Abstract
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated fibroinflammatory disorder primarily affecting adults. The disease in pediatric age is unusual and preferentially affects adolescents. In contrast to adults, who commonly exhibit the involvement of multiple organs simultaneously or sequentially over time, young patients tend to present with a localized disease, typically affecting the orbits. Proptosis, ptosis, diplopia, and restricted eye movement may be observed in these patients. Symptoms are proteiform, and the disease is chronic and indolent with a relapsing–remitting course. Diagnostic criteria have been developed for adults, which may not fully capture the pediatric disease phenotype. If untreated or poorly managed, IgG4-RD can lead to progressive fibrosis and scarring of affected organs, potentially causing irreversible damage. We conducted a narrative review using the IMRAD approach, presenting a nonsystematic analysis of the literature on pediatric IgG4-RD. Original papers, case reports/series, and relevant reviews in English were selected from PubMed, EMBASE, and Web of Science up to January 2024. Keywords included “IgG4-Related Disease” and “pediatric” and, additionally, we presented two original pediatric cases. Our purpose is to offer an overview of IgG4-RD manifestations, and challenges in diagnosing and managing this rare condition in children.
1. Introduction
Immunoglobulin G4-related disease (IgG4-RD) is a multi-organ, immune-mediated fibroinflammatory disease of unknown etiology. The typical histological findings are lymphoplasmacytic infiltration, mostly characterized by IgG4-positive plasma cells, and a fibrosis with a typical “storiform” pattern [1,2].
This is a relatively recently recognized condition, first described in the early 2000s. In recent years, there has been a progressive increase in the number of diagnoses due to improved knowledge [3,4]. In the USA, during the period 2015–2019, the incidence has raised from 0.78 to 1.39 per 100,000 person-years, with a prevalence of 5.3/100,000 persons [5,6]. A slight female predominance (57.6%) has been observed in the USA, in contrast with previous Japanese data [5]. The average age at diagnosis worldwide is 50–70 years, while pediatric cases have been rarely reported [7].
The disease presentation is protean, with an indolent and slowly progressive clinical course, affecting either a single organ or body area or manifesting as a multisystemic condition. The disease progression results in substantial tissue damage, accompanied by organomegaly and functional failure [3]. Pancreas, kidney, orbits, lacrimal, and salivary glands, lungs, pleura, and peritoneum are among the most commonly affected organs and tissues [1].
An increase in serum IgG4 levels is a common, although unspecific, sign. IgG4 levels usually correlate with the disease severity and with the affected anatomic region [8]. Elevated IgG and IgE, peripheral eosinophilia, raised Erythrocyte Sedimentation Rate (ESR) and C-reactive protein (CRP) level, positive antinuclear antibodies (ANAs) and Rheumatoid Factor (RF), and hypocomplementemia are additional common findings [9]. However, these laboratory alterations are not pathognomonic and may overlap with other immune-mediated or infectious diseases. The presence of lymphoplasmacytic infiltration, storiform fibrosis, and obliterative phlebitis is highly suggestive of the diagnosis, with an increased number of IgG4+ plasma cells in affected tissues. However, obtaining biopsy samples can be challenging, particularly in pediatric patients, where invasive procedures may not always be feasible. In such cases, a combination of clinical, serological, and radiological findings is used to support the diagnosis, provided that other conditions are excluded.
The first set of diagnostic criteria, the Comprehensive Diagnostic Criteria (CDC), was defined in 2011 by the Japanese IgG4 team. However, as IgG4-RD gained widespread acceptance in the following years, several problems arose in clinical practice, including difficulty in obtaining biopsy samples and defining the serum IgG4 cutoff level, as well as the impaired immunostaining of IgG4. In light of these challenges, the Japanese IgG4 team updated the 2011 CDC, proposing the 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. These criteria consist of three domains: (1) clinical and radiological features, (2) serological diagnosis, and (3) pathological diagnosis (Table 1) [10].

Table 1.
The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD [10].
Concurrently, in 2019, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) have developed classification criteria for research purposes (Table 2) [11]. While these criteria are useful in identifying patients for clinical studies, their applicability in routine clinical practice, particularly in children, remains uncertain. In fact, well-established and standardized criteria for pediatric patients are still lacking, and current diagnosis relies on a combination of clinical, laboratory, and imaging findings, often referencing criteria developed for adults [7]. Given these limitations, the exclusion of other diseases with overlapping features, such as infectious, neoplastic, and other immune-mediated disorders, is a crucial step before confirming the diagnosis [12]. Radiological findings, particularly on CT and MRI, can provide supportive evidence, showing soft tissue enlargement, organ hypertrophy, and perivascular involvement. PET-CT has also been proposed as a useful tool for detecting multisystemic involvement and monitoring disease activity. However, imaging findings alone are insufficient for diagnosis and must always be correlated with clinical and histological data.

Table 2.
Summary of the diagnostic criteria for IgG4-related disease, adapted from Wallace et al. 2019 ACR/EULAR classification [11].
Due to these challenges, a multidisciplinary approach involving rheumatologists, immunologists, radiologists, and pathologists is often necessary to achieve an accurate diagnosis, especially in pediatric cases where the disease is rare and may present atypically.
Early diagnosis and subsequently early treatment are crucial to preventing fibrotic changes and their implications [13]. We conducted a narrative review of the current literature on this condition in children, discussing the challenges in diagnosing and managing IgG4-RD in this population. Additionally, we describe two diagnostically challenging cases. Both required a meticulous differential diagnosis and prolonged follow-up to reach a possible diagnosis of IgG4-RD. In one case, histopathology was inconclusive, likely due to the analysis of bone tissue, while in the other, the family declined biopsy. These cases illustrate the complexity of diagnosing IgG4-related disease in children and highlight the need for a thorough, multidisciplinary approach when histological confirmation is not feasible.
2. Materials and Methods
This narrative review was performed in accordance with the IMRAD (Introduction, Methods, Results, and Discussion) approach and presented a nonsystematic analysis of the literature on the pediatric IgG4-related disease [14]. The authors considered original scientific papers, case reports/series, and reviews of major relevance in the English language published until 20 January 2024. PubMed, EMBASE, and Web of Science were utilized as electronic databases for this research.
The following keywords (alone and/or in combination) were used to select relevant articles: “IgG4-Associated Autoimmune Disease”, “IgG4-Related Disease”, “Autoimmune related systemic disease”, “IgG4 Related Disease”, “IgG4-RD” AND “pediatric” or “children” or “child” or “adolescent”.
All cases included in this review were originally classified as IgG4-RD by the authors of the respective publications. To ensure consistency and transparency, we evaluated these cases based on fundamental criteria common to the three main classification systems for IgG4-RD: the 2019 ACR/EULAR classification criteria [11], the 2011 Comprehensive Diagnostic Criteria (CDC), and the 2011 Comprehensive Clinical Diagnostic Criteria (CCDC) [15]. Specifically, we assessed the exclusion of alternative diagnoses, the presence of organ involvement with characteristic clinical and radiological manifestations, serological findings, and histopathological confirmation. These key aspects have been summarized in Table 1 for clarity. The contributions were independently reviewed by two researchers. In cases of uncertainty, a third reviewer was consulted for additional assessment. The resulting draft was then critically revised and approved by all.
Case presentation 1
A 12-year-old Caucasian girl was admitted to the emergency department for a prolonged and fluctuating asymptomatic swelling and redness of her left upper eyelid, which started about six months earlier. The girl was initially treated with local antibiotics, and then, after about two months, she was hospitalized. A computed tomography (CT) scan detected signs of palpebral cellulitis, maxillary sinusitis, and lytic lesions on the lateral side of the orbit and the left frontal bone (Figure 1).
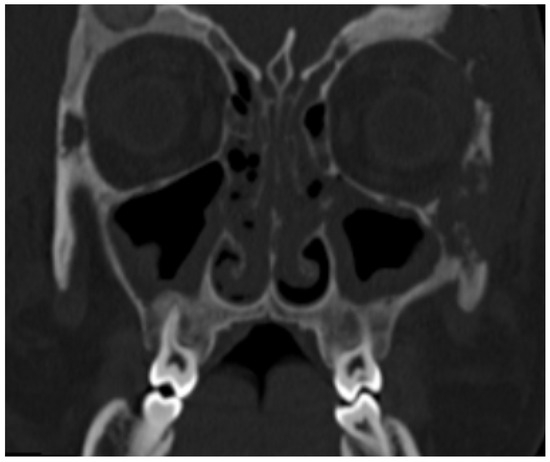
Figure 1.
