Nasal Injuries Related to Respiratory Support Interfaces in Preterm Infants: Neonatal Course and 12-Month Outcome
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Routine Care and Material
2.4. Protocol Description
2.5. Factors Associated with Injury Evolution
2.6. Statistical Analysis
2.7. Ethical Approval
3. Results
3.1. Characteristics of Studied Infants
3.2. Nasal Injuries at Inclusion
3.3. Nasal Injuries in the First Month of Follow-Up
- Severity of Injuries
- Sites of Injury
- Evolution of Injuries
3.4. Nasal Injuries at Discharge
3.5. Medium-Term Evolution of Injuries
- Evolution of Injuries
- Esthetic and Functional Consequences
- Parental Perceptions
4. Discussion
4.1. Main Results
4.2. Strengths and Limitations
4.3. Incidence of Nasal Injuries
4.4. Time of Occurrence and Severity
4.5. Factors Associated with Nasal Injury Occurrence
4.6. Evolution of Injuries and Determinants
4.7. Clinical Implications and Perspectives
4.8. Research Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, P.G.; Morley, C.J.; Owen, L.S. Non-invasive respiratory support of preterm neonates with respiratory distress: Continuous positive airway pressure and nasal intermittent positive pressure ventilation. Semin. Fetal. Neonatal. Med. 2009, 14, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Pas, A.T.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef] [PubMed]
- Newnam, K.M.; McGrath, J.M.; Estes, T.; Jallo, N.; Salyer, J.; Bass, W.T. An Integrative Review of Skin Breakdown in the Preterm Infant Associated with Nasal Continuous Positive Airway Pressure. J. Obstet. Gynecol. Neonatal Nurs. 2013, 42, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Ota, N.T.; Davidson, J.; Guinsburg, R. Early nasal injury resulting from the use of nasal prongs in preterm infants with very low birth weight: A pilot study. Rev. Bras. Ter. Intensiva 2013, 25, 245–250. [Google Scholar] [CrossRef]
- Guimarães, A.R.; Rocha, G.; Rodrigues, M.; Guimarães, H. Nasal CPAP complications in very low birth weight preterm infants. J. Neonatal-Perinat Med. 2020, 13, 197–206. [Google Scholar] [CrossRef]
- Imbulana, D.I.; Manley, B.J.; Dawson, J.A.; Davis, P.G.; Owen, L.S. Nasal injury in preterm infants receiving non-invasive respiratory support: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F29–F35. [Google Scholar] [CrossRef]
- Bonfim, S.d.F.S.F.; de Vasconcelos, M.G.L.; de Sousa, N.F.C.; da Silva, D.V.C.; Leal, L.P. Nasal septum injury in preterm infants using nasal prongs. Rev. Lat. Am. Enfermagem 2014, 22, 826–833. [Google Scholar] [CrossRef]
- de Sousa, N.F.C.; Bonfim, S.d.F.S.F.; de Vasconcelos, M.G.L.; Bezerra, J.L.d.O.; da Silva, D.V.C.; Leal, L.P. Prevalencia de lesao do septo nasal em prematuros no uso de prongas nasais. Rev. Esc. Enferm. USP 2013, 47, 1285–1290. [Google Scholar] [CrossRef]
