Role of Combined Use of Adiponectin and hsCRP in Cardiovascular Risk in Pediatric Neurogenic Bladder
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistics
2.3. Ethical Issues
3. Results
4. Discussion
5. Conclusions
- Increased hsCRP levels may be considered risk factors of CVD in MMC children.
- Altered adiponectin levels in MMC patents appear to be influenced by nutritional status, body composition, and mobility limitations.
- Overweight patients with MMC have the lowest adiponectin levels and the highest hsCRP concentrations when compared to healthy controls.
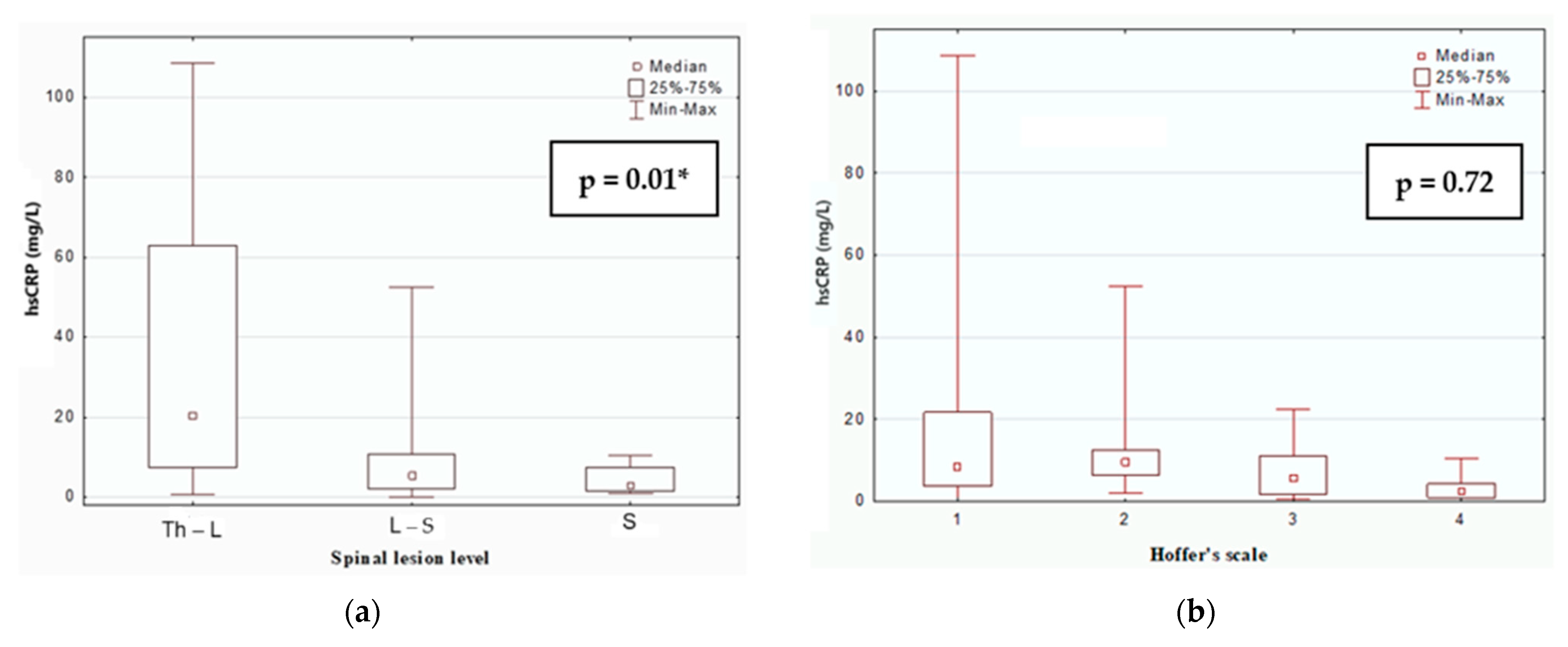
- The risk of CVD in MMC children may correlate with the level of spinal lesion, with higher lesion levels associated with increased cardiovascular risk.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MMC | myelomeningocele |
| NB | neurogenic bladder |
| CVDs | cardiovascular diseases |
| hsCRP | high-sensitivity C-reactive protein |
| BMI | body mass index |
| HDL | high-density lipoprotein |
| LDL | low-density lipoprotein |
| WHO | World Health Organization |
| HS | Hoffer scale |
| CKD | chronic kidney disease |
References
- Perak, A.M.; Benuck, I. Preserving Optimal Cardiovascular Health in Children. Pediatr. Ann. 2018, 47, e479–e486. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Mamaeghani, M.; Mohammadi, S.; Arefhosseini, S.R.; Fallah, P.; Bazi, Z. Adiponectin as a potential marker of vascular disease. Vasc. Health Risk Manag. 2015, 11, 55–70. [Google Scholar] [PubMed]
- Banait, T.; Wanjari, A.; Danade, V.; Banait, S.; Jain, J. Role of High-Sensitivity C-reactive Protein (Hs-CRP) in Non-communicable Diseases: A Review. Cureus 2022, 14, e30225. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Qiu, S.; Yang, G.; Wu, Q. Adiponectin and metabolic cardiovascular diseases: Therapeutic opportunities and challenges. Genes Dis. 2022, 10, 1525–1536. [Google Scholar] [CrossRef]
- Woodward, L.; Akoumianakis, I.; Antoniades, C. Unravelling the adiponectin paradox: Novel roles of adiponectin in the regulation of cardiovascular disease. Br. J. Pharmacol. 2017, 174, 4007–4020. [Google Scholar] [CrossRef]
- Messaaoui, A.; Willems, D.; Mélot, C.; Dorchy, H. Risk markers for cardiovascular disease in young type 1 diabetic patients: Lipoproteins, high-sensitivity C-reactive protein and adiponectin. Acta Clin. Belg. 2012, 67, 79–82. [Google Scholar]
- Koch, V.H.; Lopes, M.; Furusawa, E.; Vaz, K.; Barroso, U. Multidisciplinary management of people with spina bifida across the lifespan. Pediatr. Nephrol. 2024, 39, 681–697. [Google Scholar] [CrossRef]
- Szymanski, K.M.; Adams, C.M.; Alkawaldeh, M.Y.; Austin, P.F.; Bowman, R.M.; Castillo, H.; Castillo, J.; Chu, D.I.; Estrada, C.R., Jr.; Fascelli, M.; et al. Causes of death among people with myelomeningocele: A multi-institutional 47-year retrospective study. J. Pediatr. Rehabil. Med. 2023, 16, 605–619. [Google Scholar] [CrossRef]
- Hoffer, M.M.; Feiwell, E.; Perry, R.; Perry, J.; Bonnettet, C. Functional ambulation in patients with myelomeningocele. J. Bone Jt. Surg. Am. 1973, 55, 137–148. [Google Scholar] [CrossRef]
- Monti, R.; Mariani, F.; Mastricci, R.; Nifosì, F.M.; Palmieri, V.; Manes Gravina, E.; Capriati, M.; Rendeli, C. Spina bifida and cardiorespiratory profile: The impact of leisure sport activities on physical fitness. Child’s Nerv. Syst. 2024, 40, 205–211. [Google Scholar] [CrossRef]
