Action Observation for Children and Adolescents with Cerebral Palsy: Hope or Hype? A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Searches and Selection Process
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Meta-Analysis and Qualitative Synthesis
3. Results
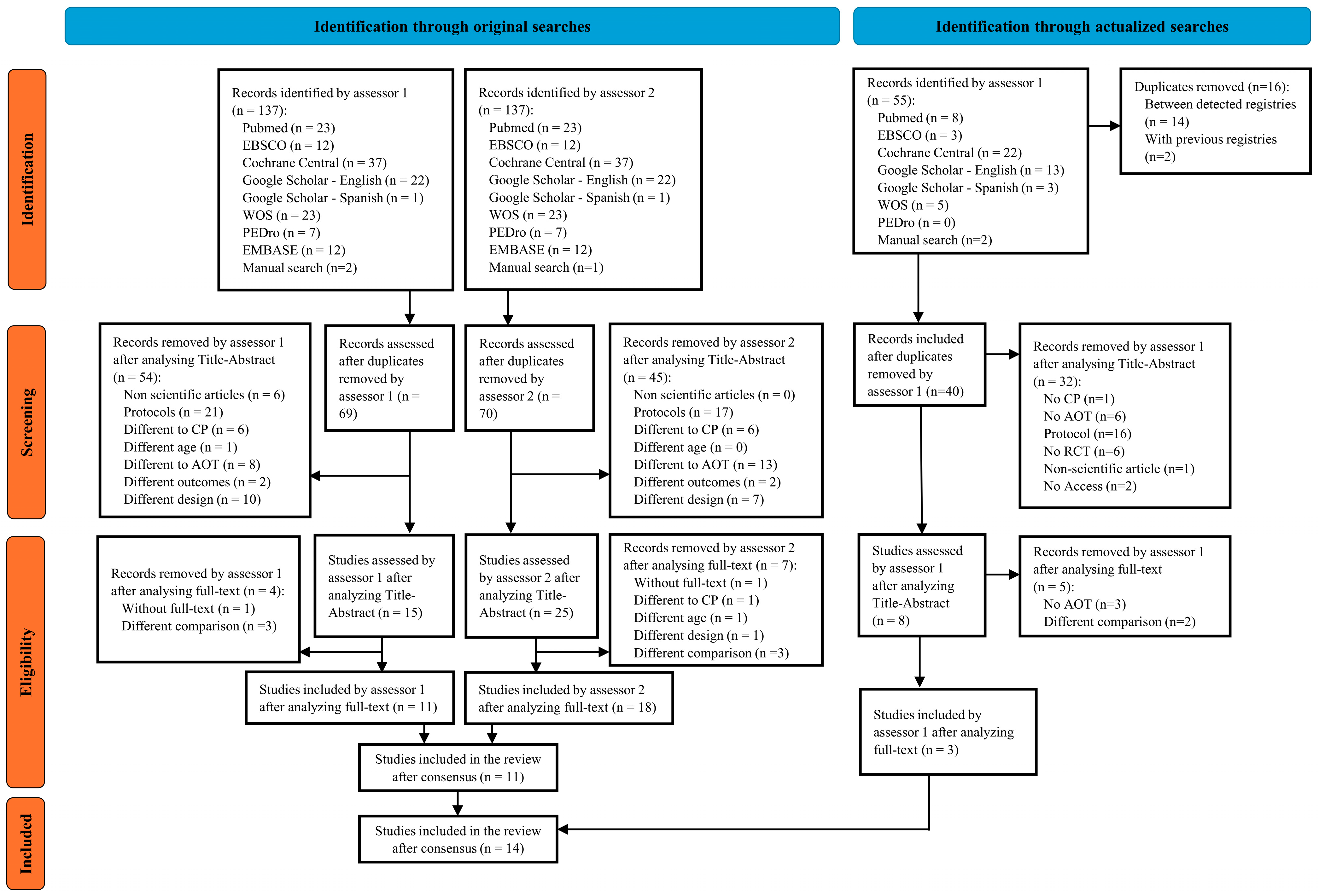
3.1. Selection Process
3.2. Study Features
3.3. AOT Prescription Parameters
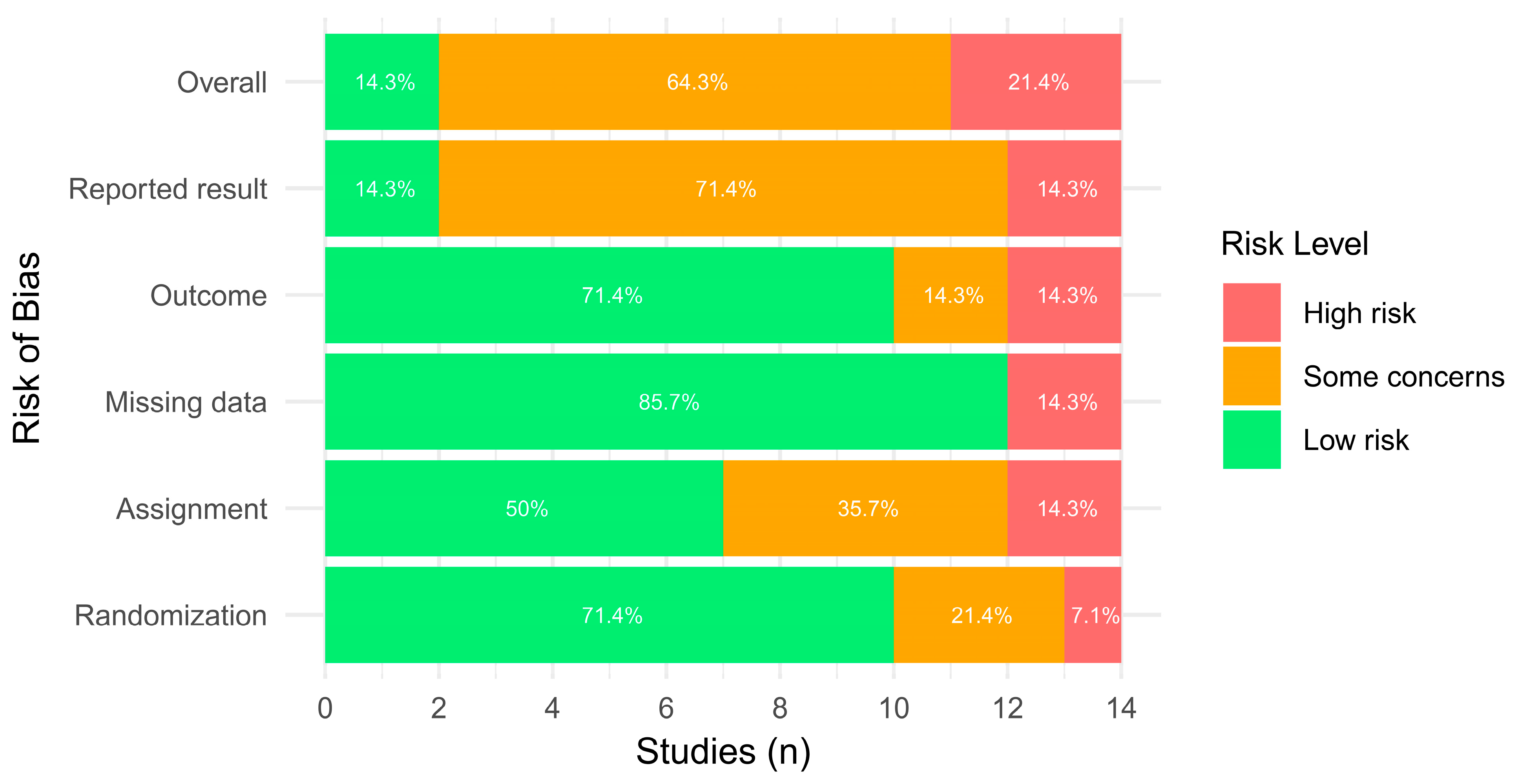
3.4. Risk of Bias Evaluation
3.5. Meta-Analysis and Qualitative Synthesis Results
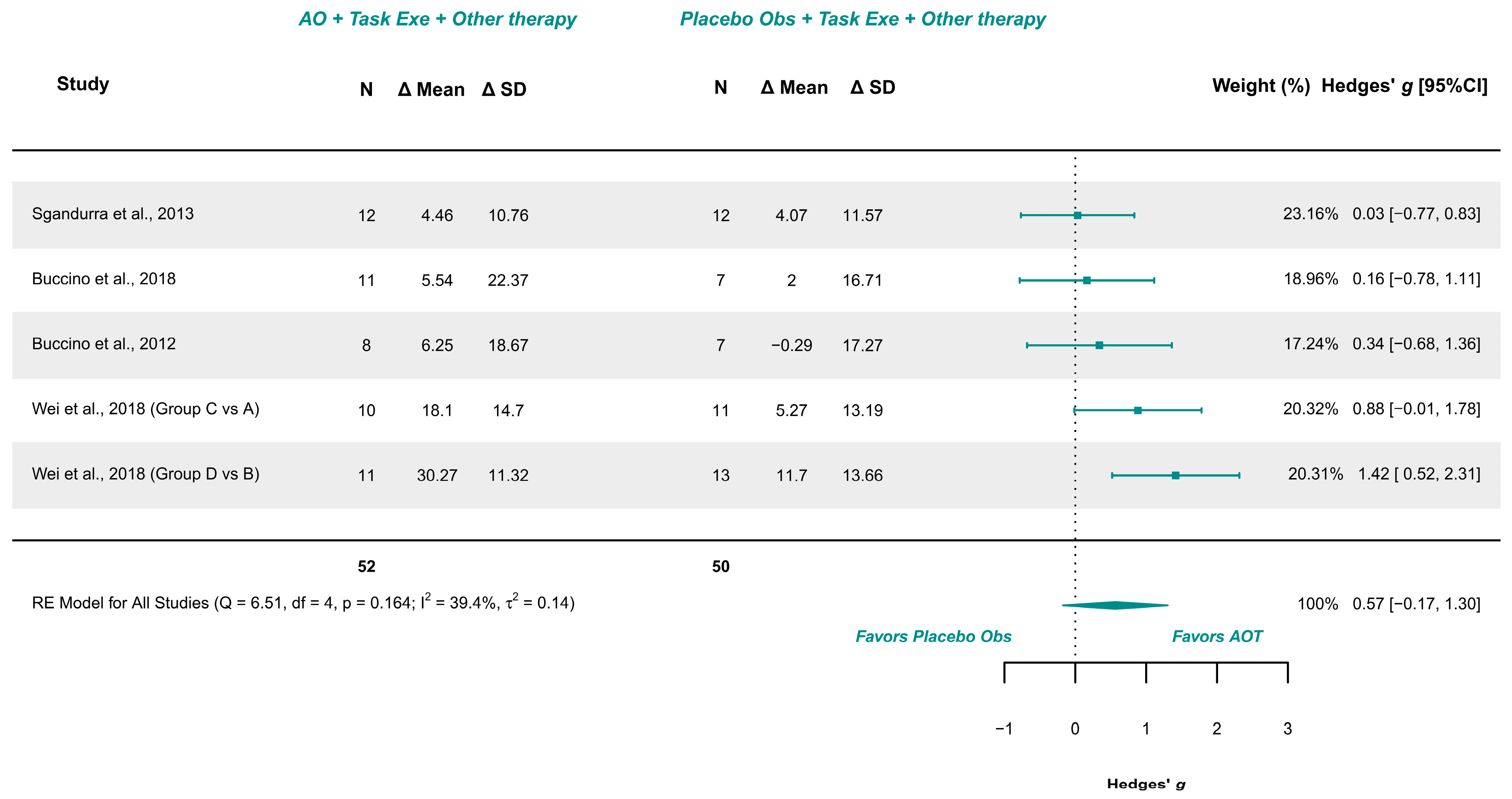
3.5.1. AOT Versus Placebo—Unilateral Upper Limb Function (More-Affected Limb)
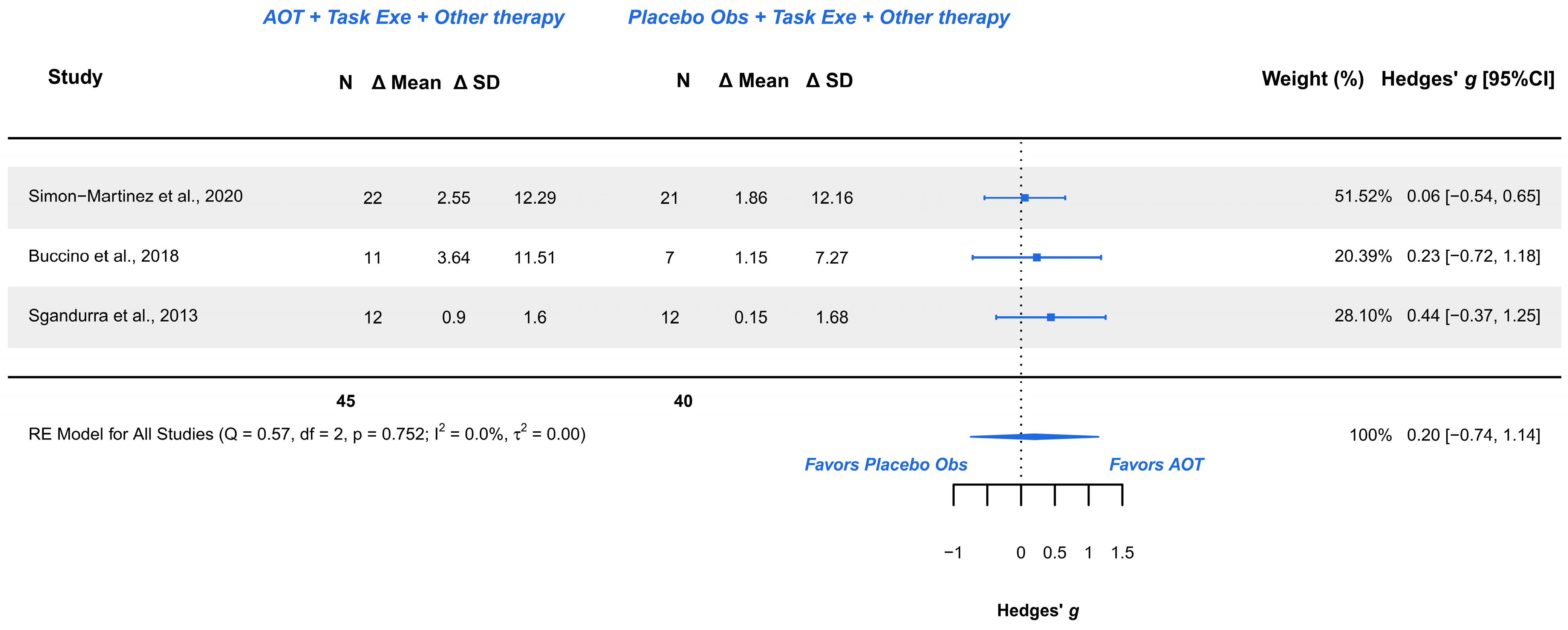
3.5.2. AOT Versus Placebo—Assisting Hand Function During Bimanual Activities
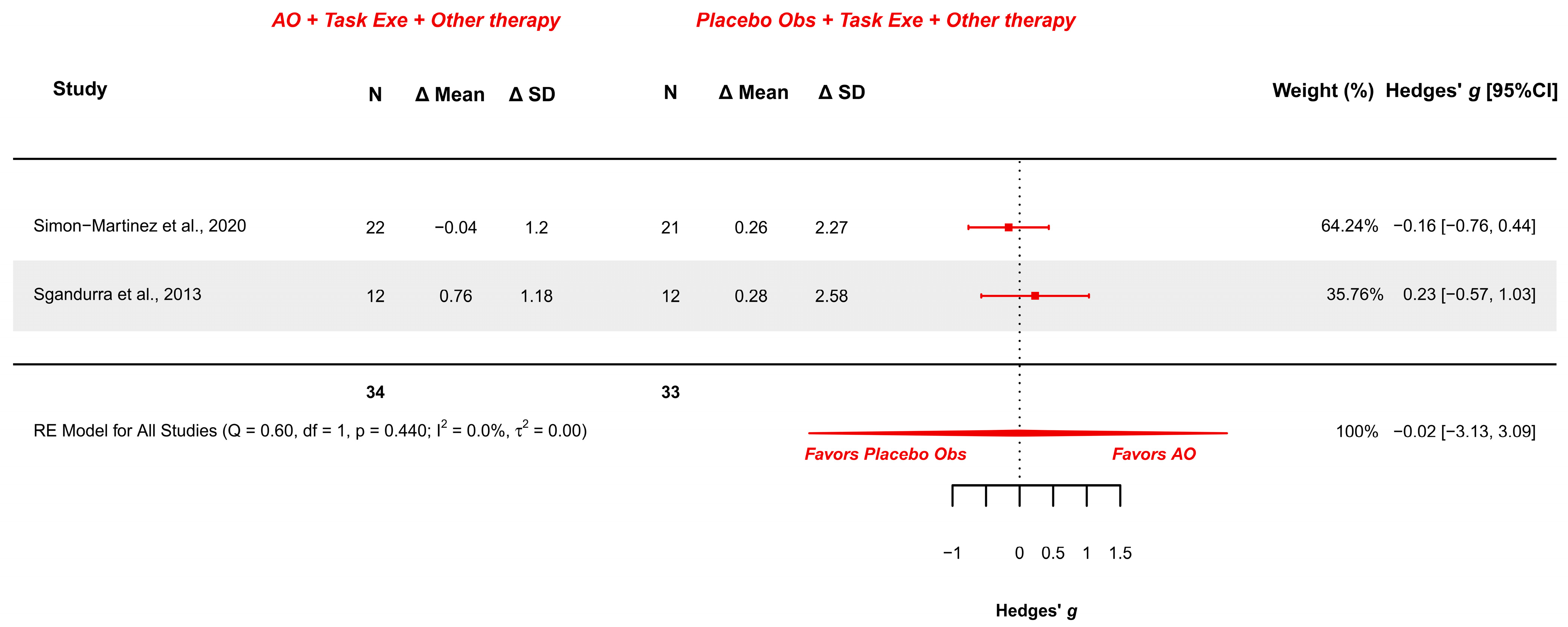
3.5.3. AOT Versus Placebo—Manual Function During Daily Activities
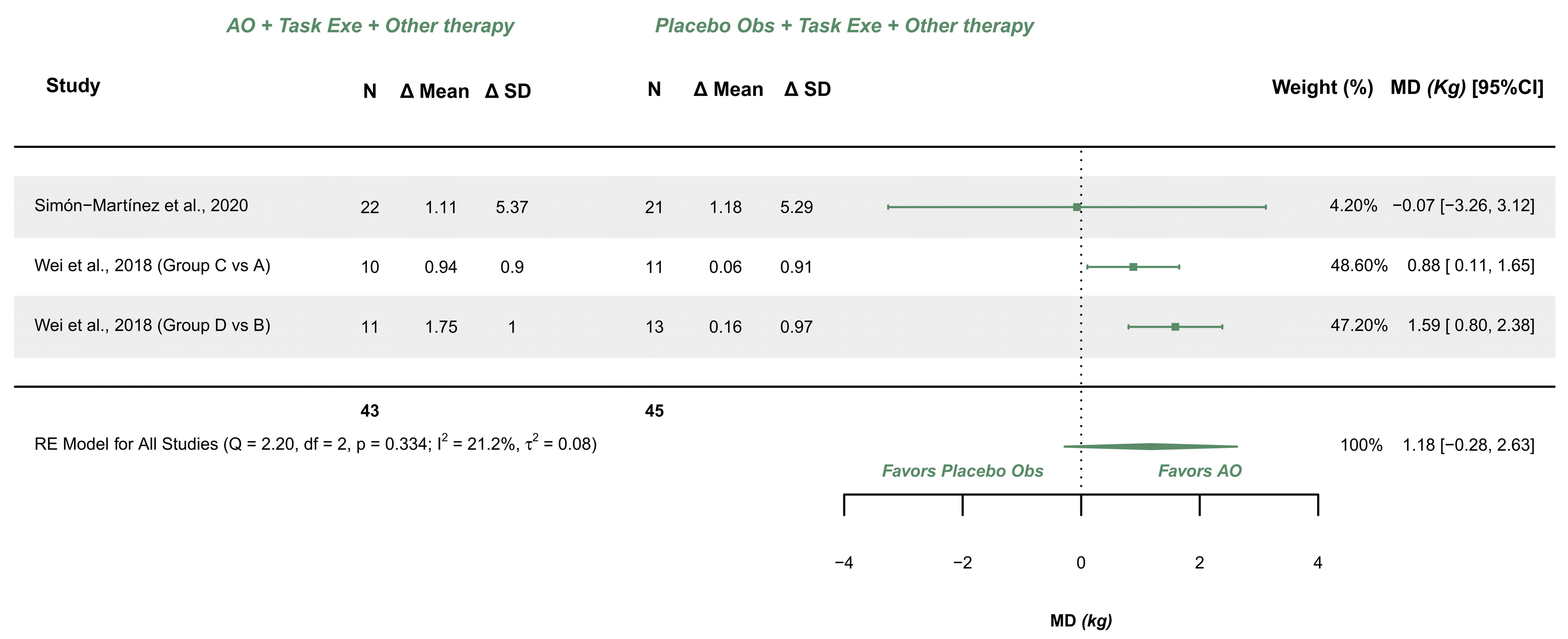
3.5.4. AOT Versus Placebo—Hand Grip Strength (More-Affected Limb)
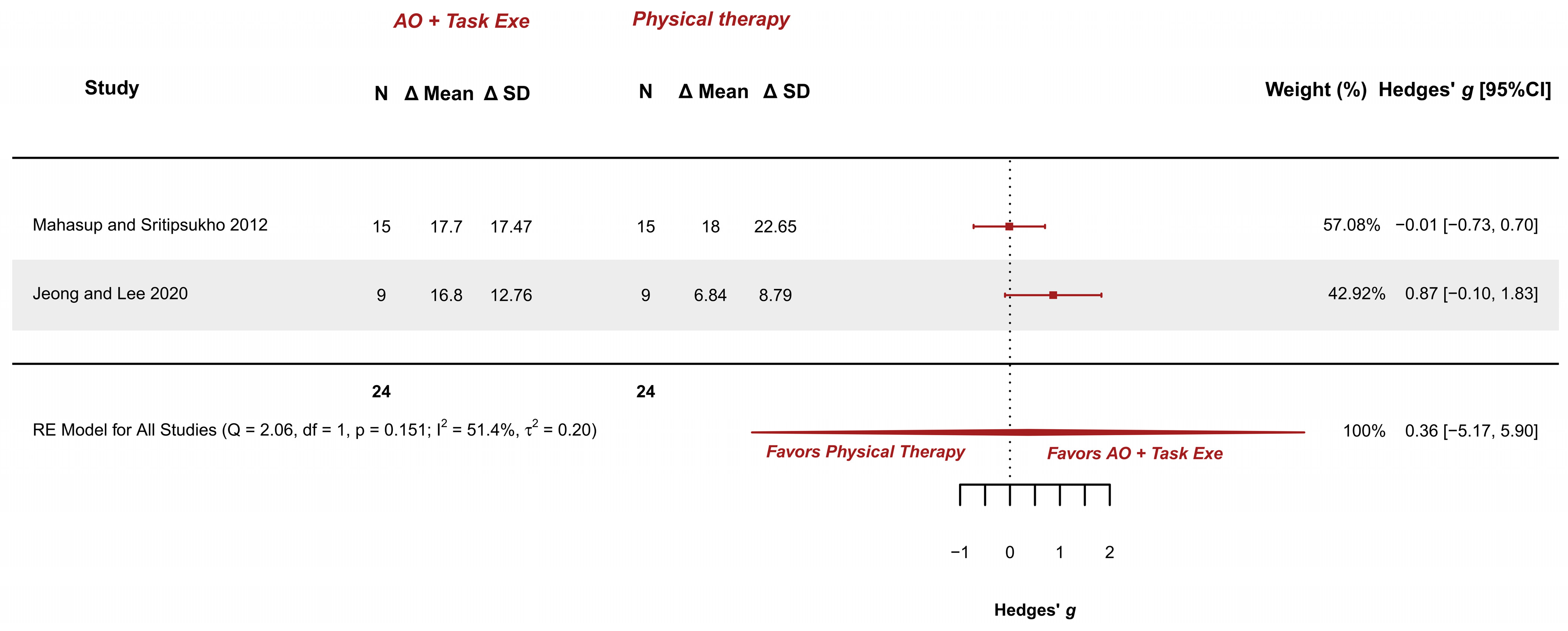
3.5.5. AOT Versus Physical Therapy—Gross Motor Function in Standing Dimension
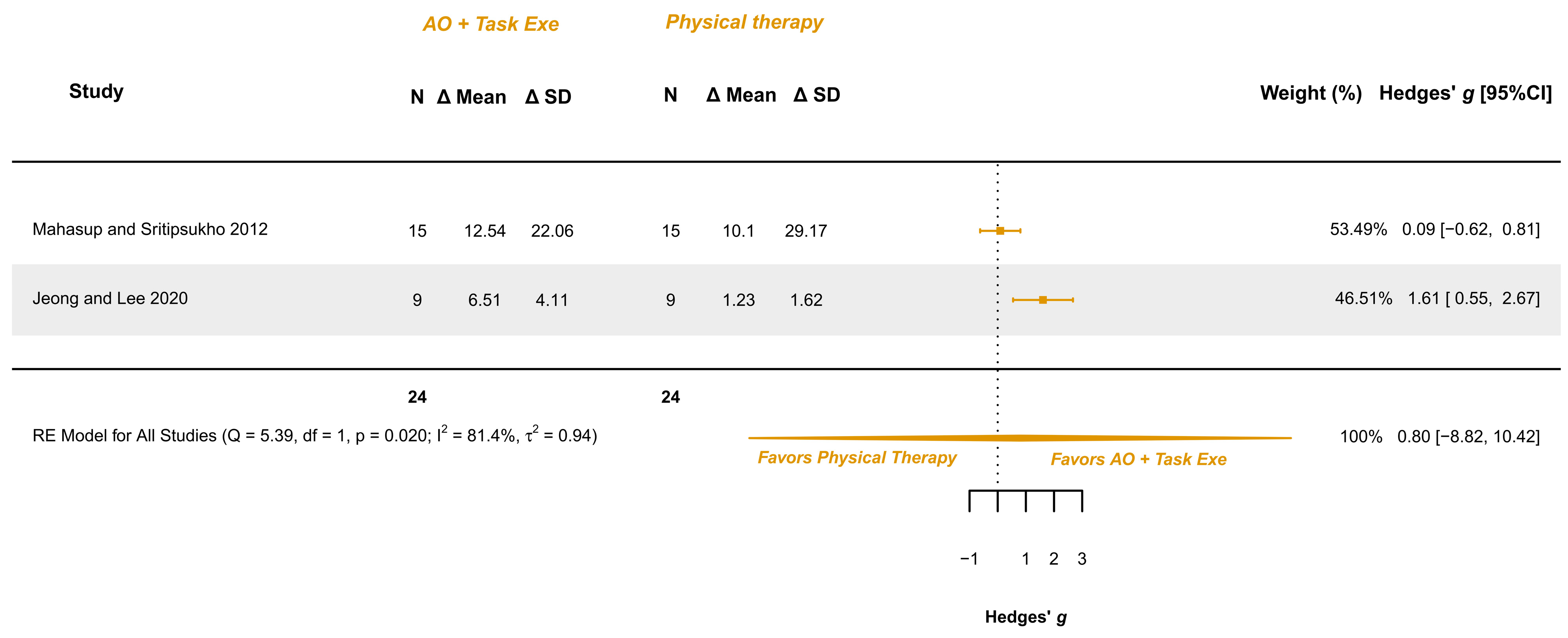
3.5.6. AOT Versus Physical Therapy—Gross Motor Function in Walking, Standing, and Jumping Dimensions
4. Discussion
4.1. Srengths and Limitations
4.2. Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, S.; Xia, J.; Gao, J.; Wang, L. Increasing Prevalence of Cerebral Palsy among Children and Adolescents in China 1988–2020: A Systematic Review and Meta-Analysis. J. Rehabil. Med. 2021, 53, jrm00195. [Google Scholar] [CrossRef] [PubMed]
- Kirby, R.S.; Wingate, M.S.; Braun, K.V.N.; Doernberg, N.S.; Arneson, C.L.; Benedict, R.E.; Mulvihill, B.; Durkin, M.S.; Fitzgerald, R.T.; Maenner, M.J.; et al. Prevalence and Functioning of Children with Cerebral Palsy in Four Areas of the United States in 2006: A Report from the Autism and Developmental Disabilities Monitoring Network. Res. Dev. Disabil. 2011, 32, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Tonmukayakul, U.; Shih, S.T.F.; Bourke-Taylor, H.; Imms, C.; Reddihough, D.; Cox, L.; Carter, R. Systematic Review of the Economic Impact of Cerebral Palsy. Res. Dev. Disabil. 2018, 80, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Fullen, B.; Rio, E.; Segurado, R.; Stokes, D.; O’Sullivan, C. Effect of Action Observation Therapy in the Rehabilitation of Neurologic and Musculoskeletal Conditions: A Systematic Review. Arch. Rehabil. Res. Clin. Transl. 2021, 3, 100106. [Google Scholar] [CrossRef]
- Cuenca-Martínez, F.; Reina-Varona, Á.; Castillo-García, J.; Touche, R.L.; Angulo-Díaz-Parreño, S.; Suso-Martí, L. Pain Relief by Movement Representation Strategies: An Umbrella and Mapping Review with Meta-Meta-Analysis of Motor Imagery, Action Observation and Mirror Therapy. Eur. J. Pain. 2021, 26, 284–309. [Google Scholar] [CrossRef]
- Herranz-Gómez, A.; Gaudiosi, C.; Angulo-Díaz-Parreño, S.; Suso-Martí, L.; Touche, R.L.; Cuenca-Martínez, F. Effectiveness of Motor Imagery and Action Observation on Functional Variables: An Umbrella and Mapping Review with Meta-Meta-Analysis. Neurosci. Biobehav. Rev. 2020, 118, 828–845. [Google Scholar] [CrossRef]
- Errante, A.; Fogassi, L. Activation of Cerebellum and Basal Ganglia during the Observation and Execution of Manipulative Actions. Sci. Rep. 2020, 10, 12008. [Google Scholar] [CrossRef]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural Correlates of Action: Comparing Meta-Analyses of Imagery, Observation, and Execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef]
- Agosta, F.; Gatti, R.; Sarasso, E.; Volonté, M.A.; Canu, E.; Meani, A.; Sarro, L.; Copetti, M.; Cattrysse, E.; Kerckhofs, E.; et al. Brain Plasticity in Parkinson’s Disease with Freezing of Gait Induced by Action Observation Training. J. Neurol. 2017, 264, 88–101. [Google Scholar] [CrossRef]
- Kemmerer, D. What Modulates the Mirror Neuron System during Action Observation?: Multiple Factors Involving the Action, the Actor, the Observer, the Relationship between Actor and Observer, and the Context. Prog. Neurobiol. 2021, 205, 102128. [Google Scholar] [CrossRef]
- Morales, S.; Bowman, L.C.; Velnoskey, K.R.; Fox, N.A.; Redcay, E. An fMRI Study of Action Observation and Action Execution in Childhood. Dev. Cogn. Neurosci. 2019, 37, 100655. [Google Scholar] [CrossRef] [PubMed]
- Bieber, E.; Smits-Engelsman, B.C.M.; Sgandurra, G.; Martini, G.; Guzzetta, A.; Cioni, G.; Feys, H.; Klingels, K. Insights on Action Observation and Imitation Abilities in Children with Developmental Coordination Disorder and Typically Developing Children. Res. Dev. Disabil. 2023, 139, 104556. [Google Scholar] [CrossRef] [PubMed]
- Foti, F.; Martone, D.; Orrù, S.; Montuori, S.; Imperlini, E.; Buono, P.; Petrosini, L.; Mandolesi, L. Are Young Children Able to Learn Exploratory Strategies by Observation? Psychol. Res. 2018, 82, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.R.; Fernandes, A.B.; Melo, L.P.; Guerra, R.O.; Campos, T.F. Action Observation for Upper Limb Rehabilitation after Stroke. Cochrane Database Syst. Rev. 2018, 10, CD011887. [Google Scholar] [CrossRef]
- Temporiti, F.; Adamo, P.; Cavalli, E.; Gatti, R. Efficacy and Characteristics of the Stimuli of Action Observation Therapy in Subjects with Parkinson’s Disease: A Systematic Review. Front. Neurol. 2020, 11, 808. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Fahey, M.; Finch-Edmondson, M.; Galea, C.; Hines, A.; Langdon, K.; Namara, M.M.; Paton, M.C.; Popat, H.; et al. State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy. Curr. Neurol. Neurosci. Rep. 2020, 20, 3. [Google Scholar] [CrossRef]
- Buccino, G.; Arisi, D.; Gough, P.; Aprile, D.; Ferri, C.; Serotti, L.; Tiberti, A.; Fazzi, E. Improving Upper Limb Motor Functions through Action Observation Treatment: A Pilot Study in Children with Cerebral Palsy. Dev. Med. Child. Neurol. 2012, 54, 822–828. [Google Scholar] [CrossRef]
- Sgandurra, G.; Ferrari, A.; Cossu, G.; Guzzetta, A.; Fogassi, L.; Cioni, G. Randomized Trial of Observation and Execution of Upper Extremity Actions versus Action Alone in Children with Unilateral Cerebral Palsy. Neurorehabilit. Neural Repair 2013, 27, 808–815. [Google Scholar] [CrossRef]
- Yang, F.-A.; Lee, T.-H.; Huang, S.-W.; Liou, T.-H.; Escorpizo, R.; Chen, H.-C. Upper Limb Manual Training for Children with Cerebral Palsy: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Clin. Rehabil. 2023, 37, 516–533. [Google Scholar] [CrossRef]