Multiple, partially confluent, osteolithic lesions in zygomatic and frontal bones. Paranasal sinus involvement.
While routine laboratory exams were inconclusive, immunological investigations revealed a reduction in Natural Killer (NK) cells (89/µL, 5%) and elevated serum IgG (1597 mg/dL) and IgG4 (223 mg/dL). Flow cytometry also showed normal levels of CD8+ T cells and no abnormal expression of PD-1. Despite the absence of fever and other systemic and laboratory signs of inflammation, suspecting a bacterial infection, broad-spectrum intravenous antibiotics were prescribed for 2 weeks, followed by an oral antibiotic treatment for an additional 3 weeks.
The follow-up CT scan showed lytic lesions at the frontal and zygomatic bones, and a magnetic resonance imaging (MRI) scan was required. One month after discontinuation of antibiotic therapy, MRI revealed a diffuse mucosal thickening of the left maxillary sinus, periorbital adipose tissue inflammation, pre-septal cellulitis, temporal and masseter muscle edema, and thickening and diffuse signal alterations of frontal bone with epicranic soft tissue involvement (Figure 2).
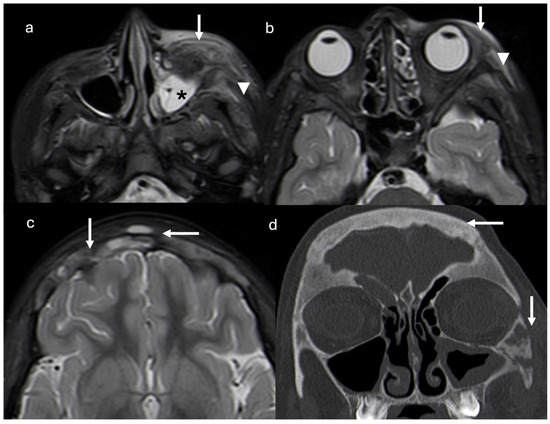
Figure 2.
Axial short tau inversion recovery (STIR) magnetic resonance images show diffuse mucosal thickening of left maxillary sinus (asterisk), periorbital adipose tissue inflammation and pre-septal cellulitis (arrows), and temporal and masseter muscle edema (arrowheads) (a,b). Thickening and diffuse signal alterations of frontal bone with epicranic soft tissue involvement (arrows) are demonstrated (c). Coronal reconstruction CT scan (d) confirmed thickening on frontal and zygomatic bones with lytic areas. Right frontal and left maxillary sinus inflammation is present (arrows).
The family sought a second opinion at our hospital.
At a physical exam, her upper left eyelid appeared hyperemic and moderately edematous, not painful or tender to palpation, with unrestricted ocular movements.
Three months later, a second MRI showed a complete muco-inflammatory pansinusitis, bilateral orbital and left zygomatic bone erosions, mild left dacryoadenitis, left masseter muscle oedema, and left pre-septal orbital fat inflammation. On the same side, inflammation was also appreciable in the periorbital adipose tissue, orbicularis, temporal, and masseter muscles, with additional lytic bone lesions present on the zygomatic and frontal bones, and fibroinflammatory dural thickening.
Routine laboratory examination results were negative or within normal ranges. Elevated levels of total IgG and IgG4 in the serum were confirmed (2360 mg/dL, normal values nv < 1909, and 353 mg/dL, nv < 135 respectively).
A functional endoscopic sinus surgery (FESS) was performed in order to treat her chronic sinusitis and biopsy the maxillary sinus mucosae and the left zygomatic bone. Mild fibroblastic and myofibroblastic mucosal proliferation were detected, along with a moderate trabecular reorganization of bone tissue and a mild fibrosclerosis of the bone marrow. The biopsy showed an osteoblastic rim around isolated bone spicules and mild perivascular lympho-monocytic infiltrate. Hemorrhagic extravasations with hemosiderin pigment were also present. Immunostains performed included CD38, IgG4, CD163, p16, CD34, and LCA, with two IgG4-positive cells and a low IgG4/IgG ratio reported. As unequivocal histological support was lacking in this patient, a careful differential diagnosis was conducted, with multiple consultations from infectious disease specialists, immunologists, and oncohematologists during her various hospital admissions. Although the presence of lytic bone lesions initially raised the suspicion of histiocytosis, a series of assessments ultimately led us to favor IgG4-RD. Factors suggesting this diagnosis included the indolent and prolonged course of the disease, characterized by intermittent episodes of mild hyperemia and swelling of the periorbital soft tissues and ipsilateral hemiface, without pain or other symptoms, and the absence of systemic and laboratory signs of inflammation. Additionally, radiological studies revealed the typical head and neck distribution of IgG4-RD observed in adolescent patients, involving areas such as the sinus mucosa, lacrimal gland, adipose tissue, and dural membrane. Bone lesions appeared adjacent to each other, partially confluent, and located near the altered paranasal sinuses. In histiocytosis, bone lesions are well-defined and less destructive and are often multiple, with a less extensive involvement of adjacent soft tissues. Elevated serum IgG and IgG4 levels, the absence of granulomas and histiocytic proliferation in the biopsy, and lack of involvement of other organs further supported IgG4-RD over histiocytosis.
Corticosteroid oral treatment was started, leading to a rapid clinical and radiological improvement. Nevertheless, the patient chose to discontinue the follow-up after three months.
Case presentation 2
A previously healthy 14-year-old male was referred to our pediatric rheumatology outpatient clinic for recurrent dacryoadenitis. This condition had been diagnosed three years earlier at a local ophthalmological center, where the patient presented with bulbar conjunctival hyperemia and eyeball pain and underwent bulbar ultrasound, optical coherence tomography (OCT), and cranial CT scan, revealing peripalpebral soft tissue swelling on the left portion of the eye. A beneficial treatment with oral and topical antibiotic therapy, along with topical corticosteroids, was initially prescribed. Additionally, corrective lenses have been prescribed for simple hypermetropic astigmatism. However, due to the recurrence of inflammatory episodes coinciding with the tapering of corticosteroids, an orbital MRI was performed, revealing an enlarged left lacrimal gland and minimal peribulbar fluid (Figure 3).
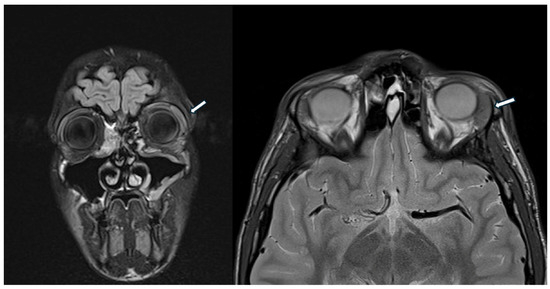
Figure 3.
The head MRI shows enlarged left lacrimal gland (white arrow) and minimal peribulbar fluid.
Routine blood exams provided uninformative results, except for increased IgG4 levels (2970 mg/L, nv < 1350 mg/L). Suspecting systemic IgG4-RD, the boy was referred to our pediatric rheumatology clinic.
At the physical examination, the patient was in good general condition, displaying swelling and mild hyperemia of the left eye. Comprehensive laboratory analyses confirmed elevated IgG4 levels (1598 mg/L). Abdominal and neck ultrasound was negative. Due to persistent ocular symptoms and signs such as prominence of the left lacrimal gland with lacrimation and conjunctival hyperemia, the ophthalmologist recommended a left lacrimal gland biopsy, which the parents refused. At the follow-up examinations, IgG4 levels were persistently elevated (2282 mg/L), with negative inflammatory indices and blood counts within a normal range.
The principal features of the two cases are reported in Table 3.

Table 3.
Principal features of the two cases we present.