- Fischer, C.; Bertelle, V.; Hohlfeld, J.; Forcada-Guex, M.; Stadelmann-Diaw, C.; Tolsa, J.F. Nasal trauma due to continuous positive airway pressure in neonates. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F447–F451. [Google Scholar] [CrossRef]
- Jatana, K.R.; Oplatek, A.; Stein, M.; Phillips, G.; Kang, D.R.; Elmaraghy, C.A. Effects of Nasal Continuous Positive Airway Pressure and Cannula Use in the Neonatal Intensive Care Unit Setting. Arch. Otolaryngol. Neck Surg. 2010, 136, 287–291. [Google Scholar] [CrossRef]
- Yong, S.C. Incidence of nasal trauma associated with nasal prong versus nasal mask during continuous positive airway pressure treatment in very low birthweight infants: A randomised control study. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F480–F483. [Google Scholar] [CrossRef]
- Dani, C.; Pratesi, S.; Migliori, C.; Bertini, G. High flow nasal cannula therapy as respiratory support in the preterm infant. Pediatr. Pulmonol. 2009, 44, 629–634. [Google Scholar] [CrossRef]
- Woodhead, D.D.; Lambert, D.K.; Clark, J.M.; Christensen, R.D. Comparing two methods of delivering high-flow gas therapy by nasal cannula following endotracheal extubation: A prospective, randomized, masked, crossover trial. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2006, 26, 481–485. [Google Scholar] [CrossRef]
- Matlock, D.N. Does using a nasal barrier dressing prevent nasal injury in premature infants receiving nasal continuous positive airway pressure? Acta Paediatr. 2018, 107, 2219. [Google Scholar] [CrossRef]
- Xie, L.H. Hydrocolloid dressing in preventing nasal trauma secondary to nasal continuous positive airway pressure in preterm infants. World J. Emerg. Med. 2014, 5, 218. [Google Scholar] [CrossRef]
- Jasani, B.; Ismail, A.; Rao, S.; Patole, S. Effectiveness and safety of nasal mask versus binasal prongs for providing continuous positive airway pressure in preterm infants—A systematic review and meta-analysis. Pediatr. Pulmonol. 2018, 53, 987–992. [Google Scholar] [CrossRef]
- Bashir, T.; Murki, S.; Kiran, S.; Reddy, V.K.; Oleti, T.P. ‘Nasal mask’ in comparison with ‘nasal prongs’ or ‘rotation of nasal mask with nasal prongs’ reduce the incidence of nasal injury in preterm neonates supported on nasal continuous positive airway pressure (nCPAP): A randomized controlled trial. PLoS ONE 2019, 14, e0211476. [Google Scholar] [CrossRef]
- McCoskey, L. Nursing care guidelines for prevention of nasal breakdown in neonates receiving nasal CPAP. Adv. Neonatal Care 2008, 8, 116–124. [Google Scholar] [CrossRef]
- Wilkinson, D.; Andersen, C.; O’Donnell, C.P.; De Paoli, A.G.; Manley, B.J. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database Syst. Rev. 2016, 22, 1465–1858. [Google Scholar] [CrossRef]
- Li, Y.; Sepulveda, A.; Buchanan, E.P. Late presenting nasal deformities after nasal continuous positive airway pressure injury: 33-year experience. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 339–343. [Google Scholar] [CrossRef]
