Med. Res. Methodol. 2019, 19, 64. [Google Scholar] [CrossRef]
- Rajula, H.S.R.; Verlato, G.; Manchia, M.; Antonucci, N.; Fanos, V. Comparison of Conventional Statistical Methods with Machine Learning in Medicine: Diagnosis, Drug Development, and Treatment. Medicina 2020, 56, 455. [Google Scholar] [CrossRef]
- Ley, C.; Martin, R.K.; Pareek, A.; Groll, A.; Seil, R.; Tischer, T. Machine learning and conventional statistics: Making sense of the differences. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 753–757. [Google Scholar] [CrossRef]
- Ghana Statistical Service (GSS) and ICF. Ghana Demographic and Health Survey 2022: Key Indicators Report; GSS and ICF: Accra, Ghana; Rockville, MD, USA, 2023. [Google Scholar]
- Grobbee, D.E.; Hoes, A.W. Clinical Epidemiology: Principles, Methods, and Applications for Clinical Research, 2nd ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2025. [Google Scholar]
- Siddiqa, M.; Shah, G.H.; Mayo-Gamble, T.L.; Zubair, A. Determinants of Child Stunting, Wasting, and Underweight: Evidence from 2017 to 2018 Pakistan Demographic and Health Survey. J. Nutr. Metab. 2023, 2023, 1–12. [Google Scholar] [CrossRef]
- Ismail, A.; Fatima, F. Maternal Nutritional Knowledge and its Association with Iron Deficiency Anemia in Children. Nurture 2017, 11, 16–20. [Google Scholar] [CrossRef]
- Alhaija, R.A.; Hasab, A.A.H.; El-Nimr, N.A.; Tayel, D.I. Impact of educational intervention on mothers of infants with iron-deficiency anemia. Health Educ. Res. 2024, 39, 254–261. [Google Scholar] [CrossRef]
- Al-Suhiemat, A.A.; Shudifat, R.M.; Obeidat, H. Maternal Level of Education and Nutritional Practices Regarding Iron Deficiency Anemia Among Preschoolers in Jordan. J. Pediatr. Nurs. 2020, 55, e313–e319. [Google Scholar] [CrossRef] [PubMed]
- Bonsu, E.O.; Addo, I.Y.; Boadi, C.; Boadu, E.F.; Okeke, S.R. Determinants of iron-rich food deficiency among children under 5 years in sub-Saharan Africa: A comprehensive analysis of Demographic and Health Surveys. BMJ Open 2024, 14, e079856. [Google Scholar] [CrossRef]
- Bamboro, S.A.; Boba, H.I.; Geberetsadik, M.K.; Gebru, Z.; Gutema, B.T. Prevalence of anemia and its associated factors among under-five children living in Arba Minch Health and Demographic Surveillance System Sites (HDSS), Southern Ethiopia. PLOS Global Public. Health 2024, 4, e0003830. [Google Scholar] [CrossRef]