- Demeco, A.; Molinaro, A.; Ambroggi, M.; Frizziero, A.; Fazzi, E.; Costantino, C.; Buccino, G. Cognitive Approaches in the Rehabilitation of Upper Limbs Function in Children with Cerebral Palsy: A Systematic Review and Meta-Analysis. Eur. J. Phys. Rehabil. Med. 2024, 60, 445–457. [Google Scholar] [CrossRef]
- Buccino, G.; Molinaro, A.; Ambrosi, C.; Arisi, D.; Mascaro, L.; Pinardi, C.; Rossi, A.; Gasparotti, R.; Fazzi, E.; Galli, J. Action Observation Treatment Improves Upper Limb Motor Functions in Children with Cerebral Palsy: A Combined Clinical and Brain Imaging Study. Neural Plast. 2018, 2018, 4843985. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, E.; Pearse, J.; James, P.; Basu, A. Effect of Parent-Delivered Action Observation Therapy on Upper Limb Function in Unilateral Cerebral Palsy: A Randomized Controlled Trial. Dev. Med. Child. Neurol. 2016, 58, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (Clin. Res. Ed.) 2021, 372, 71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ (Clin. Res. Ed.) 2019, 366, l4898. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane Training: London, UK, 2022. [Google Scholar]
- Hedges, L.V. Estimation of Effect Size from a Series of Independent Experiments. Psychol. Bull. 1982, 92, 490–499. [Google Scholar] [CrossRef]
- Cohen, J. The Statistical Power of Abnormal-Social Psychological Research: A Review. J. Abnorm. Soc. Psychol. 1962, 65, 145–153. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the Metafor Package. J. Stat. Soft. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Baig, M.O.; Piracha, S. Effect of Action Observation Therapy In Spastic Kinds Of Cerebral Palsy. J. Riphah Coll. Rehabil. Sci. 2018, 6, 84–89. [Google Scholar]
- Jeong, Y.A.; Lee, B.H. Effect of Action Observation Training on Spasticity, Gross Motor Function, and Balance in Children with Diplegia Cerebral Palsy. Children 2020, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Chung, E.J.; Chun, H.L.; Lee, B.H. Effects of Whole-Body Vibration Combined with Action Observation on Gross Motor Function, Balance, and Gait in Children with Spastic Cerebral Palsy: A Preliminary Study. J. Exerc. Rehabil. 2020, 16, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Mahasup, P.; Sritipsukho, P. Effects of Mirror Neurons Stimulation on Motor Skill Rehabilitation in Children with Cerebral Palsy: A Clinical Trial. J. Med. Assoc. Thail. 2012, 95 (Suppl. 1), S166–S172. [Google Scholar]
- Quadrelli, E.; Anzani, A.; Ferri, M.; Bolognini, N.; Maravita, A.; Zambonin, F.; Turati, C. Electrophysiological Correlates of Action Observation Treatment in Children with Cerebral Palsy: A Pilot Study. Dev. Neurobiol. 2019, 79, 934–948. [Google Scholar] [CrossRef]
- Simon-Martinez, C.; Mailleux, L.; Hoskens, J.; Ortibus, E.; Jaspers, E.; Wenderoth, N. Randomized Controlled Trial Combining Constraint-Induced Movement Therapy and Action-Observation Training in Unilateral Cerebral Palsy: Clinical Effects and Influencing Factors of Treatment Response. Ther. Adv. Neurol. Disord. 2020, 13, 1–19. [Google Scholar] [CrossRef]
- Wei, Y.-M.; Jiang, Z.-M.; Tang, J.-H.; Du, J.-Y.; Li, X.-M.; Wang, Y.-N.; Li, M.-Q. Effect of Action Observation Therapy on Upper Limb Function in Children with Spastic Hemi-Plegic Cerebral Palsy. Chin. J. Rehabil. Theory Pract. 2018, 24, 432–436. [Google Scholar] [CrossRef]
- Abdelfattah, H.E.; ElHadidy, E.I.; Al-Nemr, A.F. Effect of Action Observation Physical Training on Quality of Upper Limb and Functional Independence in Children with Hemiplegia. Egypt. J. Hosp. Med. 2023, 93, 6908–6913. [Google Scholar] [CrossRef]
- Iswarya, S.; Jagatheesan, A.; Senthil Kumar, N.; Shruthi, J. Effect of Action Observation Training and Bimanual Arm Training on Hand Function for Children with Hemiparetic Cerebral Palsy. IJPOT 2024, 18, 254–260. [Google Scholar] [CrossRef]
- Beani, E.; Menici, V.; Sicola, E.; Ferrari, A.; Feys, H.; Klingels, K.; Mailleux, L.; Boyd, R.; Cioni, G.; Sgandurra, G. Effectiveness of the Home-Based Training Program Tele-UPCAT (Tele-Monitored UPper Limb Children Action Observation Training) in Unilateral Cerebral Palsy: A Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2023, 59, 554–563. [Google Scholar] [CrossRef]
- Eliasson, A.-C.; Krumlinde-Sundholm, L.; Rösblad, B.; Beckung, E.; Arner, M.; Ohrvall, A.-M.; Rosenbaum, P. The Manual Ability Classification System (MACS) for Children with Cerebral Palsy: Scale Development and Evidence of Validity and Reliability. Dev. Med. Child. Neurol. 2006, 48, 549–554. [Google Scholar] [CrossRef] [PubMed]
- House, J.H.; Gwathmey, F.W.; Fidler, M.O. A Dynamic Approach to the Thumb-in Palm Deformity in Cerebral Palsy. J. Bone Jt. Surg. Am. 1981, 63, 216–225. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Smith, M.B. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Bodkin, A.W.; Robinson, C.; Perales, F.P. Reliability and Validity of the Gross Motor Function Classification System for Cerebral Palsy. Pediatr. Phys. Ther. 2003, 15, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Simon-Martinez, C.; Mailleux, L.; Ortibus, E.; Fehrenbach, A.; Sgandurra, G.; Cioni, G.; Desloovere, K.; Wenderoth, N.; Demaerel, P.; Sunaert, S.; et al. Combining Constraint-Induced Movement Therapy and Action-Observation Training in Children with Unilateral Cerebral Palsy: A Randomized Controlled Trial. BMC Pediatr. 2018, 18, 250. [Google Scholar] [CrossRef]
- Simon-Martinez, C.; Mailleux, L.; Jaspers, E.; Ortibus, E.; Desloovere, K.; Klingels, K.; Feys, H. Effects of Combining Constraint-Induced Movement Therapy and Action-Observation Training on Upper Limb Kinematics in Children with Unilateral Cerebral Palsy: A Randomized Controlled Trial. Sci. Rep. 2020, 10, 10421. [Google Scholar] [CrossRef]
- Kim, D.H. Comparison of Short- and Long-Time Action Observation Training (AOT) on Upper Limb Function in Children with Cerebral Palsy. Physiother. Pract. Res. 2020, 41, 53–58. [Google Scholar] [CrossRef]
- Sgandurra, G.; Cecchi, F.; Beani, E.; Mannari, I.; Maselli, M.; Falotico, F.P.; Inguaggiato, E.; Perazza, S.; Sicola, E.; Feys, H.; et al. Tele-UPCAT: Study Protocol of a Randomised Controlled Trial of a Home-Based Tele-Monitored UPper Limb Children Action Observation Training for Participants with Unilateral Cerebral Palsy. BMJ Open 2018, 8, e017819. [Google Scholar] [CrossRef]
- Sgandurra, G.; Ferrari, A.; Cossu, G.; Guzzetta, A.; Biagi, L.; Tosetti, M.; Fogassi, L.; Cioni, G. Upper Limb Children Action-Observation Training (UP-CAT): A Randomised Controlled Trial in Hemiplegic Cerebral Palsy. BMC Neurol. 2011, 11, 80. [Google Scholar] [CrossRef]
- Ertelt, D.; Small, S.; Solodkin, A.; Dettmers, C.; McNamara, A.; Binkofski, F.; Buccino, G. Action Observation Has a Positive Impact on Rehabilitation of Motor Deficits after Stroke. Neuroimage 2007, 36, T164–T173. [Google Scholar] [CrossRef]