3. Results
We collected a total of one-hundred and seventeen cases of pediatric IgG4-RD: fifty-nine cases were described in a review published in 2023 by Saad et al. [16], fifty-six cases were reported in the literature separately, and finally, two new cases were diagnosed at our facilities. Of these, twelve cases were excluded due to insufficient diagnostic confirmation: ten lacked a biopsy [17,18,19,20,21,22,23,24], one had a negative histological result [25] and one had borderline findings [26]. Thus, 105 cases were included in the final analysis. Consistently with previous data, the mean age at presentation was 11 years (range 1–18 years old), with a slight predominance of males (53%), contrasting with the findings of Bu et al., who reported a female predominance [27]. Regarding the clinical presentation, 71 patients (68%) had localized disease (with a single organ involvement), and 34 (32%) had a systemic disease. Among the one-hundred and five patients included in this study, orbital disease was present in forty-two of one-hundred and five cases (40%), with thirty cases (28%) limited to the orbits (IgG4-related orbital disease or IgG4-ROD); eight patients (7%) with hepatic involvement, nine (8%) with pancreatic manifestations, three (3%) with pulmonary disease, and nine (7%) with renal involvement.
A total of 52% of cases presented with constitutional symptoms and 52% had elevated inflammatory markers. In 80% of patients, IgG4 levels were increased, fulfilling the serological criteria. A total of 100% of cases had histological findings consistent with IgG4-related disease due to the inclusion criteria. Overall, 80% met both serological and histological criteria, supporting a definitive diagnosis. Steroid treatment was employed in 81% of patients.
The main characteristics of the one-hundred and three pediatric IgG4-RD cases described in the literature, and the two children we described are reported in Table 4.

Table 4.
Cases of pediatric IgG4-RD reported in the literature.
4. Discussion
IgG4-RD is a rare and often underestimated condition in pediatric patients, primarily affecting adolescents [3,7,102]. The clinical presentation is typically subtle, with symptoms beginning months or even years before diagnosis [102,103].
In adults, constitutional symptoms are rare. Weight loss, if present, is usually mild, with the exception of IgG4-related autoimmune pancreatitis. Fever is a highly atypical symptom [103]. ESR can be elevated, due to high serum immunoglobulin levels, while increased CRP is less common [3]. Low serum levels of ANA and positive RF are frequent, although serum positivity for more specific autoantibodies is uncommon [103].
In children, systemic constitutional symptoms are more frequently observed, and half of the cases may show an increase in inflammatory marker [7,17].
According to the existing literature, approximately 40% of the adult patients have single organ involvement, with the disease commonly affecting the pancreas, lymph nodes, orbit, and salivary tract [9,103]. Organ-specific symptoms at diagnosis frequently include abdominal pain, sicca syndrome features, respiratory symptoms, pruritus, and diarrhea [103].
Central nervous system (CNS) involvement is rare and is typically localized to the pituitary gland and meninges, sparing the parenchyma [104,105].
Single organ involvement is prevalent in children, occurring in approximately 60% of cases according to the systematic review by Karim et al. In our analysis, 68% of the 105 cases presented as localized disease [7].
The pancreato-hepatobiliary tract, lymph nodes, salivary glands, and lungs can be affected in children [21]. However, the orbits are the most frequently involved area, with a prevalence of 38% among the 59 cases described by Bu et al., and 40% among the 105 cases we analyzed [27]. According to Bu et al., pediatric IgG4-ROD rarely presents bilaterally (15%), and extra-orbital involvement is infrequent (20%), contrasting with adults, where it occurs in 70% of cases [27].
In a study of 13 children, 85% exhibited unilateral orbital protrusion/swelling, with 46% showing eyelid involvement, whereas dacryoadenitis represents the most prevalent manifestation in adults [30].
Both possible IgG4-RD adolescents described presented with localized disease. The boy had orbital involvement, while the female patient exhibited a more extensive condition affecting the lacrimal gland, maxillary and ethmoidal sinuses, periorbital adipose tissue, orbicularis, temporal, and masseter muscles, zygomatic and frontal bones, and leptomeninges. Hypertrophic pachymeningitis (IgG4-HP) is an uncommon manifestation of IgG4-RD, exceptionally reported in pediatric cases, with only two patients described so far [36,104,106,107,108]. Clinically, it may remain asymptomatic, as seen in the girl we described, or manifest with chronic headache, sometimes accompanied by a wide range of neurological symptoms resulting from mass effect, nerve compression, or vascular compression [109]. It usually presents in isolation, without other organ involvement, normal-to-low serum IgG4 levels, and no systemic symptoms, posing differential diagnosis challenges [110]. Brain MRI may reveal contrast enhancement and localized or diffused thickening of the dura mater [104,111]. The cerebrospinal fluid (CSF) usually shows only mild and unspecific alterations [112]. Increased CSF IgG4 levels have been only occasionally reported and can be diagnostically valuable when combined with suggestive histological features [30]. In the case of the girl we described, this neurological manifestation was detected on the MRI of the head, although a CSF analysis or biopsy examination was not performed.
This young girl also exhibited bone erosion in the zygomatic and frontal bones. This is another uncommon feature of IgG4-RD, rarely reported in the literature and primarily involving the skull bones and spine [113,114,115].
In the same 12-year-old patient, widespread sinusitis was documented on imaging but was completely asymptomatic. Although the typical classification or diagnostic criteria do not usually include the sinonasal regions, their involvement is reported in a percentage ranging from 32% to 65% of the cases [116,117,118]. Typically, sinusitis manifests with evocative features such as nasal congestion, facial pain or pressure, and a long-lasting diminished sense of smell. Nasal polyps may be observed on endoscopy, and neuroimaging may reveal bilateral and diffuse involvement of the paranasal sinuses with sinus wall thickening [116].
Our two patients lacked constitutional symptoms and inflammatory markers but exhibited elevated serum IgG4 levels.
Around 20% of adults classified as IgG4-RD according to the 2019 ACR/EULAR IgG4-RD classification criteria show normal serum IgG4 levels [11]. Furthermore, 9% of patients do not undergo biopsy, and in 37% of those who do undergo biopsy, the classic histopathologic findings are missing [11].
Due to the lack of specific pediatric criteria, diagnosis relies on criteria established for adults, particularly the RCD Criteria [10]. However, typical IgG4-RD features such as IgG4-positive plasma cell counts, focally dense plasma cell infiltrate at biopsy, and specific IgG4 ratio are not universally observed in children as well [27]. Normal serum IgG4 levels are reported in a significant percentage of children, mostly in those with orbital involvement (58% in pediatric IgG4-ROD vs. 33% in pediatric IgG4-RD as a whole) [27]. Additionally, typical histological features, especially storiform fibrosis and obliterative phlebitis, are observed only in a minority of young patients [17,27].
In the girl described, who underwent a biopsy, characteristic histological findings were absent, except for the presence of a modest fibrous component.
Therefore, the possible diagnosis in both reported cases was established following the careful exclusion of other similar conditions and was primarily supported by the prolonged and indolent disease course, elevated serum IgG4 levels, and orbital localization.
Early treatment is crucial to prevent organ damage, especially considering that children may have a longer disease duration and the consequences of fibrosis can be more impactful [13]. Therapy involves immunosuppressive agents for both remission induction and, given the relapsing nature of the disease, for remission maintenance as well [119]. Not all manifestations of the disease require immediate treatment, because some indolent cases can persist for decades; therefore, watchful waiting may be an option in specific situations [102].
Glucocorticoids (GCs) are nearly universally used as first-line therapy [1,102,119]. The treatment usually involves prednisone at a dosage of 0.5–2 mg/kg/day for 2–4 weeks, followed by a gradual tapering. [7,119]. Clinical improvement typically occurs rapidly, although the speed can vary depending on the affected organs and the extent of fibrosis [101]. In cases where the diagnosis is uncertain, swift improvement following GCs is often considered a valuable diagnostic indicator [1]. Recurrence rates after treatment with steroids are significant: in adults, they range from 10% to 53%, while in the pediatric population, they reach up 57% of cases [1,7]. In cases of relapse or GC resistance, second-line treatments such as mycophenolate mofetil, azathioprine, methotrexate, rituximab, or surgery should be considered [120]. Rituximab has recently been recognized as an effective treatment option, both for remission induction and for treatment of disease flares, and, together with GC, it represents the most established treatment option [121]. Immunosuppressants are usually introduced early, reducing the likelihood of relapse and the development of GC toxicity [122].