- Rima, F.S.; Kundu, S.; Tarannum, S.; Jannatul, T.; Bin Sharif , A. Spatial variations and determinants of vitamin A and iron rich food consumption among Bangladeshi children aged 6–23 months. Sci. Rep. 2025, 15, 17881. [Google Scholar]
- Nicholson, W.K.; Silverstein, M.; Wong, J.B.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Jaén, C.R.; Krousel-Wood, M.; Lee, S.; Li, L.; et al. Screening and Supplementation for Iron Deficiency and Iron Deficiency Anemia During Pregnancy. JAMA 2024, 332, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.D.; Greer, F.R. Diagnosis and Prevention of Iron Deficiency and Iron-Deficiency Anemia in Infants and Young Children (0–3 Years of Age). Pediatrics 2010, 126, 1040–1050. [Google Scholar] [CrossRef]
- Zemariam, A.B.; Yimer, A.; Abebe, G.K.; Wondie, W.T.; Abate, B.B.; Alamaw, A.W.; Yilak, G.; Melaku, T.M.; Ngusie, H.S. Employing supervised machine learning algorithms for classification and prediction of anemia among youth girls in Ethiopia. Sci. Rep. 2024, 14, 9080. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, P. Prediction of Anemia using Machine Learning Algorithms. Int. J. Comput. Sci. Inf. Technol. 2023, 15, 15–30. [Google Scholar] [CrossRef]
- Khan, J.R.; Chowdhury, S.; Islam, H.; Raheem, E. Machine Learning Algorithms To Predict The Childhood Anemia In Bangladesh. J. Data Sci. 2021, 17, 195–218. [Google Scholar] [CrossRef]
- Zahirzada, A.; Zaheer, N.; Shahpoor, M.A. Machine Learning Algorithms to Predict Anemia in Children Under the Age of Five Years in Afghanistan: A Case of Kunduz Province. J. Surv. Fish. Sci. 2023, 10, 752–762. [Google Scholar]
- Appiahene, P.; Asare, J.W.; Donkoh, E.T.; Dimauro, G.; Maglietta, R. Detection of iron deficiency anemia by medical images: A comparative study of machine learning algorithms. BioData Min. 2023, 16, 2. [Google Scholar] [CrossRef]
- El Bilbeisi, A.H. Prevalence of nutritional anemia and its risk factors in children under five in the Gaza Strip. Front. Nutr. 2025, 12. [Google Scholar] [CrossRef]
- Ewusie, J.E.; Ahiadeke, C.; Beyene, J.; Hamid, J.S. Prevalence of anemia among under-5 children in the Ghanaian population: Estimates from the Ghana demographic and health survey. BMC Public Health 2014, 14, 626. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, A.R.; Yakubu, M. Determinants of under-five anaemia in the high prevalence regions of Ghana. F1000Res 2022, 11, 724. [Google Scholar]