- Jayaratne, Y.S.N.; Zwahlen, R.A.; Htun, S.Y.; Butow, K.W. Columella pressure necrosis: A method of surgical reconstruction and its long-term outcome. BMJ Case Rep. 2014, 5, bcr2013203132. [Google Scholar] [CrossRef]
- Mamelle, N.; Munoz, F.; Martin, J.L.; Laumon, B.; Grandjean, H. Fetal growth from the AUDIPOG study. II. Application for the diagnosis of intrauterine growth retardation. J. Gynecol. Obstet. Biol. Reprod. 1996, 25, 71–77. [Google Scholar]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869. [Google Scholar] [CrossRef]
- Nascimento, R.M.D.; Ferreira, A.L.C.; Coutinho, A.C.F.P.; Veríssimo, R.C.S.S. The frequency of nasal injury in newborns due to the use of continuous positive airway pressure with prongs. Rev. Lat. Am. Enfermagem 2009, 17, 489–494. [Google Scholar] [CrossRef]
- Boyar, V. Pressure injuries of the nose and columella in preterm neonates receiving noninvasive ventilation via a specialized nasal cannula: A retrospective comparison cohort study. J. Wound Ostomy Continence Nurs. 2020, 47, 111–116. [Google Scholar] [CrossRef]
- Rezaei, P.; Jafari-Mianaei, S.; Sadeghnia, A.; Heidari, Z. Protective dressings, injury, and device failure in preterm infants receiving nasal continuous positive airway pressure: A randomized controlled trial. Adv. Skin. Wound Care 2021, 34, 1–6. [Google Scholar] [CrossRef]
- Ilhan, O.; Bor, M. Randomized trial of mask or prongs for nasal intermittent mandatory ventilation in term infants with transient tachypnea of the newborn. Pediatr. Int. 2020, 62, 484–491. [Google Scholar] [CrossRef]
- Sharma, D.; Kaur, A.; Farahbakhsh, N.; Agarwal, S. To compare nasal mask with binasal prongs in delivering continuous positive airway pressure for reducing need of invasive ventilation: Randomized controlled trial. J. Matern. Fetal Neonatal Med. 2021, 34, 1890–1896. [Google Scholar] [CrossRef]
- Razak, A.; Patel, W. Nasal mask vs binasal prongs for nasal continuous positive airway pressure in preterm infants: A systematic review and meta-analysis. Pediatr. Pulmonol. 2020, 55, 2261–2271. [Google Scholar] [CrossRef]
- Goel, S.; Mondkar, J.; Panchal, H.; Hegde, D.; Utture, A.; Manerkar, S. Nasal mask versus nasal prongs for delivering nasal continuous positive airway pressure in preterm infants with respiratory distress: A randomized controlled trial. Indian. Pediatr. 2015, 52, 1035–1040. [Google Scholar] [CrossRef]
- Magalhães, P.A.F.; D’Amorim, A.C.G.; Oliveira, E.F.A.L.; Ramos, M.E.A.; Mendes, A.P.D.A.; Barbosa, J.F.S.; Reinaux, C.M.A. Rotating nasal masks with nasal prongs reduces the incidence of moderate to severe nasal injury in preterm infants supported by noninvasive ventilation. Rev. Bras. Ter. Intensiva 2022, 34, 247–254. [Google Scholar] [CrossRef]
- Sardar, S.; Pal, S.; Ghosh, M. A three-arm randomized, controlled trial of different nasal interfaces on the safety and efficacy of nasal intermittent positive-pressure ventilation in preterm newborns. Indian. J. Pediatr. 2022, 89, 1195–1201. [Google Scholar] [CrossRef]