Beyond rituximab, alternative biologics such as adalimumab or ruxolitinib have been explored in severe or refractory cases, though evidence remains limited [123].
Combination therapy, particularly glucocorticoids with steroid-sparing agents such as azathioprine or mycophenolate mofetil, has shown improved disease control and reduced relapse rates in pediatric cases [123]. Our first case successfully responded to first-line treatment, although long-term outcome details are not available, while the girl developed a recurrence of inflammation during the tapering of GC therapy.
In both cases, histopathology was not conclusive in confirming IgG4-RD. In the adolescent girl, the biopsy did not provide definitive support for the diagnosis, possibly due to the nature of the sampled tissue, which was predominantly bone. In the boy, a histopathological sample was not obtained due to parental refusal. Nevertheless, in both patients, the diagnosis of possible IgG4-RD was supported by a prolonged and indolent clinical course, characteristic organ involvement, persistently elevated IgG4 levels, exclusion of alternative diagnoses, and a positive response to corticosteroid therapy. These elements align with recognized diagnostic frameworks for IgG4-RD. Given the challenges in obtaining biopsies in pediatric patients, particularly in delicate anatomical sites, a multidisciplinary approach integrating clinical, serological, and radiological findings is often necessary for diagnosis. The description of these two cases, despite the lack of definitive histopathological confirmation, illustrates the real-world difficulties in clinical practice. These cases underscore the importance of an integrated diagnostic strategy in pediatric IgG4-RD, where biopsy may not always be feasible.
5. Conclusions
This work offered an overview of IgG4-related disease in pediatric age, a condition that is extremely rare in this age group. IgG4-RD in children often shows a localized distribution, preferentially affecting the orbits. The disease course is chronic and indolent, occasionally accompanied by constitutional symptoms. Elevated IgG4 serum levels are an evocative laboratory sign of this condition. Although typical histological findings provide a definitive diagnosis, they may be sometimes unclear or absent. Additionally, performing biopsies in children can be challenging due to the invasiveness of the procedure and the need for sedation. These challenges underline the need for further studies to better shape diagnostic criteria in this population. GCs are the cornerstone of IgG4-RD therapy, especially in the initial stages. However, given the chronic nature of this condition, characterized by a relapsing–remitting course, the early introduction of immunosuppressive agents is often required to manage long-term treatment and minimize potential GC side effects.
Author Contributions
The contributions were independently collected by S.T. and A.M.; the review and discussion were conducted by T.G. and L.B.; S.T. and L.B.; wrote the original draft. The resulting draft was critically revised by A.P., A.T., L.B., A.M. and T.G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Institutional Review Board Statement
Ethical approval was obtained from the local ethics committees.
Informed Consent Statement
Consent to publish has been obtained from the parents of the patients described.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
Abbreviations
ACR: American College of Rheumatology, AAV: ANCA Associated vasculitis, AIHA: Autoimmune hemolytic anemia, ANA positive antinuclear antibodies, AZA: azathioprine, CDC: Comprehensive Diagnostic Criteria, CRP: C-reactive protein, CSF: Cerebrospinal fluid, CT: computed tomography, ESR: Erythrocyte Sedimentation Rate, EULAR: European League Against Rheumatism, F: female, GCs: Glucocorticoids, IBD: Inflammatory bowel disease, IgG4-HP: Immunoglobulin G4 Hypertrophic pachymeningitis, IgG4-RD: Immunoglobulin G4-related disease, IgG4-ROD: Immunoglobulin G4-related orbital disease, IMRAD: Introduction, Methods, Results, and Discussion, IRAK-4: interleukin-1 receptor-associated kinase 4, LFTs: liver function tests, M: male, mm: months old, MMF: mycophenolate mofetil, MRI: magnetic resonance imaging, MTX: methotrexate, TMP-SMX: trimethoprim/sulfamethoxazole, RCD: revised comprehensive diagnostic, RF: Rheumatoid Factor, UDCA: Ursodeoxycholic acid.
References
- Nambiar, S.; Oliver, T.I. IgG4-Related Disease. In StatPearls; StatPearls: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK499825/ (accessed on 7 April 2024).
- Zen, Y.; Nakanuma, Y. IgG4-Related Disease: A Cross-sectional Study of 114 Cases. Am. J. Surg. Pathol. 2010, 34, 1812. [Google Scholar] [CrossRef] [PubMed]
- Katz, G.; Stone, J.H. Clinical Perspectives on IgG4-Related Disease and Its Classification. Annu. Rev. Med. 2022, 73, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Hamano, H.; Kawa, S.; Horiuchi, A.; Unno, H.; Furuya, N.; Akamatsu, T.; Fukushima, M.; Nikaido, T.; Nakayama, K.; Usuda, N.; et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N. Engl. J. Med. 2001, 344, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Wallace, Z.S.; Miles, G.; Smolkina, E.; Petruski-Ivleva, N.; Madziva, D.; Cook, C.; Fu, X.; Zhang, Y.; Stone, J.H.; Choi, H.K. Incidence, prevalence and mortality of IgG4-related disease in the USA: A claims-based analysis of commercially insured adults. Ann. Rheum. Dis. 2023, 82, 957–962. [Google Scholar] [CrossRef]
- Floreani, A.; Okazaki, K.; Uchida, K.; Gershwin, M.E. IgG4-related disease: Changing epidemiology and new thoughts on a multisystem disease. J. Transl. Autoimmun. 2020, 4, 100074. [Google Scholar] [CrossRef]
- Karim, F.; Loeffen, J.; Bramer, W.; Westenberg, L.; Verdijk, R.; Van Hagen, M.; Van Laar, J. IgG4-related disease: A systematic review of this unrecognized disease in pediatrics. Pediatr. Rheumatol. 2016, 14, 18. [Google Scholar] [CrossRef]
- Wallace, Z.S.; Deshpande, V.; Mattoo, H.; Mahajan, V.S.; Kulikova, M.; Pillai, S.; Stone, J.H. IgG4-Related Disease: Baseline clinical and laboratory features in 125 patients with biopsy-proven disease. Arthritis Rheumatol. 2015, 67, 2466–2475. [Google Scholar] [CrossRef]
- Karadeniz, H.; Vaglio, A. IgG4-related disease: A contemporary review. Turk. J. Med. Sci. 2020, 50, 1616–1631. [Google Scholar] [CrossRef]
- Umehara, H.; Okazaki, K.; Kawa, S.; Takahashi, H.; Goto, H.; Matsui, S.; Ishizaka, N.; Akamizu, T.; Sato, Y.; Kawano, M.; et al. The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod. Rheumatol. 2021, 31, 529–533. [Google Scholar] [CrossRef]
- Wallace, Z.S.; Naden, R.P.; Chari, S.; Choi, H.; Della-Torre, E.; Dicaire, J.-F.; Hart, P.A.; Inoue, D.; Kawano, M.; Khosroshahi, A.; et al. The 2019 American College of Rheumatology/European League Against Rheumatism Classification Criteria for IgG4-Related Disease. Arthritis Rheumatol. 2020, 72, 7–19. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L.; Chen, F.; Yuan, G. Multi-System Langerhans Cell Histiocytosis as a Mimic of IgG4-Related Disease: A Case Report and Literature Review. Front. Endocrinol. 2022, 13, 896227. [Google Scholar] [CrossRef] [PubMed]
- Batu, E.D.; Arici, Z.S.; Orhan, D.; Kiratli, H.; Özen, S. Immunoglobulin G4-related orbital disease: Report of two pediatric cases. Clin. Exp. Rheumatol. 2015, 33, 409–410. [Google Scholar]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. J. Chiropr. Med. 2006, 5, 101–117. [Google Scholar] [CrossRef]
- Umehara, H.; Okazaki, K.; Masaki, Y.; Kawano, M.; Yamamoto, M.; Saeki, T.; Matsui, S.; Yoshino, T.; Nakamura, S.; Kawa, S.; et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod. Rheumatol. 2012, 22, 21–30. [Google Scholar] [CrossRef]
- Saad, M.A.; Ahmed, H.; Elgohary, R.; El Gendy, H.I. IgG4 related pericardium and lung disease in pediatric patient complicated with fatal massive hemoptysis: A case report and review of literature. Pediatr. Rheumatol. Online J. 2023, 21, 16. [Google Scholar] [CrossRef]
- Kaya Akca, Ü.; Atalay, E.; Kasap Cüceoğlu, M.; Şener, S.; Balık, Z.; Başaran, Ö.; Batu, E.D.; Karadağ, Ö.; Özen, S.; Bilginer, Y. IgG4-related disease in pediatric patients: A single-center experience. Rheumatol. Int. 2022, 42, 1177–1185. [Google Scholar] [CrossRef]
- Griepentrog, G.J.; Vickers, R.W.; Karesh, J.W.; Azari, A.A.; Albert, D.M.; Bukat, C.N. A clinicopathologic case study of two patients with pediatric orbital IgG4-related disease. Orbit Amst. Neth. 2013, 32, 389–391. [Google Scholar] [CrossRef]
- Kalapesi, F.B.; Garrott, H.M.; Moldovan, C.; Williams, M.; Ramanan, A.; Herbert, H.M. IgG4 orbital inflammation in a 5-year-old child presenting as an orbital mass. Orbit Amst. Neth. 2013, 32, 137–140. [Google Scholar] [CrossRef]
- Rosen, D.; Thung, S.; Sheflin-Findling, S.; Lai, J.; Rosen, A.; Arnon, R.; Chu, J. IgG4-sclerosing cholangitis in a pediatric patient. Semin. Liver Dis. 2015, 35, 89–94. [Google Scholar] [CrossRef]
- Yousefi, A.; Salehi, S.; Radgoodarzi, M.; Javid, A. Association of autoimmune pancreatitis with Raghib syndrome. Clin. Case Rep. 2023, 11, e8194. [Google Scholar] [CrossRef]
- Singla, T.; Bagri, N.K. IGG4 Related Disease-A Case Series of Four Pediatric Patients-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=1&page=1&id=L642856541 (accessed on 9 April 2024).