- Franceschini, M.; Ceravolo, M.G.; Agosti, M.; Cavallini, P.; Bonassi, S.; Dall’Armi, V. Clinical Relevance of Action Observation in Upper-Limb Stroke Rehabilitation: A Possible Role in Recovery of Functional Dexterity. A Randomized Clinical Trial. Neurorehabil. Neural Repair 2012, 26, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Dinomais, M.; Chinier, E.; Lignon, G.; Richard, I.; Minassian, A.T.; Tich, S.N.T. The Effect of Video-Guidance on Passive Movement in Patients with Cerebral Palsy: fMRI Study. Res. Dev. Disabil. 2013, 34, 3487–3496. [Google Scholar] [CrossRef] [PubMed]
- Errante, A.; Cesare, G.; Pinardi, C.; Fasano, F.; Sghedoni, S.; Costi, S. Mirror Neuron System Activation in Children with Unilateral Cerebral Palsy During Observation of Actions Performed by a Pathological Model. Neurorehabil. Neural Repair 2019, 33, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.P. Early Intervention after Perinatal Stroke: Opportunities and Challenges. Dev. Med. Child. Neurol. 2014, 56, 516–521. [Google Scholar] [CrossRef]
- Bazzini, M.C.; Nuara, A.; Scalona, E.; Marco, D.; Rizzolatti, G.; Avanzini, P. The Proactive Synergy Between Action Observation and Execution in the Acquisition of New Motor Skills. Front. Hum. Neurosci. 2022, 16, 793849. [Google Scholar] [CrossRef]
- Riddell, M.; Kuo, H.C.; Zewdie, E.; Kirton, A. Mirror Movements in Children with Unilateral Cerebral Palsy Due to Perinatal Stroke: Clinical Correlates of Plasticity Reorganization. Dev. Med. Child. Neurol. 2019, 61, 943–949. [Google Scholar] [CrossRef]
- Bae, S.-Y.; Jung, N.-H. A Systematic Review of Action Observation Therapy Intervention Program for Children with Cerebral Palsy. J. Korean Soc. Occup. Ther. 2020, 28, 85–98. [Google Scholar] [CrossRef]
- Sakzewski, L.; Ziviani, J.; Boyd, R.N. Efficacy of Upper Limb Therapies for Unilateral Cerebral Palsy: A Meta-Analysis. Pediatrics 2014, 133, e175–e204. [Google Scholar] [CrossRef]
- Oliva-Sierra, M.; Ríos-León, M.; Abuín-Porras, V.; Martín-Casas, P. Effectiveness of Mirror Therapy and Action Observation Therapy in Infantile Cerebral Palsy: A Systematic Review. An. Sist. Sanit. Navar. 2022, 45, e1003. [Google Scholar] [CrossRef]
- Abdelhaleem, N.; Taher, S.; Mahmoud, M.; Hendawy, A.; Hamed, M.; Mortada, H.; Magdy, A.; El-Din, M.R.E.; Zoukiem, I.; Elshennawy, S. Effect of Action Observation Therapy on Motor Function in Children with Cerebral Palsy: A Systematic Review of Randomized Controlled Trials with Meta-Analysis. Clin. Rehabil. 2021, 35, 51–63. [Google Scholar] [CrossRef]
- King, G.; Chiarello, L.A.; Ideishi, R.; D’Arrigo, R.; Smart, E.; Ziviani, J.; Pinto, M. The Nature, Value, and Experience of Engagement in Pediatric Rehabilitation: Perspectives of Youth, Caregivers, and Service Providers. Dev. Neurorehabil. 2020, 23, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.; Micheletti, S.; Pagani, F.; Garofalo, G.; Galli, J.; Rossi, A.; Fazzi, E.; Buccino, G. Action Observation Treatment in a Tele-Rehabilitation Setting: A Pilot Study in Children with Cerebral Palsy. Disabil. Rehabil. 2022, 44, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Langan, D.; Higgins, J.P.T.; Jackson, D.; Bowden, J.; Veroniki, A.A.; Kontopantelis, E.; Viechtbauer, W.; Simmonds, M. A Comparison of Heterogeneity Variance Estimators in Simulated Random-Effects Meta-Analyses. Res. Synth. Methods 2019, 10, 83–98. [Google Scholar] [CrossRef] [PubMed]

| Study | Population | Groups | Outcome Measures | Results | |||
|---|---|---|---|---|---|---|---|
| Narrative | Effect Direction | ||||||
| Abdelfattah et al. 2023 [40] Parallel RCT | UCP, spastic 6–9 years M (63%)/F (37%) Hand function: MACS 2–3 Spasticity: MAS 1+ or 2 Gross motor function: GMFCS 2–3 Cognitive state: NI | Exp group (n = 15): Video AOT + Task execution (repeated practice) + Conventional physical therapy; 7.46 ± 1.06 years; M (40%)/F (60%). Cont group (n = 15): Conventional physical therapy; 7.2 ± 0.92 years; M (33%)/F (67%). | Upper limb function dissociated movement: QUEST—dissociated movement domain | Experimental group presented a greater improvement than the control group MD (Exp − Cont) = 7.3, t = 4.25, p = 0.001 | AOT + Task execution + Conv. phys. ther | > | Conv. phys. ther |
| Upper limb function grasp ability: QUEST—grasp domain | Experimental group presented a greater improvement than the control group MD (Exp − Cont) = 24.64, t = 22.82, p = 0.001 | AOT + Task execution + Conv. phys. ther | > | Conv. phys. ther | |||
| Upper limb function weight bearing: QUEST − weight-bearing domain | Experimental group presented a greater improvement than the control group MD (Exp − Cont) = 4.73, t = 7.11, p = 0.001 | AOT + Task execution + Conv. phys. ther | > | Conv. phys. ther | |||
| Upper limb function protective extension: QUEST—protective extension domain | Experimental group presented a greater improvement than the control group MD (Exp − Cont) = 2.35, t = 3.53, p = 0.001 | AOT + Task execution + Conv. phys. ther | > | Conv. phys. ther | |||
| Upper limb function: QUEST total score | Experimental group presented a greater improvement than the control group MD (Exp − Cont) = 9.62, t = 11.63, p = 0.001 | AOT + Task execution + Conv. phys. ther | > | Conv. phys. ther | |||
| Baig and Piracha 2018 [33] Parallel RCT | UCP or BCP, spastic 8.36 ± 3.5 years (5–15) M (77%)/F (23%) Hand function: NI Spasticity: MAS 1–2 Gross motor function: NI Cognitive state: NI | Exp group (n = 11): Video AOT + Task execution + Conventional physical therapy; 9 ± 3.77 years; M (90.9%)/F (9.1%). Cont group (n = 11): Conventional physical therapy; 7.73 ± 3.26 years; M (63.6%)/F (36.4%). | Unimanual dexterity with dominant hand: BBT | Between-group comparisons not conducted | NI | ||
| Unimanual dexterity with non-dominant hand: BBT | Between-group comparisons not conducted | NI | |||||
| Manual function during daily activities: ABILHAND-Kids | Between-group comparisons not conducted | NI | |||||
| Beani et al., 2023 [42] Parallel RCT | UCP, spastic 7–18 years M (53%)/F (47%) Hand function: HFCS ≥ 2 Spasticity: NI Gross motor function: NI Cognitive state: IQ ≥ 70 | Exp group (n = 15): Video AOT + Task execution; 11.45 ± 3.70 years; M (53.3%)/F (46.6%). Cont group (n = 15): Conventional physical therapy; 11.77 ± 3.53 years; M (53.3%)/F (46.6%). | Spontaneous use of assisting hand: AHA | Between-group comparisons not conducted | NI | ||
| Unimanual dexterity with dominant hand: BBT | Between-group comparisons not conducted | NI | |||||