- Spitzer, A.R.; Fox, W.W. Postextubation atlectasis—The role of oral versus nasal endotracheal tubes. J. Pediatr. 1982, 100, 806–810. [Google Scholar] [CrossRef]
- Reina Ferragut, C.; López-Herce, J. Complicaciones de la ventilación mecánica. Pediatría 2003, 59, 160–165. [Google Scholar] [CrossRef]
- Gowdar, K.; Bull, M.J.; Schreiner, R.L.; Lemons, J.A.; Gresham, E.L. Nasal deformities in neonates. Their occurrence in those treated with nasal continuous positive airway pressure and nasal endotracheal tubes. Am. J. Dis. Child. 1980, 34, 954–957. [Google Scholar] [CrossRef]
- Collins, C.L.; Barfield, C.; Horne, R.S.C.; Davis, P.G. A comparison of nasal trauma in preterm infants extubated to either heated humidified high-flow nasal cannulae or nasal continuous positive airway pressure. Eur. J. Pediatr. 2014, 173, 181–186. [Google Scholar] [CrossRef]
- Hong, H.; Li, X.-X.; Li, J.; Zhang, Z.-Q. High-flow nasal cannula versus nasal continuous positive airway pressure for respiratory support in preterm infants: A meta-analysis of randomized controlled trials. J. Matern. Fetal Neonatal Med. 2021, 34, 259–266. [Google Scholar] [CrossRef]
- Soonsawad, S.; Tongsawang, N.; Nuntnarumit, P. Heated Humidified high-flow nasal cannula for weaning from continuous positive airway pressure in preterm infants: A randomized controlled trial. Neonatology 2016, 110, 204–209. [Google Scholar] [CrossRef]
- Yengkhom, R.; Suryawanshi, P.; Gupta, B.; Deshpande, S. Heated humidified high-flow nasal cannula vs. nasal continuous positive airway pressure for post-extubation respiratory support in preterm infants: A randomized controlled trial. J. Trop. Pediatr. 2021, 67, fmaa082. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, S.J.; Adappa, R.; Gupta, N.; Watkins, W.J.; Kotecha, S.; Chakraborty, M. Safety and efficacy of high-flow nasal cannula therapy in preterm infants: A meta-analysis. Pediatrics 2015, 136, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Fleeman, N.; Mahon, J.; Bates, V.; Dickson, R.; Dundar, Y.; Dwan, K.; Ellis, L.; Kotas, E.; Richardson, M.; Shah, P.; et al. The clinical effectiveness and cost-effectiveness of heated humidified high-flow nasal cannula compared with usual care for preterm infants: Systematic review and economic evaluation. Health Technol. Assess. 2016, 20, 1–68. [Google Scholar] [CrossRef] [PubMed]
- Bruet, S.; Butin, M.; Dutheil, F. Systematic review of high-flow nasal cannula versus continuous positive airway pressure for primary support in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 56–59. [Google Scholar] [CrossRef]
- Junior, J.C.; de Azevedo, R.; Araujo, O.; de Carvalho, W.B. High-flow nasal cannula as a post-extubation respiratory support strategy in preterm infants: A systematic review and meta-analysis. J. Pediatr. 2020, 96, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Maram, S.; Murki, S.; Nayyar, S.; Kadam, S.; Oleti, T.P.; Anne, R.P.; Deshobhotla, S.; Sharma, D.; Arun, S.; Vadije, P.R. RAM cannula with Cannulaide versus Hudson prongs for delivery of nasal continuous positive airway pressure in preterm infants: An RCT. Sci. Rep. 2021, 11, 23527. [Google Scholar] [CrossRef]