- Islam, M.A.; Afroja, S.; Khan, M.S.; Alauddin, S.; Nahar, M.T.; Talukder, A. Prevalence and Triggering Factors of Childhood Anemia: An Application of Ordinal Logistic Regression Model. Int. J. Clin. Pract. 2022, 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- New Series Highlights the Importance of a Positive Postnatal Experience for All Women and Newborns; WHO: Geneva, Switzerland, 2024.
- Gotine, A.R.E.M.; Xavier, S.P.; Vasco, M.D.; Alfane, N.W.A.; Victor, A. Prevalence and predictors of anemia in children under 5 years of age in sub-Saharan Africa: A systematic review and meta-analysis. medRxiv 2024. [Google Scholar] [CrossRef]
- Gemechu, K.; Asmerom, H.; Sileshi, B.; Belete, R.; Ayele, F.; Nigussie, K.; Bete, T.; Negash, A.; Sertsu, A.; Mekonnen, S.; et al. Anemia and associated factors among under-five children attending public Hospitals in Harari Regional State, eastern Ethiopia: A cross-sectional study. Medicine 2024, 103, e38217. [Google Scholar] [CrossRef]
- Sorsa, A.; Habtamu, A.; Kaso, M. Prevalence and Predictors of Anemia Among Children Aged 6–23 Months in Dodota District, Southeast Ethiopia: A Community-Based Cross-Sectional Study. Pediatric Health Med. Ther. 2021, 12, 177–187. [Google Scholar] [CrossRef]




| Sr. No | Attributes | Categories | freq. | Percentage % | OR (C-I) | p Value |
|---|---|---|---|---|---|---|
| Societal Characteristics | ||||||
| 1 | Region | Western * | 454 | 4.85 | - | |
| Central | 510 | 5.45 | 0.002 (−0.001–0.383) | 0.991 | ||
| Greater Accra | 455 | 4.86 | 0.369 (0.170–0.530) | 0.070 | ||
| Volta | 383 | 4.09 | 0.252 (−0.156–0.661) | 0.227 | ||
| Eastern | 436 | 4.66 | 0.309 (−0.212–0.692) | 0.132 | ||
| Ashanti | 592 | 6.33 | 0.241 (−0.612–0.329) | 0.202 | ||
| Western North | 434 | 4.64 | 0.002 (0.001–0.415) | 0.991 | ||
| Ahafo | 497 | 5.31 | 0.398 (−0.788–0.728) | 0.045 | ||
| Bono | 427 | 4.57 | 0.338 (0.149–0.471) | 0.106 | ||
| Bono East | 659 | 7.05 | 0.212 (−0.154–0.579) | 0.256 | ||
| Oti | 632 | 6.76 | 0.522 (0.146–0.898) | 0.006 | ||
| Northern | 970 | 10.37 | 1.996 (1.646–2.345) | <0.001 | ||
| Savannah | 797 | 8.52 | 0.639 (0.284–0.995) | <0.001 | ||
| North East | 868 | 9.28 | 0.835 (0.4804- 1.190) | <0.001 | ||
| Upper East | 638 | 6.82 | 0.770 (0.393–1.1476) | <0.001 | ||
| Upper West | 601 | 6.43 | 0.803 (0.432–1.174) | <0.001 | ||
| 2 | Household members | <4 * | 298 | 3.19 | - | |
| 4–6 | 117 | 1.25 | 1.149 (0.604–2.187) | 0.671 | ||
| 7–9 | 233 | 2.49 | 1.238 (0.731–2.097) | 0.425 | ||
| >9 | 8705 | 93.07 | 1.041 (0.729–1.487) | 0.823 | ||