- Singh, S.; Singh, A.; Ahluwalia, J. IgG4 Related Disorder: A Clinical Possibility in Children with Peripheral Gangrene-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=4&page=8&id=L624232028 (accessed on 22 February 2024).
- Navetta-Modrov, B.; Stone, J.; Bonagura, V. IgG4 Related Disease (IgG4RD) in an Adolescent Male Misdiagnosed as Autoimmune lymphoproliferative Syndrome-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=2&page=1&id=L622389217 (accessed on 9 April 2024).
- Bolia, R.; Chong, S.Y.; Coleman, L.; MacGregor, D.; Hardikar, W.; Oliver, M.R. Autoimmune Pancreatitis and IgG4 Related Disease in Three Children. ACG Case Rep. J. 2016, 3, e115. [Google Scholar] [CrossRef] [PubMed]
- Jamee, M.; Fallahi, M.; Enayat, J. Chronic Granulomatous Disease with Associated IgG4-Related Disease Masquerading as Pulmonary Pseudotumor-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=3&page=5&id=L634909463 (accessed on 21 February 2024).
- Bu, F.; Koo, S.C. Clinicopathologic Characterization of IgG4-Rich Pediatric Head and Neck Lesions. Arch. Pathol. Lab. Med. 2022, 146, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Tille, L.; Schnabel, A.; Laass, M.W.; Hahn, G.; Taut, H.; Leszczynska, A.; Pablik, J.; Berner, R.; Brück, N.; Hedrich, C.M. Orbital inflammation and colitis in pediatric IgG4-related disease: A case report and review of the literature. Eur. J. Rheumatol. 2020, 7, S21–S27. [Google Scholar] [CrossRef] [PubMed]
- Dylewska, K.; Kobusińska, K.; Kurylak, A. Tumour of the orbit and pterygopalatine fossa: Delayed recognition of possible IgG4-related disease. Contemp. Oncol. Onkol. 2020, 24, 136–139. [Google Scholar] [CrossRef]
- Smerla, R.G.; Rontogianni, D.; Fragoulis, G.E. Ocular manifestations of IgG4-related disease in children. More common than anticipated? Review of the literature and case report. Clin. Rheumatol. 2018, 37, 1721–1727. [Google Scholar] [CrossRef]
- Aydemir, Y.; Akcoren, Z.; Demir, H.; Saltik Temizel, I.N.; Ozen, H.; Yuce, A. Clinical and histopathological features of immunoglobulin G4-associated autoimmune hepatitis in children. J. Gastroenterol. Hepatol. 2019, 34, 742–746. [Google Scholar] [CrossRef]
- Keidar, E.; Shermetaro, J.; Kwartowitz, G. Pediatric Parotid Chronic Sclerosing Sialadenitis in an African-American Female: A Rare Case and Review of the Literature. Cureus 2020, 12, e8846. [Google Scholar] [CrossRef]
- Namireddy, M.K.; Consul, N.; Sher, A.C. FDG-Avid Pulmonary Nodules and Tracheobronchial Mural Inflammation in IgG4-Related Disease. Clin. Nucl. Med. 2021, 46, e125–e126. [Google Scholar] [CrossRef]
- Corujeira, S.; Ferraz, C.; Nunes, T.; Fonseca, E.; Vaz, L.G. Severe IgG4-Related Disease in a Young Child: A Diagnosis Challenge. Case Rep. Pediatr. 2015, 2015, 140753. [Google Scholar] [CrossRef]
- Nastri, M.M.F.; Novak, G.V.; Sallum, A.E.M.; Campos, L.M.A.; Teixeira, R.A.P.; Silva, C.A. Immunoglobulin G4-related disease with recurrent uveitis and kidney tumor mimicking childhood polyarteritis nodosa: A rare case report. Acta Reumatol. Port. 2018, 43, 226–229. [Google Scholar]
- Nambirajan, A.; Sharma, M.C.; Garg, K.; Sriram, S.; Boorgula, M.T.; Suri, V. Large dural-based mass with bony hyperostosis in a 16-year-old male: IgG4-related disease mimicking lymphoplasmacyte-rich meningioma. Childs Nerv. Syst. 2019, 35, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.M.; Aydin, F.; Acar, B.; Kurt, T.; Poyraz, A.; Kiremitci, S.; Gülleroglu, B.; Azili, M.N.; Bayrakci, U.S. IgG4-related disease and ANCA positive vasculitis in childhood: A case-based review. Clin. Rheumatol. 2021, 40, 3817–3825. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Pal, P.; Dutta, S.; Nada, R. IgG4-related Disease at Rectovesical Pouch Mimicking Inflammatory Myofibroblastic Tumor. Indian. Pediatr. 2019, 56, 1059–1061. [Google Scholar] [CrossRef] [PubMed]
- Özdel, S.; Ekim, M.; Kaygusuz, G.; Çelikel, E.; Vatansever, G.; Taçyıldız, N. A new location for pediatric immunoglobulin G4 related disease: The biceps muscle. Turk. J. Pediatr. 2020, 62, 495–497. [Google Scholar] [CrossRef]
- Akkelle, B.Ş.; Tutar, E.; Ergelen, R.; Çelikel, Ç.A.; Ertem, D. IgG4 related disease in a seven year old girl with multiple organ involvement: A rare presentation. Turk. Arch. Pediatr. Pediatri Arş. 2020, 55, 191–194. [Google Scholar] [CrossRef]
- Szczawinska-Poplonyk, A.; Wojsyk-Banaszak, I.; Jonczyk-Potoczna, K.; Breborowicz, A. Pulmonary manifestation of immunoglobulin G4-related disease in a 7-year-old immunodeficient boy with Epstein-Barr virus infection: A case report. Ital. J. Pediatr. 2016, 42, 58. [Google Scholar] [CrossRef]
- Ferreira da Silva, R.C.; Lieberman, S.M.; Hoffman, H.T.; Policeni, B.; Bashir, A.; Smith, R.J.H.; Sato, T.S. IgG4-related disease in an adolescent with radiologic-pathologic correlation. Radiol. Case Rep. 2017, 12, 196–199. [Google Scholar] [CrossRef]
- Raab, E.L.; Moayedpardazi, H.S.; Naids, S.M.; Friedman, A.H.; Meltzer, M.A. Lacrimal gland abscess in a child as a rare manifestation of IgG4-related disease. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2018, 22, 73–75. [Google Scholar] [CrossRef]
- Gabrovska, N.; Velizarova, S.; Spasova, A.; Kostadinov, D.; Yanev, N.; Shivachev, H.; Rangelov, E.; Pahnev, Y.; Antonova, Z.; Kartulev, N.; et al. A Case of Tracheal Stenosis as an Isolated Form of Immunoproliferative Hyper-IgG4 Disease in a 17-Year-Old Girl. Children 2021, 8, 589. [Google Scholar] [CrossRef]