- Drescher, G.S.; Hughes, C.W. Comparison of Interfaces for the Delivery of Noninvasive Respiratory Support to Low Birthweight Infants. Respir. Care 2018, 63, 1197–1206. [Google Scholar] [CrossRef]
- Hochwald, O.; Riskin, A.; Borenstein-Levin, L.; Shoris, I.; Dinur, G.P.; Said, W.; Jubran, H.; Littner, Y.; Haddad, J.; Mor, M.; et al. Cannula with Long and Narrow Tubing vs Short Binasal Prongs for Noninvasive Ventilation in Preterm Infants: Noninferiority Randomized Clinical Trial. JAMA Pediatr. 2021, 175, 36. [Google Scholar] [CrossRef] [PubMed]
- Imbulana, D.I.; Owen, L.S.; Dawson, J.A.; Bailey, J.L.; Davis, P.G.; Manley, B.J. A Randomized Controlled Trial of a Barrier Dressing to Reduce Nasal Injury in Preterm Infants Receiving Binasal Noninvasive Respiratory Support. J. Pediatr. 2018, 201, 34–39.e3. [Google Scholar] [CrossRef]
- Morris, L.D.; Behr, J.H.; Smith, S.L. Hydrocolloid to Prevent Breakdown of Nares in Preterm Infants. MCN Am. J. Matern. Nurs. 2015, 40, 39–43. [Google Scholar] [CrossRef]
- Loftus, B.C.; Ahn, J.; Haddad, J. Neonatal Nasal Deformities Secondary to Nasal Continuous Positive Airway Pressure. Laryngoscope 1994, 104, 1019–1022. [Google Scholar] [CrossRef]
- Priyanka, M.; Khadijah, M.N.; Jeyanthi, K. Columella necrosis in a child secondary to nasal continuous positive airway pressure during neonatal period. Med. J. Malaysia 2021, 76, 771–773. [Google Scholar]
- Ottinger, D.; Hicks, J.; Wilson, S.; Sperber, K.; Power, K. The pressure is on!: Neonatal skin and nasal continuous positive airway pressure. Adv. Neonatal Care 2016, 16, 420–423. [Google Scholar] [CrossRef]
- Maruccia, M.; Fanelli, B.; Ruggieri, M.; Onesti, M.G. Necrosis of the columella associated with nasal continuous positive airway pressure in a preterm infant. Int. Wound J. 2014, 11, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Aykanat, A.; Çelik, H.T.; Günaydın, R.Ö.; Yiğit, Ş. Iatrogenic nasal synechiae in a premature newborn. Turk. J. Pediatr. 2020, 62, 505. [Google Scholar] [CrossRef]
- Fjaeldstad, A.; Cipliene, R.; Ramsgaard-Jensen, T.; Ebbesen, F. Septum necrosis following CPAP treatment of preterm infant. Ugeskr. Laeger 2014, 176, V12120735. [Google Scholar] [PubMed]
- Borràs-Novell, C.; Causapié, M.G.; Murcia, M.; Djian, D.; García-Algar, Ó. Development of a 3d individualized mask for neonatal non-invasive ventilation. Int. J. Bioprinting 2022, 8, 516. [Google Scholar] [CrossRef]
- Wu, Y.; Lv, J.; Xu, J.; Zhang, S.; Zhang, L.; Fu, L. Application of a photoelectric magnifier to nasal injury in preterm infants receiving non-invasive ventilation: A prospective observational study. J. Tissue Viability 2022, 31, 130–134. [Google Scholar] [CrossRef]