| 3 | Place of residence | Urban * | 3857 | 41.24 | - | |
| Rural | 5496 | 58.76 | 1.516 (1.335–1.722) | <0.001 | ||
| 4 | Source of drinking water | Unimproved * | 3862 | 41.29 | - | |
| Improved | 5491 | 58.71 | 1.191 (1.049–1.352) | 0.176 | ||
| 5 | Sex of household head | Male * | 6880 | 73.56 | - | |
| Female | 2473 | 26.44 | 0.831 (0.722–0.956) | 0.010 | ||
| 6 | Socioeconomic status | Poor * | 5308 | 56.75 | - | |
| Middle | 1681 | 17.97 | 0.746 (0.629–0.885) | 0.001 | ||
| Rich | 2364 | 25.28 | 0.449 (0.386–0.522) | <0.001 | ||
| Parental Characteristics | ||||||
| 7 | Mother’s education | No education * | 2917 | 31.19 | - | |
| Primary | 1496 | 15.99 | 0.918 (0.752–1.120) | 0.402 | ||
| Secondary | 4237 | 45.3 | 0.529 (0.457–0.614) | <0.001 | ||
| Higher | 703 | 7.52 | 0.400 (0.309–0.519) | <0.001 | ||
| 8 | Father’s education | No education * | 2715 | 33.51 | - | |
| Primary | 868 | 10.71 | 0.862 (0.676–1.100) | 0.233 | ||
| Secondary | 3419 | 42.2 | 0.543 (0.464–0.636) | <0.001 | ||
| Higher | 1099 | 13.57 | 0.485 (0.390–0.603) | <0.001 | ||
| 9 | Maternal age | 15–19 * | 353 | 3.77 | - | |
| 20–24 | 1671 | 17.87 | 0.810 (0.540–1.217) | 0.312 | ||
| 25–29 | 2276 | 24.33 | 0.657 (0.442–0.976) | 0.038 | ||
| 30–34 | 2266 | 24.23 | 0.643 (0.433–0.953) | 0.028 | ||
| 35–39 | 1713 | 18.31 | 0.541 (0.362–0.809) | 0.003 | ||
| 40–44 | 819 | 8.76 | 0.548 (0.358–0.839) | 0.006 | ||
| 45–49 | 255 | 2.73 | 0.780 (0.460–1.321) | 0.357 | ||
| 10 | Maternal smoking | No * | 9287 | 99.29 | - | |
| Yes | 66 | 0.71 | 1.293 (0.626–2.671) | 0.487 | ||
| 11 | Breastfeed ever | No * | 4195 | 44.85 | - | |
| Yes | 5158 | 55.15 | 1.754 (1.546–1.991) | <0.001 | ||
| 12 | Initiation of breastfeeding | Immediately * | 3667 | 63.84 | - | |
| Within the first hour | 1805 | 31.42 | 0.926 (0.778–1.104) | 0.395 | ||
| Within 1 day | 272 | 4.74 | 0.988 (0.675–1.445) | 0.951 | ||
| 13 | Mother's occupation | Not working * | 1537 | 16.43 | - | |
| Working | 7816 | 83.57 | 0.756 (0.632–0.904) | 0.002 | ||
| 14 | Intake of iron during pregnancy | No * | 469 | 8.99 | - | |
| Yes | 4749 | 91.01 | 0.575 (0.410–0.807) | 0.001 | ||
| 15 | Consumption of drugs for intestinal parasites during pregnancy | No * | 2382 | 45.65 | - | |
| Yes | 2836 | 54.35 | 0.680 (0.568–0.814) | <0.001 | ||
| Child Characteristics | ||||||
| 16 | Birth order number | 1st born * | 2386 | 25.51 | - | |
| 2–4 | 4782 | 51.13 | 1.164 (0.999–1.355) | 0.051 | ||