| Unimanual dexterity with non-dominant hand: BBT | Between-group comparisons not conducted | NI | |||||
| Unilateral upper limb function: MA2 (ROM) | Between-group comparisons not conducted | NI | |||||
| Unilateral upper limb function: MA2 (Acc) | Between-group comparisons not conducted | NI | |||||
| Unilateral upper limb function: MA2 (Flu) | Between-group comparisons not conducted | NI | |||||
| Unilateral upper limb function: MA2 (Dex) | Between-group comparisons not conducted | NI | |||||
| Buccino et al., 2012 [17] Parallel RCT | UCP or BCP, spastic 7.93 ± 1.83 years (6–11) M (60%)/F (40%) Hand function: NI Spasticity: NI Gross motor function: NI Cognitive state: 90.7 ± 15.1 IQ (IQ > 70) | Exp group (n = 8): Video AOT + Task execution + Conventional rehabilitation; 7.5 mdn years; M (50%)/F (50%) Cont group (n = 7): No motor content observation (video) + Task execution + Conventional rehabilitation; 8 mdn years; M (71.4%)/F (28.6%) | Unilateral upper limb function: MUUL | Experimental group presented a greater improvement than the control group t13 (ΔExp − ΔCont) = 2.518, p = 0.026. | AOT + Task execution + Conv.rehab | > | Placebo Obs. + Task execution + Conv.rehab |
| Buccino et al., 2018 [21] Parallel RCT | UCP or BCP, spastic 7.44 ± 1.98 years (5–11 years) M (50%)/F (50%) Hand function: MACS ≤ 4 Spasticity: NI Gross motor function: NI Cognitive state: 89.8 ± 12.7 IQ (IQ > 70) | Exp group (n = 11): Video AOT + Task execution + Conventional rehabilitation; 8.23 ± 2.3 years; M (45.5%)/F (54.5%) Cont group (n = 7): No motor content observation (video) + Task execution + Conventional rehabilitation; 7.63 ± 1.47 years; M (57.14%)/F (42.86%) | Spontaneous use of assisting hand: AHA | No observable differences were detected between groups after the intervention (indicated in Figure 2) | AOT + Task execution + Conv.rehab | ≈ | Placebo Obs. + Task execution + Conv.rehab |
| Unilateral upper limb function: MUUL | No observable differences were detected between groups after the intervention (indicated in Figure 2) | AOT + Task execution + Conv.rehab | ≈ | Placebo Obs. + Task execution + Conv.rehab | |||
| Iswarya et al., 2024 [41] Parallel RCT | UCP, spastic 6–9 years NI for sex Hand function: MACS ≤ 2 Spasticity: NI Gross motor function: NI Cognitive state: MMSE ≥ 24 | Exp group (n = 13): AOT + Task execution; NI for years; NI for sex Cont group (n = 12): Bimanual arm training; NI for years; NI for sex | Unimanual dexterity with dominant hand: BBT | Between-group comparisons not conducted | NI | ||
| Hand sensorimotor function: Fugl Meyer | Between-group comparisons not conducted | NI | |||||
| Jeong and Lee 2020 [34] Parallel RCT | BCP, spastic 5–11 years M (44%)/F (56%) Hand function: MACS ≤ 4 Spasticity: MAS ≤ 1+ Gross motor function: GMFCS 1–3 Cognitive state: NI | Exp group (n = 9): Video AOT + Task execution (Repeated practice); 7.44 ± 1.88 years; M (33.3%)/F (66.7%) Cont group (n = 9): Conventional physical therapy; 6.90 ± 1.79 years; M (55%)/F (45%) | Gross motor function in sitting: GMFM-88 Domain B | No observable differences were detected between groups after the intervention t (ΔExp − ΔCont) = 1.99, p = 0.064 | AOT + Task execution | ≈ | Conventional phys. therapy |
| Gross motor function in crawling and kneeling: GMFM-88 Domain C | No observable differences were detected between groups after the intervention t (ΔExp − ΔCont) = 1.74, p = 0.102 | AOT + Task execution | ≈ | Conventional phys. therapy | |||
| Gross motor function in standing: GMFM-88 Domain D | No observable differences were detected between groups after the intervention t (ΔExp − ΔCont) = 1.93, p = 0.072 | AOT + Task execution | ≈ | Conventional phys. therapy | |||
| Gross motor function in walking, running, and jumping: GMFM-88 Domain E | Experimental group presented a greater improvement than the control group t (ΔExp − ΔCont) = 3.58, p = 0.002 | AOT + Task execution | > | Conventional phys. therapy | |||
| Balance: PRT frontal-right | Experimental group presented a greater improvement than the control group t (ΔExp − ΔCont) = 2.33, p = 0.033 | AOT + Task execution | > | Conventional phys. therapy | |||
| Balance: PRT frontal-left | Experimental group presented a greater improvement than the control group t (ΔExp − ΔCont) = 3.55, p = 0.003 | AOT + Task execution | > | Conventional phys. therapy | |||
| Balance: PRT lateral-right | Experimental group presented a greater improvement than the control group t (ΔExp − ΔCont) = 2.15, p = 0.047 | AOT + Task execution | > | Conventional phys. therapy | |||
| Balance: PRT lateral-left | Experimental group presented a greater improvement than the control group t (ΔExp − ΔCont) = 2.34, p = 0.033 | AOT + Task execution | > | Conventional phys. therapy | |||
| Jung et al., 2020 [35] Parallel RCT | UCP or BCP, spastic. 4–12 years M (43%)/F (57%) Hand function: NI Spasticity: MAS ≤ 2 Gross motor function: GMFCS: 1–3 Cognitive state: NI | Exp group (n = 7): Video AOT + Task execution + Whole body vibration + Conventional physical therapy; 9.00 ± 3.26 years; M (43%)/F (57%) Cont group (n = 7): Task execution + Whole body vibration + Conventional physical therapy; 8.71 ± 3.19 years; M (43%)/F (57%) | Gross motor function in standing: GMFM-66 Domain D | No observable differences were detected between groups after the intervention t (Exp − Cont) = 0.54, p = 0.599 | AOT + Task execution + Body Vibration + Conv. Phys. ther | ≈ | Task execution + Body Vibration + Conv. Phys. ther |
| Gross motor function in walking, running, and jumping: GMFM-66 Domain E | No observable differences were detected between groups after the intervention t (Exp − Cont) = 0.51, p = 0.621 | AOT + Task execution + Body Vibration + Conv. Phys. ther | ≈ | Task execution + Body Vibration + Conv. Phys. ther | |||
| Balance: PBS | No observable differences were detected between groups after the intervention t (Exp − Cont) = 0.78, p = 0.449 | AOT + Task execution + Body Vibration + Conv. Phys. ther | ≈ | Task execution + Body Vibration + Conv. Phys. ther | |||
| Balance: PRT | No observable differences were detected between groups after the intervention t (Exp − Cont) = 0.71, p = 0.494 | AOT + Task execution + Body Vibration + Conv. Phys. ther | ≈ | Task execution + Body Vibration + Conv. Phys. ther | |||
| Function in sit-to-stand and walking tasks: TUG | No observable differences were detected between groups after the intervention t (Exp − Cont) = 0.54, p = 0.602 | AOT + Task execution + Body Vibration + Conv. Phys. ther | ≈ | Task execution + Body Vibration + Conv. Phys. ther | |||