- Timeus, F.; Calvo, M.M.; Caci, A.M.; Gallone, G.O.; Vittone, F. IgG4-related chronic sclerosing sialadenitis in a child with recurrent parotitis: A case report. BMC Pediatr. 2021, 21, 586. [Google Scholar] [CrossRef]
- Hoshiyama, S.; Maruyama, Y.; Iwaya, M.; Uehara, T.; Nakazawa, Y. Immunoglobulin G4-related orbital disease in an 8-year-old girl. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2022, 64, e15014. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.Y.; Leahy, K.E.; Wong, M.; Krivanek, M.; Tumuluri, K. IgG4-related disease of the orbit in an infant. J. AAPOS Off. Publ. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2021, 25, 255–257. [Google Scholar] [CrossRef]
- Miglani, R.K.; Murthy, D.; Bhat, R.; Kumar, A.K.V. Immunoglobulin G4-associated cholangitis mimicking cholangiocarcinoma in a young boy. J. Postgrad. Med. 2010, 56, 140–142. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Zhang, L.; Freese, D.K. A 3-year-old with immunoglobulin G4-associated cholangitis. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 109–111. [Google Scholar] [CrossRef]
- Mannion, M.; Cron, R.Q. Successful treatment of pediatric IgG4 related systemic disease with mycophenolate mofetil: Case report and a review of the pediatric autoimmune pancreatitis literature. Pediatr. Rheumatol. Online J. 2011, 9, 1. [Google Scholar] [CrossRef]
- Zakeri, H.; Kashi, Z. Variable Clinical Presentations of Riedel’s Thyroiditis: Report of Two Cases. Case Rep. Med. 2011, 2011, 709264. [Google Scholar] [CrossRef]
- Melo, J.C.; Kitsko, D.; Reyes-Múgica, M. Pediatric chronic sclerosing sialadenitis: Küttner tumor. Pediatr. Dev. Pathol. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. Soc. 2012, 15, 165–169. [Google Scholar] [CrossRef]
- Naghibi, M.; Ahmed, A.; al Badri, A.M.; Bateman, A.C.; Shepherd, H.A.; Gordon, J.N. The successful treatment of IgG4-positive colitis with adalimumab in a patient with IgG4-related sclerosing disease--a new subtype of aggressive colitis? J. Crohns Colitis 2013, 7, e81–e84. [Google Scholar] [CrossRef]
- Pifferi, M.; Di Cicco, M.; Bush, A.; Caramella, D.; Chilosi, M.; Boner, A.L. Uncommon pulmonary presentation of IgG4-related disease in a 15-year-old boy. Chest 2013, 144, 669–671. [Google Scholar] [CrossRef]
- Sane, M.; Chelnis, J.; Kozielski, R.; Fasiuddin, A. Immunoglobulin G4-related sclerosing disease with orbital inflammation in a 12-year-old girl. J. AAPOS Off. Publ. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2013, 17, 548–550. [Google Scholar] [CrossRef]
- Caso, F.; Fiocco, U.; Costa, L.; Sfriso, P.; Punzi, L.; Doria, A. Successful use of rituximab in a young patient with immunoglobulin G4-related disease and refractory scleritis. Jt. Bone Spine 2014, 81, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Hasosah, M.Y.; Satti, M.B.; Yousef, Y.A.; Alzahrani, D.M.; Almutairi, S.A.; Alsahafi, A.F.; Sukkar, G.A.; Alzaben, A.A. IgG4-related sclerosing mesenteritis in a 7-year-old Saudi girl. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2014, 20, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, M.P.; Agarwal, M.; Mulay, K.; Sawhney, S. IgG4-related orbital inflammation presenting as unilateral pseudotumor. Indian. J. Pediatr. 2014, 81, 1108–1110. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Ganguly, A.; Rath, S.; Das, B.; Mishra, A. IgG4-related orbital inflammation presenting as bilateral proptosis in a child. Eye 2014, 28, 1264–1266. [Google Scholar] [CrossRef]
- Notz, G.; Intili, A.; Bilyk, J.R. IgG4-related dacryoadenitis in a 13-year-old girl. Ophthal. Plast. Reconstr. Surg. 2014, 30, e161–e163. [Google Scholar] [CrossRef]
- Prabhu, S.M.; Yadav, V.; Irodi, A.; Mani, S.; Varghese, A.M. IgG4-related disease with sinonasal involvement: A case series. Indian. J. Radiol. Imaging 2014, 24, 117–120. [Google Scholar] [CrossRef]
- Gillispie, M.C.; Thomas, R.D.; Hennon, T.R. Successful treatment of IgG-4 related sclerosing disease with rituximab: A novel case report. Clin. Exp. Rheumatol. 2015, 33, 549–550. [Google Scholar]
- Nada, R.; Gupta, A.; Kang, M.; Rawat, A.; Sood, A.; Ahluwalia, J.; Singh, S. Hepatic Mass and Coagulopathy in a Ten-Year-Old Boy With Fever. Arthritis Rheumatol. 2015, 67, 1977. [Google Scholar] [CrossRef]
- Meli, M.; Arrabito, M.; Salvatorelli, L.; Soma, R.; Presti, S.; Licciardello, M.; Miraglia, V.; Scuderi, M.G.; Belfiore, G.; Magro, G.; et al. Report of Two Cases of Pediatric IgG4-Related Lymphadenopathy (IgG4-LAD): IgG4-Related Disease (IgG4-RD) or a Distinct Clinical Pathological Entity? Children 2022, 9, 1472. [Google Scholar] [CrossRef]
- Kato, D.; Uchida, H.; Hinoki, A.; Sumida, W.; Shirota, C.; Makita, S.; Okamoto, M.; Takimoto, A.; Takada, S.; Nakagawa, Y. IgG4-related disease of duodenal obstruction due to multiple ulcers in a 12-year-old girl. BMC Pediatr. 2023, 23, 376. [Google Scholar] [CrossRef]
- Ewing, D.E.; Hammer, R.D. IgG4-related disease simulating Hodgkin lymphoma in a child. Hum. Pathol. Case Rep. 2016, 4, 42–45. [Google Scholar] [CrossRef][Green Version]
- Rojas-Ramirez, O.; Nunez-Velazquez, M.; Acosta-Jimenez, E.; Vargas-Caro, A. O016 IgG4-related ophthalmic disease in children: A case report. Ann. Allergy. Asthma. Immunol. 2016, 117, S6. [Google Scholar] [CrossRef]
- Marissen, J.; Pagel, J.; Steinmetz, A.; Härtel, C.; Lauten, M. Germany IgG4-Related Disease and Successful Treatment with Rituximab in a Three-Year-Old Boy. Ann. Hematol. Oncol. 2021, 8, 1334. Available online: http://austinpublishinggroup.com/hematology/fulltext/hematology-v8-id1334.php (accessed on 20 June 2024).