| >5 | 2185 | 23.36 | 1.476 (0.999–1.355) | <0.001 | ||
| 17 | Birth type | Single birth * | 8907 | 95.23 | - | |
| Multiple births | 446 | 4.77 | 0.873 (0.637–1.196) | 0.398 | ||
| 18 | Sex of child | Male * | 4804 | 51.36 | - | |
| Female | 4549 | 48.64 | 0.854 (0.753–0.968) | 0.014 | ||
| 19 | Size of child at birth | Small * | 792 | 13.73 | - | |
| Average | 2329 | 40.38 | 0.957 (0.737–1.243) | 0.747 | ||
| Large | 2647 | 45.89 | 0.912 (0.705–1.179) | 0.484 | ||
| 20 | Formula milk consumption | No * | 5123 | 90.5 | - | |
| Yes | 538 | 9.5 | 0.771 (0.589–1.009) | 0.058 | ||
| 21 | Child's age in months | 0–6 * | 609 | 13.61 | - | |
| 7–12 | 490 | 10.95 | 1.035 (0.649–1.650) | 0.884 | ||
| 13–24 | 1017 | 22.73 | 1.032 (0.660–1.612) | 0.889 | ||
| 25–36 | 845 | 18.89 | 0.571 (0.365–0.893) | 0.014 | ||
| 37–48 | 834 | 18.64 | 0.473 (0.302–0.740) | 0.001 | ||
| 49–60 | 679 | 15.18 | 0.342 (0.217–0.539) | <0.001 | ||
| 22 | Stunting | No * | 199 | 4.45 | - | |
| Moderate | 618 | 13.83 | 0.700 (0.481–1.017) | 0.062 | ||
| Severe | 3653 | 81.72 | 1.423 (0.301–1.594) | <0.001 | ||
| 23 | Underweight | No * | 107 | 2.39 | - | |
| Moderate | 477 | 10.67 | 0.958 (0.595–1.544) | 0.863 | ||
| Severe | 3886 | 86.94 | 0.540 (0.348–0.839) | 0.636 | ||
| 24 | Wasting | No * | 48 | 1.07 | - | |
| Moderate | 213 | 4.77 | 0.873 (0.439–1.736) | 0.699 | ||
| Severe | 4209 | 94.16 | 0.777 (0.413–1.460) | 0.434 | ||
| 25 | Intake of fruits and vegetables | No * | 3087 | 62.29 | - | |
| Yes | 1869 | 37.71 | 0.268 (0.055–1.523) | 0.011 | ||
| 26 | Baby postnatal checkup within 2 months | No * | 222 | 5.04 | - | |
| Yes | 4183 | 94.96 | 0.572 (0.358–0.915) | 0.020 | ||
| 27 | Given zinc | No * | 747 | 61.89 | - | |
| Yes | 460 | 38.11 | 1.219 (0.855–1.739) | 0.273 | ||
| Sr No. | Attributes | Categories | AOR (C-I) | p Value |
|---|---|---|---|---|
| Societal Characteristics | ||||
| 1 | Region | Western * | - | |
| Central | 0.363 (−2.044–2.772) | 0.767 | ||
| Greater Accra | −2.485 (−5.557–0.587) | 0.113 | ||
| Volta | 1.690 (−1.203–4.584) | 0.252 | ||
| Eastern | −2.033 (−4.890–0.823) | 0.163 | ||
| Ashanti | −1.042 (−3.341–1.256) | 0.374 | ||
| Western North | −2.099 (−4.950–0.751) | 0.149 | ||
| Ahafo | −0.717 (−3.111–1.676) | 0.557 | ||
| Bono | −1.729 (−4.769–1.309) | 0.265 | ||
| Bono East | −0.610 (−3.009–1.787) | 0.618 | ||
| Oti | −0.392 (−2.806–2.021) | 0.750 | ||
| Northern | 0.364 (−1.873–2.601) | 0.750 | ||