| Function in sit-to-stand tasks: FTSTS | No observable differences were detected between groups after the intervention t (Exp − Cont) = −0.62, p = 0.549 | AOT + Task execution + Body Vibration + Conv. Phys. ther | ≈ | Task execution + Body Vibration + Conv. Phys. ther | |||
| Function in walking tasks: 10 mWT | No observable differences were detected between groups after the intervention t (Exp − Cont) = −0.09, p = 0.930 | AOT + Task execution + Body Vibration + Conv. Phys. ther | ≈ | Task execution + Body Vibration + Conv. Phys. ther | |||
| Function in walking tasks: 6 MWT | No observable differences were detected between groups after the intervention t (Exp − Cont) = 0.37, p = 0.721 | AOT + Task execution + Body Vibration + Conv. Phys. ther | ≈ | Task execution + Body Vibration + Conv. Phys. ther | |||
| Function in stair climbing tasks: TUDS | No observable differences were detected between groups after the intervention t (Exp − Cont) = −0.43, p = 0.674 | AOT + Task execution + Body Vibration + Conv. Phys. ther | ≈ | Task execution + Body Vibration + Conv. Phys. ther | |||
| Kirkpatrick et al., 2016 [22] Parallel RCT | UCP, NI of type 3–10 years M (56%)/F (44%) Hand function: NI Spasticity: NI Gross motor function: NI Cognitive state: NI | Exp group (n = 35): Life AOT + Task execution (Repeated practice); 5.17 mdn (IQR 4) years; M (48.6%)/F (51.4%) Cont group (n = 35): Task execution (Repeated practice); 5.33 mdn (IQR 3.33) years; M (62.9%)/F (37.1%) | Spontaneous use of assisting hand: AHA | No observable differences were detected between groups after the intervention Δmean (95% CI): Exp = 2.2 (1.3, 3.1), Cont = 1.6 (0.6, 2.6) | AOT + Task execution | ≈ | Task execution |
| Manual function during daily activities: ABILHAND-Kids | No observable differences were detected between groups after the intervention Δmdn (95% CI): Exp = 0.67 (−1.7, 0.2), Cont = 0.67 (−0.4, 1.4) | AOT + Task execution | ≈ | Task execution | |||
| Unilateral upper limb function: MA2 (ROM) | No observable differences were detected between groups after the intervention Δmdn (95% CI): Exp = 7.4 (4.4, 10.7), Cont = 7.4 (3.7, 11.8) | AOT + Task execution | ≈ | Task execution | |||
| Unilateral upper limb function: MA2 (Acc) | No observable differences were detected between groups after the intervention Δmdn (95% CI): Exp = 4.8 (1.2, 12.0), Cont = 5.9 (5.0, 16.1) | AOT + Task execution | ≈ | Task execution | |||
| Unilateral upper limb function: MA2 (Flu) | No observable differences were detected between groups after the intervention Δmdn (95% CI): Exp = 2.4 (−0.6, 9.5), Cont = 4.8 (2.4, 11.9) | AOT + Task execution | ≈ | Task execution | |||
| Unilateral upper limb function: MA2 (Dex) | No observable differences were detected between groups after the intervention Δmdn (95% CI): Exp = 8.8 (3.1, 18.8), Cont = 0.0 (0.0, 12.5) | AOT + Task execution | ≈ | Task execution | |||
| Mahasup and Sritipsukho 2012 [36] Parallel RCT | BCP, spastic 5.9 ± 2.2 years 2–10 years M (63%)/F (37%) Hand function: NI Spasticity: NI Gross motor function: GMFCS 1–3 Cognitive state: NI | Exp group (n = 15): Video AOT + Task execution; 6.2 ± 2.2 years; M (60%)/F (40%) Cont group (n = 15): Conventional physical therapy; 5.5 ± 2.2 years; M (67%)/F (34%) | Gross motor function in lying and rolling: GMFM-66 Domain A | No relevant differences were detected between groups after the intervention MD adjusted for baseline values (Exp − Cont) = −0.3, 95% CI: −3.4, 2.7. | AOT + Task execution | ≈ | Conv. Phys. Ther |
| Gross motor function in sitting: GMFM-66 Domain B | No relevant differences were detected between groups after the intervention MD adjusted for baseline values (Exp − Cont) = 4.9, 95% CI: −0.6, 10.5. | AOT + Task execution | ≈ | Conv. Phys. Ther | |||
| Gross motor function in crawling and kneeling: GMFM-66 Domain C | No relevant differences were detected between groups after the intervention MD adjusted for baseline values (Exp − Cont) = 3.9, 95% CI: −3.0, 10.8. | AOT + Task execution | ≈ | Conv. Phys. Ther | |||
| Gross motor function in standing: GMFM-66 Domain D | No relevant differences were detected between groups after the intervention MD adjusted for baseline values (Exp − Cont) = −0.3, 95% CI: −10.1, 9.4. | AOT + Task execution | ≈ | Conv. Phys. Ther | |||
| Gross motor function in walking, running, and jumping: GMFM-66 Domain E | No relevant differences were detected between groups after the intervention MD adjusted for baseline values (Exp − Cont) = 2.8, 95% CI: −7.1, 12.8. | AOT + Task execution | ≈ | Conv. Phys. Ther | |||
| Gross motor function across several domains: GMFM-66 total score | No relevant differences were detected between groups after the intervention MD adjusted for baseline values (Exp − Cont) = 2.1, 95% CI: −2.3, 6.5. | AOT + Task execution | ≈ | Conv. Phys. Ther | |||
| Quadrelli et al., 2019 [37] Cross-over RCT | UCP or BCP, spastic 7.25 ± 3.8 years (4–14 years) M (75%)/F (25%) Manual function: MACS ≤ 4 Spasticity: NI Gross motor function: GMFCS 1–4 Cognitive state: 88.3 ± 14 IQ (IQ > 70) | Cross-over group 1 (n = 4): Video AOT + Task execution (Exp)—No motor content observation (videogame) + Task execution (Cont) Cross-over group 2 (n = 4): No motor content observation (videogame) + Task execution (Cont) − Video AOT + Task execution (Exp) | Spontaneous use of assisting hand: AHA | No relevant differences were detected between interventions after the treatments U = 4.50, p = 0.38 (AOT-VOT: M = 67.30, SD = 6.34; VOT-AOT: M = 60.50, SD = 11.90) | AOT + Task execution | ≈ | Placebo Obs. + Task execution |
| Unilateral upper limb function (more-affected limb): MUUL | No relevant differences were detected between interventions after the treatments U = 5.00, p = 0.49 (AOT-VOT: M = 76.10, SD = 13.85; VOT-AOT: M = 68.70, SD = 18.90) | AOT + Task execution | ≈ | Placebo Obs. + Task execution | |||
| Unilateral upper limb function (less-affected limb): MUUL | No relevant differences were detected between interventions after the treatments U = 5.00, p = 0.41 (AOT-VOT: M = 95.30, SD = 5.70; VOT-AOT: M = 99.00, SD = 2.05) | AOT + Task execution | ≈ | Placebo Obs. + Task execution | |||
| Sgandurra et al., 2013 [18] Parallel RCT | UCP, spastic 5–15 years M (67%)/F (33%) Manual function: HFCS 4–8 Spasticity: MAS ≤ 2 Gross motor function: NI Cognitive state: “within normal limits” | Exp group (n = 12): Video AOT + Task execution (Repeated practice); 9.48 ± 2.12 years; M (66.7%)/F (33.3%) Cont group (n = 12): No motor content observation (videogame) + Task execution (Repeated practice); 9.94 ± 2.77 years; M (66.7%)/F (33.3%) | Spontaneous use of assisting hand: AHA | Experimental group presented a greater improvement than the control group Mann–Whitney U test (ΔExp − ΔCont) p = 0.033 | AOT + Task execution | > | Placebo Obs. + Task execution |