- Woo, W. M195 IGG4-Related Disease with Nasopharyngeal Masses in the Pediatric Patient. Ann. Allergy Asthma. Immunol. 2021, 127, S107. [Google Scholar] [CrossRef]
- Zeybek, G.; Kalin, S.; Karakayali, B.; Zemheri, E.; Naderi, S.; Sözeri5, B. Unusual presentation of immunoglobulin G4-related disease: A case report. Arch. Rheumatol. 2021, 36, 129–134. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Houshmand, G.; Pouraliakbar, H.; Soltani, Z.; Salehabadi, G.; Azimi, A.; Shabanian, R. Coronary artery involvement in a patient with IgG4-related disease. Radiol. Case Rep. 2023, 18, 3699–3703. [Google Scholar] [CrossRef]
- Pac, M.; Obrycki, L.; Sarnecki, J.; Szymanska, S.; Litwin, M. A Rare Cause of Acute Tubulointerstitial Nephritis in a 16.5-Year-Old Boy-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=22&page=1&id=L641736945 (accessed on 20 February 2024).
- Rodrigues, S.S.S.; Salazar, L.; Lima, J.B.; Fraga, J.; Alves, S.; Zilhao, C. Mikulicz Disease-a Sjogren’s Mimicker-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=3&page=1&id=L642856169 (accessed on 9 April 2024).
- Hsueh, J.; Kumar, S. More Than Meets the Eye: IgG4-related Disease presentation as fluctuating ocular symptoms. Clin. Immunol. 2023, 250, 109455. [Google Scholar] [CrossRef]
- Ma, B.; Li, Y.; Wang, X.; Du, L.; Wang, S.; Ma, H.; Zhou, D.; Usman, T.; Lu, L.; Qu, S. Association Between Abdominal Adipose Tissue Distribution and Obstructive Sleep Apnea in Chinese Obese Patients. Front. Endocrinol. 2022, 13, 847324. [Google Scholar] [CrossRef]
- Farha, G.; Derouen, J. Pediatric IgG4 Related Disease of the Orbit-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=4&page=2&id=L2022350567 (accessed on 20 February 2024).
- Niksic, S.; Slak, P.; Brajovic, S.M.; Plut, D. Unusual Case of an IgG4-Related Disease-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=24&page=1&id=L641875120 (accessed on 21 February 2024).
- Tsygin, A.; Dmitrienko, S.; Ananin, P.; Vorobyova, O.; Savostyanov, K.; Vashurina, T.; Fisenko, A. Case of IGG-4 Related Disease with Kidney Involvement in Child. Efficacy of Long Term Immunosuppressive Treatment-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=17&page=2&id=L639293233 (accessed on 21 February 2024).
- Qing, P.; Lu, C.; Yan, B.; Liu, C.; Fox, D.A.; Zhao, Y.; Liu, Y.; Tan, C. Case report: IgG4-related intracranial lesions mimicking multiple sclerosis in a 14-year-old girl. Front. Neurol. 2022, 13, 1007153. Available online: https://www.frontiersin.org/journals/neurology/articles/10.3389/fneur.2022.1007153 (accessed on 21 February 2024). [CrossRef]
- Kasap-Demir, B. Rapid Response to Pulse Methylprednisolone in an Adolescent Girl with IGG4 Disease Related Orbital Mass-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=3&page=3&id=L639228502 (accessed on 21 February 2024).
- Qi, S.R.; Hebert, M.; You, E.; Proulx-Gauthier, J.-P.; Legare, M.E. Conjunctival Infiltration in a Child as a Rare Manifestation of IgG4-Related Disease-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=20&page=1&id=L2017431387 (accessed on 9 April 2024).
- Jesus, O.D.; Sandoval-Consuegra, J.; Correa-Rivas, M.; Oliver-Ricart, M. IgG4-related disease at the foramen magnum and craniovertebral junction compressing the medulla. BMJ Case Rep. 2021, 14, e244202. [Google Scholar] [CrossRef]
- Vasudevan, A.K.; Kumar, G.A.; Rajesh, S.; Ahamed, M.Z. IgG4-Related Coronary Aneurysm in a Child. Indian. J. Pediatr. 2021, 88, 593. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-T.; Jeng, Y.-M.; Wu, J.-F. Immunoglobulin G4-related sclerosing cholangitis in a 3 years of age boy. Adv. Dig. Med. 2021, 8, 59–63. [Google Scholar] [CrossRef]
- Raja, M.K.H.; Raja, M.H.K.; Amjad, S.M.D. Resolution of IgG4-related retroperitoneal fibrosis with rituximab in a paediatric patient. Int. J. Rheum. Dis. 2020, 23, 109–353. [Google Scholar] [CrossRef]
- La Porta, E.; Pierri, F.; Pisani, I.; Pilato, F.P.; Lanino, E.; Lanino, L.; Sementa, A.R.; Verrina, E.E. IgG4 related disease: Nephropathy and bone marrow failure in a 2 year-old child. Nephrol. Dial. Transplant. 2020, 35 (Suppl. S3), 1832. [Google Scholar] [CrossRef]
- Tanzifi, P.; Wong, M.; Krivanek, M. Orbital IgG4 Related disease in a patient with irka variant gene: A case report. Pathology 2020, 52, S90–S91. [Google Scholar] [CrossRef]
- Cinar, O.K.; Khaosut, P.; Sebire, N.; Eleftheriou, D.; Al-Obaidi, M. P43 Immunoglobulin G4-related disease in a 10 year-old girl with multisystem involvement. Rheumatology 2019, 58, kez416.010. [Google Scholar] [CrossRef]
- Kumar, R.; Rawat, A.; Gupta, A.; Guleria, S.; Nameirakpam, J.; Suri, D.; Gupta, A.; Nada, R.; Singh, S. Sat0504 Igg4 Related Disease in Children: A Single Centre Experience from North-West India. Ann. Rheum. Dis. 2019, 78 (Suppl. S2), 1341. [Google Scholar] [CrossRef]
- Johnson, J.S.; Saltzman, A.F.; Treece, A.L.; Cost, N.G. A case of IgG4-related renal pseudotumor in a child with history of Wilms tumor. Urol. Case Rep. 2018, 21, 107–109. [Google Scholar] [CrossRef]
- Deepak, S.; Warrier, K.; Camina, N.; Eveleigh, C. P22 Orbital mass in a 9 year old girl. Rheumatology 2018, 57, key273.024. [Google Scholar] [CrossRef][Green Version]
- Kozlova, A.; Burlakov, V.; Abramov, D.; Laberko, A.; Sagoyan, G.; Shcherbina, A. AB1082 Characterisation of a group of patients with igg4-related disease: Single centre experience. Ann. Rheum. Dis. 2018, 77 (Suppl. S2), 1651. [Google Scholar] [CrossRef]
- Chen, C.; Chen, K.; Huang, X.; Wang, K.; Qian, S. Concurrent eosinophilia and IgG4-related disease in a child: A case report and review of the literature. Exp. Ther. Med. 2018, 15, 2739–2748. Available online: http://www.spandidos-publications.com/10.3892/etm.2018.5743 (accessed on 23 February 2024). [CrossRef] [PubMed]
- Parvaneh, V.J.; Shiari, R.; Rahmani, K. Orbital Mass: The Presentation of IgG4-Related Disease in 12- Year-Old Children-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=1&page=1&id=L625168244 (accessed on 9 April 2024).
- Eng, V.; Jean, T.; Pourang, D.; Ramanathan, A.; Eichhorn, K.; Hever, A. IGG4-Related Orbital Disease with Anca Positivity: A Case Report-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=21&page=8&id=L619349156 (accessed on 23 February 2024).
- Ozdemir, K.; Basaran, C.; Selver, O.B.; Palamar, M.; Kutukculer, N.; Yagci, A.; Serdaroglu, E. IgA Nephropathy and IgG4 Related Orbital Disease: A Child Case-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=15&page=1&id=L618119403 (accessed on 9 April 2024).
- Diaz, T.; Ramirez, Y.; Osorio, S.; Brana, M.T.; Aparicio, L.; Rodriguez, A.; Faugier, E.; Maldonado, R. Successful Treatment of Pediatric IGG4 Related Ophthalmic Disease with Mycophenolate Mofetil: Case Report and a Review of the Pediatric Autoimmune Dacryoadenitis Literature-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&id=L618421202 (accessed on 23 February 2024).