| Savannah | 1.446 (−1.176–4.069) | 0.280 | ||
| North East | 0.678 (−1.680–3.037) | 0.573 | ||
| Upper East | 1.996 (−1.180–5.173) | 0.218 | ||
| Upper West | 2.016 (−1.217–5.250) | 0.222 | ||
| 2 | Household members | <4 * | - | |
| 4–6 | 0.143 (0.103–3.420) | 0.432 | ||
| 7–9 | 0.654 (0.521–2.543) | 0.596 | ||
| >9 | 0.342 (0.832–5.321) | 0.104 | ||
| 3 | Place of residence | Urban * | - | |
| Rural | 1.791 (0.247–2.951) | 0.564 | ||
| 4 | Source of drinking water | Unimproved * | - | |
| Improved | 2.312 (0.272–9.613) | 0.442 | ||
| 5 | Sex of household head | Male * | - | |
| Female | 0.457 (0.078–2.672) | 0.385 | ||
| 6 | Socioeconomic status | Poor * | - | |
| Middle | 0.010 (0.0001–0.568) | 0.025 | ||
| Rich | 0.041 (0.001–1.077) | 0.046 | ||
| Parental Characteristics | ||||
| 7 | Mother’s education | No education * | - | |
| Primary | 0.040 (0.0009–1.608) | 0.088 | ||
| Secondary | 0.068 (0.003–1.173) | 0.064 | ||
| Higher | 0.002 (0.0009–0.529) | 0.029 | ||
| 8 | Father’s education | No education * | - | |
| Primary | 4.945 (0.850–7.058) | 0.062 | ||
| Secondary | 0.652 (0.288–2.126) | 0.009 | ||
| Higher | 0.156 (3.582–5.457) | 0.012 | ||
| 9 | Maternal age | 15–19 * | - | |
| 20–24 | 9.766 (0.396–9.946) | 0.163 | ||
| 25–29 | 6.270 (0.255–4.070) | 0.261 | ||
| 30–34 | 2.134 (0.083–5.762) | 0.647 | ||
| 35–39 | 0.042 (0.0004–4.554) | 0.186 | ||
| 40–44 | 0.793 (0.009–6.967) | 0.919 | ||
| 45–49 | 0.486 (0.765–3.210) | 0.659 | ||
| 10 | Maternal smoking | No * | - | |
| Yes | 0.672 (0.383–1.895) | 0.085 | ||
| 11 | Breastfeed ever | No * | - | |
| Yes | 3.586 (0.228–6.299) | 0.363 | ||
| 12 | Initiation of breastfeeding | Immediately * | - | |
| Within the first hour | 3.445 (1.540–7.313) | 0.119 | ||
| Within 1 day | 4.071 (0.104–5.180) | 0.065 | ||
| 13 | Mother occupation | Not working * | - | |
| Working | 0.673 (0.056–8.018) | 0.755 | ||
| 14 | Intake of iron during pregnancy | No * | - | |
| Yes | 0.017 (0.004–6.122) | 0.033 | ||
| 15 | Consumption of drugs for intestinal parasites during pregnancy | No * | - | |
| Yes | 0.761 (0.122–4.715) | 0.769 | ||
| Child Characteristics | ||||
| 16 | Birth order number | 1st born * | - | |
| 2–4 | 0.132 (0.009–1.943) | 0.140 | ||
| >5 | 0.434 (0.014–3.224) | 0.632 | ||
| 17 | Birth type | Single birth * | - | |
| Multiple births | 7.641 (0.009–8.456) | 0.552 | ||
| 18 | Sex of child | Male * | - | |
| Female | 0.877 (0.170–4.520) | 0.876 | ||
| 19 | Size of child at birth | Small * | - | |