| Manual function during daily activities: ABILHAND-Kids | No relevant differences were detected between groups after the interventions Mann–Whitney U test (ΔExp − ΔCont) p = 0.15 | AOT + Task execution | ≈ | Placebo Obs. + Task execution | |||
| Unilateral upper limb function with more-affected limb: MUUL | No relevant differences were detected between groups after the interventions Mann–Whitney U test (ΔExp − ΔCont) p = 0.93 | AOT + Task execution | ≈ | Placebo Obs. + Task execution | |||
| Simon-Martinez et al., 2020 [38] Parallel RCT Information extracted from Simon-Martinez et al., 2018 [47], and Simon-Martinez et al., 2020 [48] | UCP, NI of type 9.5 ± 1.83 years (6–12 years) M (61%)/F (39%) Manual function: HFCS 4–8, MACS: ≤ 3 Spasticity: MAS (mean) 4.6 and 5.05 Gross motor function: NI Cognitive state: NI | Exp group (n = 22): Video AOT + Task execution (Repeated practice) + mCIMT; 9.3 ± 1.92 years; M (68%)/F (32%) Cont group (n = 22): No motor content observation (videogame) + Task execution (Repeated practice) + mCIMT; 9.3 ± 1.83 years; M (55%)/F (45%) | Unimanual dexterity: JTHF | Between-group comparisons not conducted | NI | ||
| Unimanual dexterity: TPT large pegs | Between-group comparisons not conducted | NI | |||||
| Unimanual dexterity: TPT medium pegs | Between-group comparisons not conducted | NI | |||||
| Unimanual dexterity: TPT small pegs | Between-group comparisons not conducted | NI | |||||
| Bimanual dexterity: TPT large pegs when more-affected towards less-affected hand and vice versa | Between-group comparisons not conducted | NI | |||||
| Spontaneous use of assisting hand: AHA | Between-group comparisons not conducted | NI | |||||
| Manual function during daily activities: ABILHAND-Kids | Between-group comparisons not conducted | NI | |||||
| Strength, Hand grip strength: Dynamometer | Between-group comparisons not conducted | NI | |||||
| Unilateral upper limb function: MA2 (ROM, Acc, Flu, Dex) | Between-group comparisons not conducted | NI | |||||
| Strength, Upper limb (9 muscle groups, each with 0–8 points): MMT (0–45 points) | Between-group comparisons not conducted | NI | |||||
| Wei et al., 2018 [39] Parallel RCT | UCP, spastic 5–12 years M (44%)/F (56%) Hand function: MACS ≤ 3 Spasticity: MAS ≤ 3 Gross motor function: GMFCS 1–2 Cognitive state: NI | Exp groups (C and D groups): Video AOT + Task execution + Conventional rehabilitation for 20 and 30 min, respectively. Group C (n = 10): 6.17 ± 1.34 years; M (50%)/F (50%). Group D (n = 11): 6.34 ± 1.27 years; M (36.7%)/F (63.3%) Cont groups (A and B groups): No motor content observation (video) + Task execution + Conventional rehabilitation for 20 and 30 min, respectively. A group (n = 11): 6.73 ± 1.33 years; M (45.5%)/F (54.5%) B group (n = 13): 6.56 ± 1.23 years; M (46.2%)/F (53.8%) | Strength, Hand grip strength: Dynamometer (kg) | Group C vs. A (20 min): Experimental group presented a greater improvement than the control group t (C − A) = 2.27, p = 0.035 | AOT (20 min) + Task execution (20 min) + Conv. Phys. rehab | ≈ | Placebo Obs (20 min) + Task execution (20 min) + Conv. Phys. rehab |
| Group D vs. B (30 min): Experimental group presented a greater improvement than the control group t (D – B )= 3.98, p = 0.001 | AOT (30 min) + Task execution (30 min) + Conv. Phys. rehab | ≈ | Placebo Obs (30 min) + Task execution (30 min) + Conv. Phys. rehab | ||||
| Group D vs. C: Longer session AOT group (30 min) presented a greater improvement than the shorter session AOT group (20 min) t (D − C) = 2.18, p = 0.042 | AOT (30 min) + Task execution (30 min) + Conv. Phys. rehab | ≈ | AOT (20 min) + Task execution (20 min) + Conv. Phys. rehab | ||||
| Unilateral upper limb function (more-affected limb): UEFT | Group C vs. A (20 min): Experimental group presented a greater improvement than the control group t (C − A) = 2.31, p = 0.032 | AOT (20 min) + Task execution (20 min) + Conv. Phys. rehab | ≈ | Placebo Obs (20 min) + Task execution (20 min) + Conv. Phys. rehab | |||
| Group D vs. B (30 min): Experimental group presented a greater improvement than the control group t (D − B) = 4.08, p = 0.001 | AOT (30 min) + Task execution (30 min) + Conv. Phys. rehab | ≈ | Placebo Obs (30 min) + Task execution (30 min) + Conv. Phys. rehab | ||||
| Group D vs. C: Longer session AOT group (30 min) presented a greater improvement than the shorter session AOT group (20 min) t (D − C) = 2.18, p = 0.042 | AOT (30 min) + Task execution (30 min) + Conv. Phys. rehab | ≈ | AOT (20 min) + Task execution (20 min) + Conv. Phys. rehab | ||||
| Study | Filmación/Presentación | Dosis | Dosis Adaptation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Perspective and Actor | Parts Perf the Action and Visible Body Parts | N° of Activities | Type of Actions | Session Distribution (Watching Time; Performing Time; Rest Time) | Session Duration (Watching Time; Performing Time; Rest Time) | Frequency | Adapted to Functional Level | Progression | Ludical | |
| Abdelfattah et al. 2023 [40] (part of information extracted from Kim 2020 [49]) | Video AOT Third person: Front, lateral, and back point of view Actor: NI Treatment by health-care professional | Unimanual and bimanual tasks. ULs and face visible (NI if others). | 12 act | Goal-directed actions: Pressing, stacking cups, drinking water, etc. | 3 act/sess. 3 act/videoclip. For each videoclip: 3 min (NI of reps); NI; NI | Sess duration: 60 min Each sess: 30 min; NI; NI | 3 sess/wk for 12 wk. | NI | NI | Yes |
| Baig and Piracha 2018 [33] | Video AOT Perspective: NI Actor: NI Treatment by health-care professional | Unimanual and bimanual tasks. ULs visible (NI if others). | 12 act | Goal-directed actions: Gripping, buttoning, filling a cup of water, etc. | 4 videoclips/sess. 3 act/videoclip. For each videoclip: 9–12 min (NI of reps); NI; NI | Sess duration: 45 min. Each sess: 36–48 min; NI; NI | 3 sess/wk for 8 wk. | NI | NI | Yes |
| Beani et al., 2023 [42] (part information extracted from Sgnadurra 2018 [50]) | Video AOT First person Actor: NI Treatment by parents | Unimanual and bimanual tasks. ULs and face visible (NI if others). | 15 act | Goal-directed actions: Opening a bottle, filling a glass of water, manipulating toys, etc. | 2 videos/act. 3 act/sess. For each videoclip: 3 min (NI of reps); 3 min (NI of reps); NI. | Sess duration: 60 min Each sess: 18 min; 18 min; NI | 5 sess/wk for 3 wk. | Activities selected by research staff based on HFCS level | First 8 sess with only unimanual and following 7 sess with only bimanual. | Yes |