- Krzyzewski, J.J.; Rogers, K.K.; Ritchey, A.M.; Farmer, C.R.; Harman, A.S.; Machry, J.S. Reducing device-related pressure injuries associated with noninvasive ventilation in the neonatal intensive care unit. Respir. Care 2022, 67, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.; Mohr, L.D.; Geistkemper, A.; Murphy, S.; Fleming, K. Sustained reduction of nasal pressure injuries in the neonatal intensive care unit with the use of bubble continuous positive airway pressure: A quality improvement project. J. Wound Ostomy Continence Nurs. 2021, 48, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Naha, N.; Pournami, F.; Prabhakar, J.; Jain, N. Nasal injury with continuous positive airway pressure: Need for “privileging” nursing staff. Indian. J. Pediatr. 2019, 86, 595–598. [Google Scholar] [CrossRef]
- Mariam, S.; Buddhavarapu, S. Impact of systematic training and CPAP checklist in the prevention of ncpap related nasal injuries in neonates- a quality improvement study. Indian. J. Pediatr. 2020, 87, 256–261. [Google Scholar] [CrossRef]
- Ribeiro, D.d.F.C.; Barros, F.S.; Fernandes, B.L.; Nakato, A.M.; Nohama, P. Nasal prongs: Risks, injuries incidence and preventive approaches associated with their use in newborns. J. Multidiscip. Healthc. 2020, 13, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.d.F.C.; Barros, F.S.; Fernandes, B.L.; Nakato, A.M.; Nohama, P. Incidence and severity of nasal injuries in preterm infants associated to non-invasive ventilation using short binasal prong. Glob. Pediatr. Health 2021, 8, 2333794X2110104. [Google Scholar] [CrossRef] [PubMed]
- Squires, A.J.; Hyndman, M. Prevention of nasal injuries secondary to ncpap application in the elbw infant. Neonatal Netw. 2009, 28, 13–27. [Google Scholar] [CrossRef]
- Zores, C.; Zana-Taïeb, E.; Caeymaex, L.; Fumeaux, C.F.; Kuhn, P.; the Group of Reflection and Evaluation of the Environment of Newborns Study Group of the French Neonatology Society. French Neonatal Society issues recommendations on preventing nasal injuries in preterm newborn infants during non-invasive respiratory support. Acta Paediatr. 2023, 112, 1849–1859. [Google Scholar] [CrossRef] [PubMed]


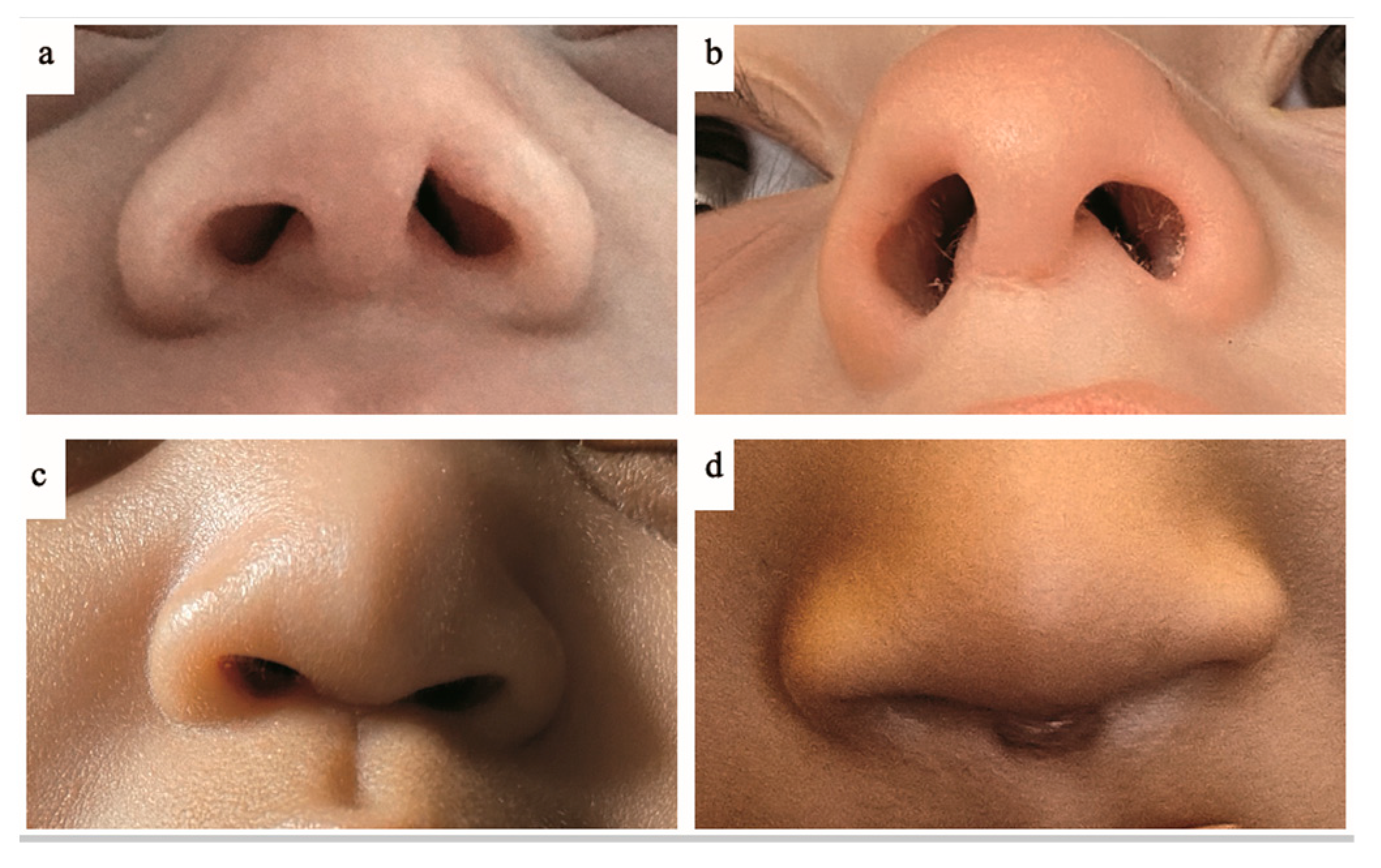
| Gestational age (weeksdays), median (range) | 276/7 (234/7–326/7) |
| Birth weight (g), median (range) | 986 (485–2090) |
| Small for gestational age, n/N infants (%) | 7/64 (10.9) |
| Sex, n Male/female | 29/35 |
| Delivery mode, n Vaginal/C section | 19/45 |
| Apgar, median (range) | |
| At 1 min | 5 (0–10) |
| At 5 min | 8 (1–10) |
| Antenatal corticosteroid therapy, n/N infants (%) | 50/64 (78.1) |
| Skin phenotype according to Fitzpatrick classification, n/N infants (%) | |
| I–III | 39/64 (60.9) |
| IV | 17/64 (26.6) |
| V–VI | 8/64 (12.5) |
| Incubator humidification rate (%), median (range) | 72 (0–95) |
| Insufflation air heater temperature (°C), median (range) | 37 (35–39) |