| Average | 2.760 (0.397–4.549) | 0.150 | ||
| Large | 6.603 (0.330–7.788) | 0.217 | ||
| 20 | Formula milk consumption | No * | - | |
| Yes | 2.845 (0.073–3.518) | 0.575 | ||
| 21 | Child age in months | 0–6 * | - | |
| 7–12 | 0.096 (0.0011–8.606) | 0.308 | ||
| 13–24 | 0.053 (0.0003–8.935) | 0.262 | ||
| 25–36 | 1.024 (0.780–1.317) | 0.929 | ||
| 37–48 | 0.704 (0.472–1.0464) | 0.087 | ||
| 49–60 | 0.710 (0.435–1.034) | 0.074 | ||
| 22 | Stunting | No * | - | |
| Moderate | 3.063 (1.106–4.611) | 0.146 | ||
| Severe | 0.747 (0.851–3.825) | 0.758 | ||
| 23 | Underweight | No * | - | |
| Moderate | 1.224 (0.048–3.627) | 0.902 | ||
| Severe | 0.969 (0.995–2.714) | 0.840 | ||
| 24 | Wasting | No * | - | |
| Moderate | 0.067 (0.002–1.920) | 0.114 | ||
| Severe | 1.692 (0.383–2.012) | 0.555 | ||
| 25 | Intake of fruits and Vegetables | No * | - | |
| Yes | 4.755 (0.639–5.364) | 0.128 | ||
| 26 | Baby postnatal checkup within 2 months | No * | - | |
| Yes | 0.732 (3.452–8.076) | 0.010 | ||
| 27 | Given zinc | No * | - | |
| Yes | 0.505 (0.081–3.130) | 0.521 | ||
| Evaluation Parameters | Random Forest | Decision Tree | Logistic Regression | K-Nearest Neighbor | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Confusion matrix | Predicted | Predicted | Predicted | Predicted | ||||||||
| No anemia | Anemia | No anemia | Anemia | No anemia | Anemia | No anemia | Anemia | |||||
| No Anemia | 507 | 1829 | No anemia | 1716 | 1169 | No anemia | 1607 | 1260 | No anemia | 1216 | 863 | |
| Anemia | 1888 | 2330 | Anemia | 1143 | 2520 | Anemia | 877 | 2803 | Anemia | 1651 | 2817 | |
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |||||||||
| Accuracy | 94.74 (90.58–95.84) | 64.69 (63.51–65.84) | 67.35 (66.20–68.49) | 61.60 (60.41–62.78) | ||||||||
| Sensitivity | 82.50 (80.47–86.85) | 68.79 (67.27–70.29) | 76.16 (74.76–77.54) | 63.04 (61.61–64.47) | ||||||||
| Specificity | 50.78 (48.54–58.92) | 59.48 (57.66–61.28) | 56.05 (54.21–57.88) | 58.48 (56.34–60.62) | ||||||||
| Positive predictive value | 75.23 (73.65–76.24) | 68.31 (66.78–69.810) | 68.98 (67.54–70.41) | 76.54 (75.15–77.91) | ||||||||
| Negative predictive value | 56.81 (51.31–58.81) | 60.02 (58.2–61.82) | 64.69 (62.78–66.58) | 42.41 (40.6–44.25) | ||||||||
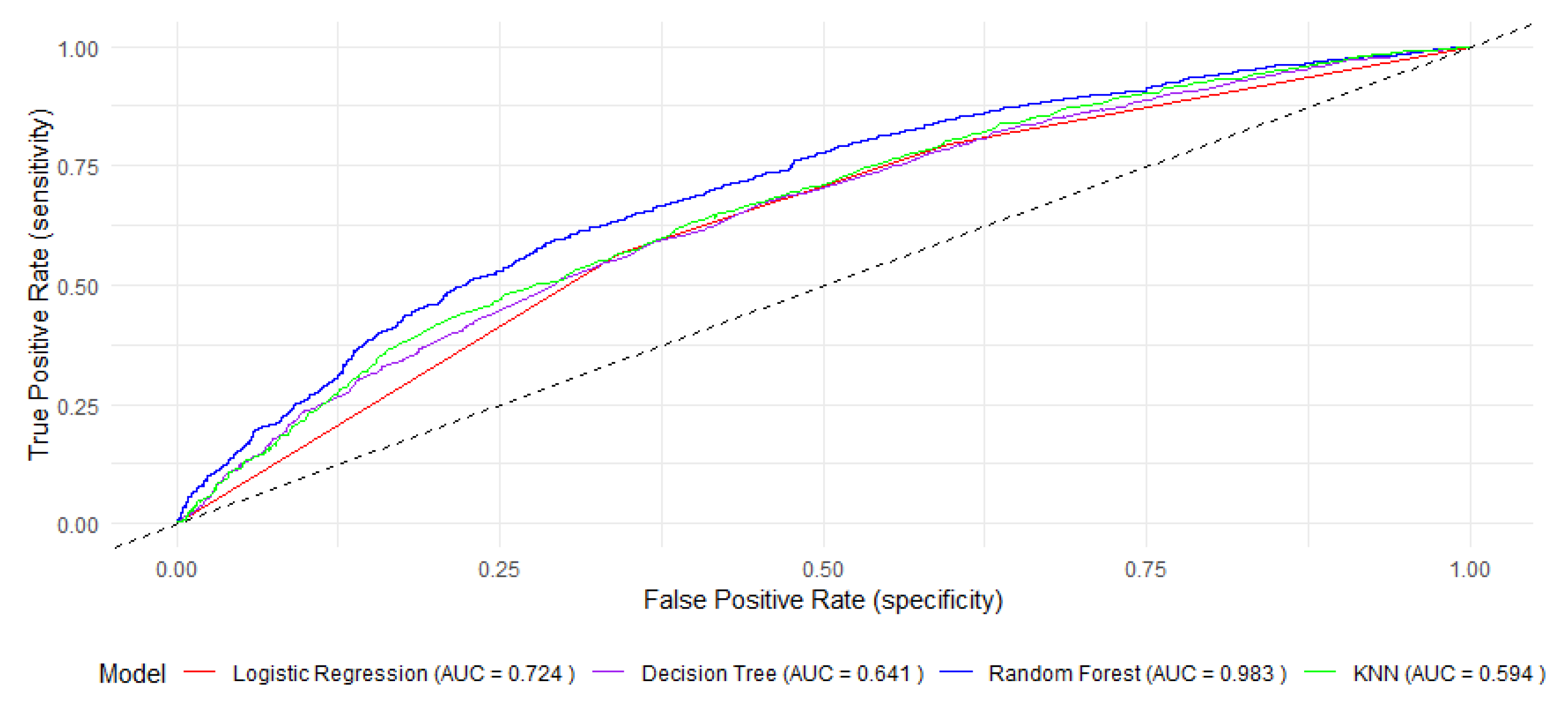
| AUC | 86.62 (80.6–88.86) | 64.16 (63.61–65.34) | 72.47 (71.26–73.7) | 59.48 (58.35–60.62) | ||||||||
| F1 scores | 96.94 | 57.36 | 67.82 | 51.03 | ||||||||