| Buccino et al., 2012, 2018 [17,21] | Video AOT Several perspectives (not specified) Actor: TD child and healthy adult Treatment by health-care professional | Unimanual and bimanual tasks. ULs visible (NI if others). | 15 act | Goal-directed actions: Grasping, writing, eating, opening and closing objects, etc. | 1 act/sess. 3–4 motor segment videos per act. For each motor segment: 3 min (NI of reps); 2 min (NI of reps); NI. | Sess duration: 15–20 min Each sess: 9–12 min; 6–8 min; NI | 5 sess/wk for 3 wk. | NI | Increasing complexity throughout activities. | Yes |
| Iswarya et al., 2024 [41] | Video AOT Perspective: NI Actor: NI Treatment by health-care professional | Unimanual or bimanual tasks. ULs visible (NI if others). | 7 act | Goal-directed actions: Opening and closing a box, folding a towel, drinking juice, etc. | 4 subact videos per act. 2 act/sess. For each motor segment: 3 min (NI of reps); 2 min (NI of reps); NI. | Sess duration min: 60 min Each sess: 24 min; 16 min; NI | 6 sess/wk for 12 wk. | NI | NI | Yes |
| Jeong and Lee 2020 [34] | Video AOT Third person: Frontal and Lateral point of views Actor: Healthy adult Treatment by health-care professional | Both LLs LLs, trunk, and face visible. | 12 act (divided in 4 volumes) | 1st vol: Sitting balance. 2nd vol: Sit-to-stand. 3rd vol: Standing balance. 4th vol: Sideway walking. | 1 vol/sess. Volume repetead 3 times/sess. Same volume for 1 wk. Performed 3 times the following sequence: 5 min (NI of reps); 5 min (NI of reps); NI. | Sess duration: 30 min Each sess: 15 min; 15 min; NI | 3 sess/wk for 6 wk. | NI | Increased complexity in activities throughout volumes. Movement retraining if difficulties seen during performance. | No |
| Jung et al., 2020 [35] | Video AOT Perspective: NI Actor: Healthy adult Treatment by health-care professional | Both LLs LLs visible (NI if others). | 6 act | 1st act: Parallel feet standing position with bent knees. 2nd act: Sit-to-stand over a limited ROM. 3rd act: Standing rotations and shifting weight side to side. 4th act: Split stance with right foot forward, shifting weight forward and backwards. 5th act: Same act as the previous with the left foot forward. 6th act: Similar act as the first one. | 6 act/sess. Each act: 1 min (NI of reps); 3 min (NI of reps); 1 min | Sess duration: 30 min Each sess: 6 min; 18 min; 6 min | 3 sess/wk for 4 wk. | NI | Increased complexity throughout activities. Proceeded to the next activity when able to perform the required action. | No |
| Kirkpatrick et al., 2016 [22] | Life AOT First person Actor: Healthy adult (parents) Treatment by parents | Symetrical and asymmetrical bimanual tasks. ULs visible (only). | 12 act (overall) | Goal-directed actions: Children games, including new games at 6th week. | 1 act/sess. Varying act throughout the week. Repeating the process taking turns: 1 rep (NI of time); 1 rep (NI of time); NI. | Sess duration: 15 min. Each sess: NI; NI; NI. | 5 sess/wk for 3 months. | Patient should try perf the task with the disabled hand. If patient continued struggling or became frustrated, patient should use their less-affected hand and move on with the therapy session. | NI | Yes |
| Mahasup and Sritipsukho 2012 [36] | Video AOT Perspective: NI Actor: TD child Treatment by parents | Both LLs LLs and trunk visible (NI if others). | 4 volumes of videos | 1st vol: Sitting balance. 2nd vol: Sit to stand. 3rd vol: Standing balance. 4th vol: Sideway walking. | 1 vol/sess. Same volume for 2 wk. Each volume: NI; NI; NI. | Sess duration: 30 min. Each sess: NI; NI; NI. | 3 sess/day for 2 months. | NI | Increasing complexity in activities throughout volumes. | No |
| Quadrelli et al., 2019 [37] | Video AOT First person Actor: NI Treatment by health-care professional | Unimanual and bimanual tasks. ULs visible (NI if others). | 15 act | Goal-directed actions: Grasping, pouring water, opening different objects, etc. | N° act/sess: NI. 3 sequences/act. Each act: 1 min (3 sequencies of 20 min) (NI of reps); 2 min (NI of reps); NI | Sess duration: 18 min Each sess: NI; NI; NI | 3 sess/wk for 6 wk. | NI | Increasing complexity throughout the rehabilitation sessions. | Yes |
| Sgandurra et al., 2013 [50] Information extracted from Sgandurra et al., [50] and [51]. | Video AOT First person Actor: NI Treatment by health-care professional | Unimanual and bimanual tasks. ULs visible (only). | 15 act | Goal-directed actions: Pouring water, picking, rolling objects, etc. | 1 act/sess, 3 subact/act. Twice each subact. Each subact: 3 min (≥15 reps); 3 min (NI of reps); Yes (NI of time). | Sess duration: 60 min. Each sess: 18 min (≥90 until completing 18 min); 18 min; NI. | 5 sess/wk (consecutive days) for 3 wk. | HFCS 4–5: Lower difficulty task variations. HFCS 6–8: Higher difficulty task variations. | Increasing complexity throughout the 3 sequential subact, and throughout act. First 8 actions were unimanual and consecutive 7 actions were bimanual. | Yes |
| Simon-Martinez et al., 2020 [38] | Video AOT First person Actor: NI Treatment by health-care professional | Unimanual tasks. Affected UL visible (only). | 15 act | Goal-directed actions with mCIMT: Grasping different objects with varying orientations and realizing. | 1 act/sess. 3 subact/act. Twice each subact Each subact: 3 min (NI of reps); 3 min (NI of reps); NI. | Sess duration: 60 min. Each sess: 18 min; 18 min; NI. | 1 or 2 sess/day (to complete 15). 5 consecutive days 1st wk, 4 consecutive days on the 2nd wk. | HFCS 4: Lower difficulty task variations. More information at Additional File 1 of Simon-Martinez et al., 2018. HFCS 6–8: Higher difficulty task variations. More information at Additional File 2 of Simon-Martinez et al., 2018. | Increasing complexity throughout the 3 sequential subact, and throughout act. | Yes |
| Wei et al., 2018 [39] | Video AOT First person Actor: NI Treatment by health-care professional | Unimanual or bimanual tasks. ULs visible (NI if others). | 60 act | Goal-directed actions: Pinching and placing coins, picking and placing spoons, etc. | 60 act grouped in 58 act video packs according to similar difficulty. 3–4 fragments of video in each action For each video pack: 4 min (NI of reps); 2 min (NI of reps); NI. | C and D groups Sess duration: 20 and 30 min, respectively. Each sess: NI: NI; NI. | 5 sess/wk for 12 wk | MACS I-II: Difficulty-enhancing version of the tasks. MACS III: Tasks easy version. Difficulty varied for range of motion or grip type. | Increasing difficulty through every video and video packs, the first being the easiest and N°60 being the most difficult. Perform the following video pack if achieving independency when perf the action in the video pack. | Yes |