| Total ventilation duration (days), median (range) | |
| Invasive | 2.5 (0–24) |
| Continuous positive airway pressure | 37 (3–68) |
| Mask | 26 (0–53) |
| Prongs | 10 (0–25) |
| Ram cannula | 0 (0–10) |
| High-flow nasal cannulas | 11 (0–31) |
| Bronchopulmonary dysplasia, n/N infants (%) | 25/64 (39) |
| Duration of caffeine treatment (days), median (range) | 58 (13–107) |
| Duration of doxapram treatment (days), median (range) | 0 (0–61) |
| Favorable Evolution | ||||||
|---|---|---|---|---|---|---|
| Day 3 | p | Day 7 | p | Day 28 | p | |
| Total, n/N infants (%) | 22/64 (34.4) | - | 29/63 (46) | - | 24/60 (40) | - |
| Type of ventilation at time of analysis, n/N infants (%) | 0.10 | 0.41 | 0.04 | |||
| Invasive ventilation | 1/11 (9) | 4/9 (44.4) | 1/5 (20) | 0.58 | ||
| Continuous positive airway pressure | 20/49 (40.8) | 20/47 (42.6) | 7/27 (25.9) | 0.06 | ||
| High-flow nasal cannulas or ventilator weaning | 1/4 (25) | 5/7 (71.4) | 16/28 (57.1) | 0.64 | ||
| Main injury site, n/N infants (%) | 0.12 | 0.26 | 0.009 | |||
| Columella | 8/26 (30.8) | 10/23 (43.5) | 9/27 (33.3) | 0.15 | ||
| Internal part of the nostril orifice | 4/19 (21.1) | 6/19 (31.6) | 5/16 (31.3) | 0.22 | ||
| External part of the nostril orifice | 1/2 (50) | 1/3 (33.3) | 0/1 (0) | 1 | ||
| Intranasal mucosa | 3/7 (42.9) | 5/7 (71.4) | 3/7 (42.9) | 1 | ||
| Cartilaginous dorsum | 6/8 (75) | 6/8 (75) | 7/7 (100) | 0.047 | ||
| Philtrum | 0/1 (0) | 0/1 (0) | 0/0 (0) | 1 | ||
| Other | 0/1 (0) | 1/2 (50) | 0/2 (0) | 0.47 | ||
| Secondary protection measure, n/N infants (%) | 0.88 | 0.68 | 0.056 | |||
| None | 9/27 (33.3) | 16/33 (43.3) | 22/47 (46.8) | |||
| Presence | 13/37 (35.1) | 13/30 (48.5) | 2/13 (15.4) | |||
| Hydrocolloid | 8/23 (34.8) | 0.23 | 4/15 (26.7) | 0.20 | 1/4 (25) | 0.58 |
| Sequelae | No Sequelae | p | |
|---|---|---|---|
| Main injuries, n/N infants (%) | 34/58 (58.6) | 24/58 (41.4) | 0.28 |
| Total injuries, n/N injuries (%) | 48/97 (49.5) | 49/97 (50.5) | 0.93 |
| Type of ventilation at injury detection, n/N infants (%) | 1 | ||
| Continuous positive airway pressure | 29/50 (58) | 21/50 (42) | |
| Invasive ventilation | 4/7 (57.1) | 3/7 (42.9) | |
| High-flow nasal cannulas | 1/1 (100) | 0/1 (0) | |
| Initial ventilation time (days), mean (SD) | |||
| Continuous positive airway pressure | 9.3 (5.7) | 10.8 (10.1) | 0.71 |
| Invasive ventilation | 7.8 (6) | 7.2 (2.9) | 0.09 |
| Ventilation duration (days) | |||
| Total, median (range) | 61 (11–101) | 44 (10–85) | 0.02 |
| Invasive ventilation, median (range) | 2.5 (0–24) | 3 (0–24) | 0.37 |
| Continuous positive airway pressure, mean (SD) | 43.8 (12.9) | 29.9 (13) | <0.001 |
| Total non-invasive, mean (SD) | 54.7 (14.7) | 41.4 (14.2) | 0.005 |
| Site, n/N injuries (%) | 0.09 | ||
| Internal part of the nostril orifice | 10/22 (45.5) | 12/22 (54.5) | |
| External part of the nostril orifice | 2/5 (40) | 3/5 (60) | |
| Columella | 21/36 (58.3) | 15/36 (41.7) | |