| Performance time | 1.5913 s | 1.1774 s | 1.1448 s | 1.81 s | ||||||||
| Evaluation Parameters | Random Forest | Decision Tree | Logistic Regression | K-nearest Neighbor | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Confusion matrix | Predicted | Predicted | Predicted | Predicted | ||||||||
| No anemia | Anemia | No anemia | Anemia | No anemia | Anemia | No anemia | Anemia | |||||
| No Anemia | 280 | 366 | No anemia | 1716 | 1169 | No anemia | 1607 | 1260 | No anemia | 1216 | 863 | |
| Anemia | 772 | 1387 | Anemia | 1143 | 2520 | Anemia | 877 | 2803 | Anemia | 1651 | 2817 | |
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |||||||||
| Accuracy | 95.75 (99.48–99.89) | 64.69 (63.51–65.84) | 67.35 (66.20–68.49) | 61.60 (60.41–62.78) | ||||||||
| Sensitivity | 98.74 (99.35–99.93) | 68.79 (67.27–70.29) | 76.16 (74.76–77.54) | 63.04 (61.61–64.47) | ||||||||
| Specificity | 67.89 (99.29–99.95) | 59.48 (57.66–61.28) | 56.05 (54.21–57.88) | 58.48 (56.34–60.62) | ||||||||
| Positive predictive value | 88.81 (99.45–99.96) | 68.31 (66.78–69.810) | 68.98 (67.54–70.41) | 76.54 (75.15–77.91) | ||||||||
| Negative predictive value | 80.67 (99.17–99.91) | 60.02 (58.2–61.82) | 64.69 (62.78–66.58) | 42.41 (40.6–44.25) | ||||||||
| AUC | 98.34 (99.55–99.93) | 64.16 (63.21–65.34) | 72.47 (71.26–73.7) | 59.48 (58.35–60.62) | ||||||||
| F1 score | 98.20 | 59.56 | 68.27 | 54.48 | ||||||||
| Performance time | 0.06 s | 0.75 s | 0.08 s | 0.2 s | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqa, M.; Shah, G.; Butt, M.S.; Kamal, A.; Opoku, S.T. Early Childhood Anemia in Ghana: Prevalence and Predictors Using Machine Learning Techniques. Children 2025, 12, 924. https://doi.org/10.3390/children12070924
Siddiqa M, Shah G, Butt MS, Kamal A, Opoku ST. Early Childhood Anemia in Ghana: Prevalence and Predictors Using Machine Learning Techniques. Children. 2025; 12(7):924. https://doi.org/10.3390/children12070924
Chicago/Turabian StyleSiddiqa, Maryam, Gulzar Shah, Mahnoor Shahid Butt, Asifa Kamal, and Samuel T. Opoku. 2025. "Early Childhood Anemia in Ghana: Prevalence and Predictors Using Machine Learning Techniques" Children 12, no. 7: 924. https://doi.org/10.3390/children12070924
APA StyleSiddiqa, M., Shah, G., Butt, M. S., Kamal, A., & Opoku, S. T. (2025). Early Childhood Anemia in Ghana: Prevalence and Predictors Using Machine Learning Techniques. Children, 12(7), 924. https://doi.org/10.3390/children12070924