| Comparison | Eligibility for Meta-Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group N° 1 | Group N° 2 | Studies (k) | Study Design | Outcome Measure | Text/Table or Plot (k) | Included in the Meta-Analysis | |||
| Values Extracted | Raw Extraction as Mean and SD (k) | Measurement Units | Effect Size | ||||||
| AOT + Task execution + Another therapy (Phys ther, conv ther, motor learning, or MCIMT) | Placebo observation + Task execution + Another therapy (Phys ther, conv ther, motor learning, or MCIMT). | 6 studies [17,18,21,37,38,39] | Parallel RCT: 5 studies [17,18,21,38,39] | Unilateral upper limb function (more-affected limb): 4 studies [17,18,21,39] | Text/Table: 3 studies [18,21,39] | Calculated from pre and post values: 3 studies [18,21,39] | Yes: 3 studies [18,21,39] | MUUL (0–122 points): Buccino et al. [17,21] MUUL (%): Sgandurra et al. [18] UEFT (0–99 points): Wei et al. [39] | Hedges’ g |
| Text (means) and Plot (SE): 1 study [17] | Calculated from pre and post values: 1 studies [17] | No: 1 study [17] ‡ | |||||||
| Spontaneous use of assisting hand: 3 studies [18,21,38] | Text/Table: 3 studies [18,21,38] | Calculated from pre and post values: 3 studies [18,21,38] | Yes: 2 studies [18,21] No: 1 study [38] ‡ | AHA (logarithmic transformation score): Sgandurra et al. [18] AHA (22–88 points): Buccino et al. [21] AHA (0–100 points): Simon-Martinez et al. [38] | Hedges’ g | ||||
| Manual function during daily activities: 2 studies [18,38] | Text/Table: 2 studies [18,38] | Calculated from pre and post values: 2 studies [18,38] | Yes: 1 study [18] No: 1 study [38] ‡ | ABILHAND-Kids (logarithmic transformation score): Sgandurra et al. [18]; Simon-Martinez et al. [38] | Hedges’ g | ||||
| Grip strength (more-affected limb): 2 studies [38,39] | Text/Table: 2 studies [38,39] | Calculated from pre and post values: 2 studies [38,39] | Yes: 1 study [39] No: 1 study [38] ‡ | Hand grip dynamometer (kg): Simon-Martinez et al. [38]; Wei et al. [39] | MD (kg) | ||||
| No other common outcome measures | |||||||||
| Cross-over RCT: 1 study [37] | |||||||||
| AOT + Task execution | Phys ther | 3 studies [34,36,42] | Parallel RCT: 3 studies [34,36,42] | Gross motor function in standing: 2 studies [34,36] | Text/Table: 2 studies [34,36] | Presented Δ mean and Δ SD: 1 study [34] Calculated from pre and post values: 1 study [36] | Yes: 2 studies [34,36] | GMFM-88 Domain D: Jeong and Lee [34] GMFM-66 Domain D: Mahasup and Sritipsukho [36] | Hedges’ g |
| Gross motor function in walking, running, and jumping: 2 studies [34,36] | Text/Table: 2 studies [34,36] | Presented Δ mean and Δ SD: 1 study [34] Calculated from pre and post values: 1 study [36] | Yes: 2 studies [34,36] | GMFM-88 Domain E: Jeong and Lee [34] GMFM-66 Domain E: Mahasup and Sritipsukho [36] | Hedges’ g | ||||
| No other common outcome measures | |||||||||
| AOT + Task execution + Phys ther | Phys thery | 2 studies [33,40] | Parallel RCT: 2 studies [33,40] | No common outcome measures | |||||
| AOT + Task execution | Task execution | 1 study [22] | |||||||
| AOT + Task execution + Body vibration + Phys ther | Task execution + Body vibration + Phys ther | 1 study [35] | |||||||
| AOT + Task execution | Bimanual arm training | 1 study [41] | |||||||
| Certainty Assessment | Comparison | Effect | Certainty | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome (Studies/Pairwise Comparisons) | Study Designs | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Experimental (n) | Control (n) | Hedges’ g [95% CI] | |
| Unilateral Upper Limb Function (4/5) | RCTs | Not serious (Some Concerns to Low) | Not serious | Not serious | Very serious | Serious | AOT (52) | Placebo (50) | 0.57 [−0.17, 1.30] | Very low (+) |
| Assisting Hand Function (3/3) | RCTs | Not serious (Some Concerns to Low) | Not serious | Not serious | Very serious | Serious | AOT (45) | Placebo (40) | 0.20 [−0.74, 0.14] | Very low (+) |
| Manual Function in Daily Activities (2/2) | RCTs | Not serious (Some Concerns to Low) | Not serious | Not serious | Very serious | Not serious | AOT (34) | Placebo (33) | −0.02 [−3.13, 3.09] | Low (+) (+) |
| Grip Strength (2/3) | RCTs | Not serious (Some Concerns) | Not serious | Not serious | Very serious | Serious | AOT (43) | Placebo (45) | 1.18 [−0.28, 2.63] | Very low (+) |
| Gross motor function. Standing dimension (2/2) | RCTs | Not serious (Some Concerns to Low) | Not serious | Not serious | Very serious | Not serious | AOT + Task execution (24) | Conv. Phys. Therapy (24) | 0.36 [−5.17, 5.90] | Low (+) (+) |
| Gross motor function. Walking, jumping, and running dimensions (2/2) | RCTs | Not serious (Some Concerns to Low) | Not serious | Not serious | Very serious | Not serious | AOT + Task execution (24) | Conv. Phys. Therapy (24) | 0.80 [−8.82, 10.42] | Low (+) (+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fierro-Marrero, J.; Cabrera-López, C.D.; Rodríguez de Rivera-Romero, B.; López-Mejías, A.; Ochandorena-Acha, M.; Lerma-Lara, S.; La Touche, R. Action Observation for Children and Adolescents with Cerebral Palsy: Hope or Hype? A Systematic Review with Meta-Analysis. Children 2025, 12, 810. https://doi.org/10.3390/children12070810
Fierro-Marrero J, Cabrera-López CD, Rodríguez de Rivera-Romero B, López-Mejías A, Ochandorena-Acha M, Lerma-Lara S, La Touche R. Action Observation for Children and Adolescents with Cerebral Palsy: Hope or Hype? A Systematic Review with Meta-Analysis. Children. 2025; 12(7):810. https://doi.org/10.3390/children12070810
Chicago/Turabian StyleFierro-Marrero, José, Carlos Donato Cabrera-López, Borja Rodríguez de Rivera-Romero, Alejandro López-Mejías, Mirari Ochandorena-Acha, Sergio Lerma-Lara, and Roy La Touche. 2025. "Action Observation for Children and Adolescents with Cerebral Palsy: Hope or Hype? A Systematic Review with Meta-Analysis" Children 12, no. 7: 810. https://doi.org/10.3390/children12070810
APA StyleFierro-Marrero, J., Cabrera-López, C. D., Rodríguez de Rivera-Romero, B., López-Mejías, A., Ochandorena-Acha, M., Lerma-Lara, S., & La Touche, R. (2025). Action Observation for Children and Adolescents with Cerebral Palsy: Hope or Hype? A Systematic Review with Meta-Analysis. Children, 12(7), 810. https://doi.org/10.3390/children12070810