- Okamoto, N.; Sugita, Y.; Shabana, K.; Murata, T.; Tamai, H. A Fourteen-Year-Old Girl with Immunoglobulin G4-Related Disease-Record Details-Embase. Available online: https://www.embase.com/records?subaction=viewrecord&rid=25&page=1&id=L618532420 (accessed on 9 April 2024).
- Goag, E.K.; Park, J.E.; Lee, E.H.; Park, Y.M.; Kim, C.Y.; Lee, J.M.; Kim, Y.J.; Kim, Y.S.; Kim, S.K.; Chang, J.; et al. A Case of Extensive IgG4-Related Disease Presenting as Massive Pleural Effusion, Mediastinal Mass, and Mesenteric Lymphadenopathy in a 16-Year-Old Male. Tuberc. Respir. Dis. 2015, 78, 396. [Google Scholar] [CrossRef] [PubMed]
- Cabrales-Escobar, I.E.; Murcio-Pérez, E.; Albarrán-Sánchez, A. IgG4-related disease manifesting as symptoms of appendicitis: Case report and literature review. Clin. J. Gastroenterol. 2021, 14, 626–632. [Google Scholar] [CrossRef]
- Creze, M.; Boussebaa, S.; Lazure, T.; Briand, S.; Court, C. IgG4-related disease: Rare presentation as a soft-tissue mass in the thigh of an adolescent. Skelet. Radiol. 2020, 49, 155–160. [Google Scholar] [CrossRef]
- Stone, J.H.; Zen, Y.; Deshpande, V. IgG4-Related Disease. N. Engl. J. Med. 2012, 366, 539–551. [Google Scholar] [CrossRef]
- Stone, J.H.; Brito-Zerón, P.; Bosch, X.; Ramos-Casals, M. Diagnostic Approach to the Complexity of IgG4-Related Disease. Mayo Clin. Proc. 2015, 90, 927–939. [Google Scholar] [CrossRef]
- Levraut, M.; Cohen, M.; Bresch, S.; Giordana, C.; Burel-Vandenbos, F.; Mondot, L.; Sedat, J.; Fontaine, D.; Bourg, V.; Martis, N.; et al. Immunoglobulin G4-related hypertrophic pachymeningitis: A case-oriented review. Neurol. Neuroimmunol. Neuroinflammation 2019, 6, e568. [Google Scholar] [CrossRef]
- Sapkota, B.; Rampure, R.; Gokden, M.; Kanuru, S. IgG4-Related Disease Presenting as Hypertrophic Pachymeningitis. Cureus 2022, 14, e21850. [Google Scholar] [CrossRef]
- Vakrakou, A.G.; Evangelopoulos, M.-E.; Boutzios, G.; Tzanetakos, D.; Tzartos, J.; Velonakis, G.; Toulas, P.; Anagnostouli, M.; Andreadou, E.; Koutsis, G.; et al. Recurrent myelitis and asymptomatic hypophysitis in IgG4-related disease: Case-based review. Rheumatol. Int. 2020, 40, 337–343. [Google Scholar] [CrossRef]
- Cação, G.; Calejo, M.; Alves, J.E.; Medeiros, P.B.; Vila-Cha, N.; Mendonça, T.; Taipa, R.; Silva, A.M.; Damásio, J. Clinical features of hypertrophic pachymeningitis in a center survey. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2019, 40, 543–551. [Google Scholar] [CrossRef]
- Esmaeilzadeh, M.; Dadak, M.; Atallah, O.; Möhn, N.; Skripuletz, T.; Hartmann, C.; Banan, R.; Krauss, J.K. IgG4-related hypertrophic pachymeningitis with tumor-like intracranial and intracerebral lesions. Acta Neurochir. 2022, 164, 2781–2787. [Google Scholar] [CrossRef]
- Matias, T.B.; Cordeiro, R.A.; Duarte, J.A.; De Jarry, V.M.; Appenzeller, S.; Villarinho, L.; Reis, F. Immune-Mediated Hypertrophic Pachymeningitis and its Mimickers: Magnetic Resonance Imaging Findings. Acad. Radiol. 2023, 30, 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, H.; Horie, R.; Kanai, M.; Suzuki, R.; Yi, E.S.; Ryu, J.H. IgG4-Related Disease: Retrospective Analysis of One Hundred Sixty-Six Patients. Arthritis Rheumatol. 2016, 68, 2290–2299. [Google Scholar] [CrossRef] [PubMed]
- Mekinian, A.; Maisonobe, L.; Boukari, L.; Melenotte, C.; Terrier, B.; Ayrignac, X.; Scheinlitz, N.; Sène, D.; Hamidou, M.; Konaté, A.; et al. Characteristics, outcome and treatments with cranial pachymeningitis. Medicine 2018, 97, e11413. [Google Scholar] [CrossRef] [PubMed]
- Della-Torre, E.; Galli, L.; Franciotta, D.; Bozzolo, E.P.; Briani, C.; Furlan, R.; Roveri, L.; Sessa, M.; Passerini, G.; Sabbadini, M.G. Diagnostic value of IgG4 Indices in IgG4-related hypertrophic pachymeningitis. J. Neuroimmunol. 2014, 266, 82–86. [Google Scholar] [CrossRef]
- Li, L.; Ward, B.; Cocks, M.; Kheradmand, A.; Francis, H.W. IgG4-Related Disease of Bilateral Temporal Bones. Ann. Otol. Rhinol. Laryngol. 2017, 126, 236–240. [Google Scholar] [CrossRef]
- Vuncannon, J.R.; Panella, N.J.; Magliocca, K.R.; Mattox, D.E. Diagnostic Challenges in a Case of IgG4-RD Affecting the Temporal Bone. Ann. Otol. Rhinol. Laryngol. 2017, 126, 241–244. [Google Scholar] [CrossRef]
- Detiger, S.E.; Karim, F.; Monserez, D.; Verdijk, R.; Van Hagen, M.; Paridaens, D.; Van Laar, J. IgG4-Related Disease of Skull Base: Case Series of 3 Patients with Headache. World Neurosurg. 2020, 134, 536–539. [Google Scholar] [CrossRef]
- Takano, K.; Kamekura, R.; Okuni, T.; Yamamoto, K. New insights into chronic rhinosinusitis associated with IgG4-related disease. Auris. Nasus. Larynx 2024, 51, 356–360. [Google Scholar] [CrossRef]
- Takano, K.; Abe, A.; Yajima, R.; Kakuki, T.; Jitsukawa, S.; Nomura, K.; Himi, T. Clinical Evaluation of Sinonasal Lesions in Patients With Immunoglobulin G4-Related Disease. Ann. Otol. Rhinol. Laryngol. 2015, 124, 965–971. [Google Scholar] [CrossRef]
- Moteki, H.; Yasuo, M.; Hamano, H.; Uehara, T.; Usami, S. IgG4-related chronic rhinosinusitis: A new clinical entity of nasal disease. Acta Otolaryngol. 2011, 131, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Khosroshahi, A.; Wallace, Z.S.; Crowe, J.L.; Akamizu, T.; Azumi, A.; Carruthers, M.N.; Chari, S.T.; Della-Torre, E.; Frulloni, L.; Goto, H.; et al. International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease. Arthritis Rheumatol. 2015, 67, 1688–1699. [Google Scholar] [CrossRef]
- Khosroshahi, A.; Carruthers, M.N.; Deshpande, V.; Unizony, S.; Bloch, D.B.; Stone, J.H. Rituximab for the treatment of IgG4-related disease: Lessons from 10 consecutive patients. Medicine 2012, 91, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, M.N.; Topazian, M.D.; Khosroshahi, A.; Witzig, T.E.; Wallace, Z.S.; Hart, P.A.; Deshpande, V.; Smyrk, T.C.; Chari, S.; Stone, J.H. Rituximab for IgG4-related disease: A prospective, open-label trial. Ann. Rheum. Dis. 2015, 74, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease. Available online: https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.39132 (accessed on 13 March 2014).
- Sapountzi, E.; Kotanidou, E.P.; Tsinopoulou, V.-R.; Fotis, L.; Fidani, L.; Galli-Tsinopoulou, A. The Management of IgG4-Related Disease in Children: A Systematic Review. Children 2025, 12, 213. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).