| Philtrum | 0/1 (0) | 1/1 (100) | |
| Cartilaginous dorsum | 3/14 (21.4) | 11/14 (78.6) | |
| Intranasal mucosa | 10/17 (58.8) | 7/17 (41.2) | |
| Other | 2/2 (100) | 0/2 (0) | |
| Severity of injury at inclusion, n/N infants (%) | 0.25 | ||
| Stage 1 | 11/24 (45.8) | 13/24 (54.2) | |
| Stage 2 | 19/29 (65.5) | 10/29 (34.5) | |
| Stage 3 | 4/5 (80) | 1/5 (20) | |
| Maximum severity of injury, n/N infants (%) | <0.001 | ||
| Stage 1 | 2/11 (18.2) | 9/11 (81.8) | 0.13 |
| Stage 2 | 5/19 (26.3) | 14/19 (73.7) | 0.09 |
| Stage 3 | 27/28 (96.4) | 1/28 (3.6) | <0.001 |
| Secondary protection measure, n/N infants (%) | 0.08 | ||
| Presence | 27/41 (65.9) | 14/41 (34.1) | |
| None | 7/17 (41.2) | 10/17 (58.8) | |
| Hydrocolloid, n/N infants (%) | 0.03 | ||
| Presence | 18/24 (75) | 6/24 (25) | 0.04 |
| None | 16/34 (47.1) | 18/34 (52.9) | 0.78 |
| Birth weight (g), median (range) | 957 (560–1544) | 1047 (485–2090) | 0.06 |
| Growth, n/N infants (%) | 1 | ||
| Small for gestational age | 4/6 (66.7) | 2/6 (33.3) | |
| Eutrophic | 30/52 (57.7) | 22/52 (42.3) | |
| Age at inclusion (days), median (range) | 10.5 (1–38) | 11 (1–49) | 0.45 |
| Gestational age (weeksdays), median (range) | 274/7 (234/7–322/7) | 284/7 (234/7–326/7) | 0.06 |
| Skin phenotype, n/N infants (%) | 0.93 | ||
| I–III | 19/34 (55.9) | 15/34 (44.1) | |
| IV | 10/16 (62.5) | 6/16 (37.5) | |
| V–VI | 5/8 (62.5) | 3/8 (37.5) | |
| Sex, n/N infants (%) | 0.37 | ||
| Female | 21/33 (63.6) | 12/33 (36.4) | |
| Male | 13/25 (52) | 12/25 (48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamaux, M.; Gibier, C.; Dillenseger, L.; Fourie, G.; Langlet-Muteau, C.; Rondel, J.; Matis, J.; Matz, B.; Schmitt, V.; Meyer, N.; et al. Nasal Injuries Related to Respiratory Support Interfaces in Preterm Infants: Neonatal Course and 12-Month Outcome. Children 2025, 12, 840. https://doi.org/10.3390/children12070840
Jamaux M, Gibier C, Dillenseger L, Fourie G, Langlet-Muteau C, Rondel J, Matis J, Matz B, Schmitt V, Meyer N, et al. Nasal Injuries Related to Respiratory Support Interfaces in Preterm Infants: Neonatal Course and 12-Month Outcome. Children. 2025; 12(7):840. https://doi.org/10.3390/children12070840
Chicago/Turabian StyleJamaux, Marielle, Corisande Gibier, Laurence Dillenseger, Gwenaelle Fourie, Claire Langlet-Muteau, Jennifer Rondel, Jacqueline Matis, Bénédicte Matz, Valérie Schmitt, Nicolas Meyer, and et al. 2025. "Nasal Injuries Related to Respiratory Support Interfaces in Preterm Infants: Neonatal Course and 12-Month Outcome" Children 12, no. 7: 840. https://doi.org/10.3390/children12070840
APA StyleJamaux, M., Gibier, C., Dillenseger, L., Fourie, G., Langlet-Muteau, C., Rondel, J., Matis, J., Matz, B., Schmitt, V., Meyer, N., Kuhn, P., & Zores, C. (2025). Nasal Injuries Related to Respiratory Support Interfaces in Preterm Infants: Neonatal Course and 12-Month Outcome. Children, 12(7), 840. https://doi.org/10.3390/children12070840