- Leonardi-Figueiredo, M.M.; de Queiroz Davoli, G.B.; Avi, A.E.; Crescêncio, J.C.; Moura-Tonello, S.C.; Manso, P.H.; Júnior, L.G.; Martinez, E.Z.; Catai, A.M.; Mattiello-Sverzut, A.C. Correction: Cardiac Autonomic Modulation of Heart Rate Recovery in Children with Spina Bifida. Int. J. Sports Med. 2021, 42, e10. [Google Scholar] [CrossRef] [PubMed]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Beauloye, V.; Zech, F.; Tran, H.T.; Clapyut, P.; Maes, M.; Brichard, S.M. Determinants of early atherosclerosis in obese children and adolescents. J. Clin. Endocrinol. Metab. 2007, 92, 3025–3032. [Google Scholar] [CrossRef]
- Zhang, M.H.; Spies, C.; Ali, S.; Kanaya, A.M.; Schiller, N.B.; Whooley, M.A. Adiponectin and inducible ischemia in patients with stable coronary heart disease: Data from the Heart and Soul Study. Atherosclerosis 2009, 205, 233–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Korczyńska, J.; Czumaj, A.; Chmielewski, M.; Śledziński, M.; Mika, A.; Śledziński, T. Increased adiponectin gene expression in adipose tissue may be related to an abnormal serum fatty acid profile in patients with chronic kidney disease. Pol. Arch. Intern. Med. 2020, 130, 1013–1016. [Google Scholar] [CrossRef]
- Kazumi, T.; Kawaguchi, A.; Hirano, T.; Yoshino, G. Serum adiponectin is associated with high-density lipoprotein cholesterol, triglycerides, and low-density lipoprotein particle size in young healthy men. Metabolism 2004, 53, 589–593. [Google Scholar] [CrossRef]
- Eslamian, M.; Mohammadinejad, P.; Aryan, Z.; Nakhjavani, M.; Esteghamati, A. Positive Correlation of Serum Adiponectin with Lipid Profile in Patients with Type 2 Diabetes Mellitus is Affected by Metabolic Syndrome Status. Arch. Iran. Med. 2016, 19, 269–274. [Google Scholar]
- Izadi, V.; Farabad, E.; Azadbakht, L. Epidemiologic evidence on serum adiponectin level and lipid profile. Int. J. Prev. Med. 2013, 4, 133–140. [Google Scholar]
- Mohammed, A.G.M.; Gafar, H.S.; Elmalah, A.A.; Elhady, M.; Abd Elgalil, H.M.; Bayoumy, E.S.M. Cardiac Biomarkers and Cardiovascular Outcome in Children with Chronic Kidney Disease. Iran. J. Kidney Dis. 2019, 13, 120–128. [Google Scholar]
- Weaver, D.J.; Mitsnefes, M. Cardiovascular Disease in Children and Adolescents with Chronic Kidney Disease. Semin. Nephrol. 2018, 38, 559–569. [Google Scholar] [CrossRef]
- Goriya, S.; Priya, N. Association of hs-CRP with Serum Creatinine Levels in Chronic Kidney Diseases. Indian J. Clin. Med. 2024, 14, 7–12. [Google Scholar] [CrossRef]
- Sung, B.M.; Oh, D.J.; Choi, M.H.; Choi, H.M. Chronic kidney disease in neurogenic bladder. Nephrology 2018, 23, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Filler, G.; Gharib, M.; Casier, S.; Lödige, P.; Ehrich, J.H.; Dave, S. Prevention of chronic kidney disease in spina bifida. Int. Urol. Nephrol. 2012, 44, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Kupelian, V.; Rosen, R.C.; Roehrborn, C.G.; Tyagi, P.; Chancellor, M.B.; McKinlay, J.B. Association of overactive bladder and C-reactive protein levels. Results from the Boston Area Community Health (BACH) Survey. BJU Int. 2012, 110, 401–407. [Google Scholar] [CrossRef]
- Chung, S.D.; Liu, H.T.; Lin, H.; Kuo, H.C. Elevation of serum c-reactive protein in patients with OAB and IC/BPS implies chronic inflammation in the urinary bladder. Neurourol. Urodyn. 2011, 30, 417–420. [Google Scholar] [CrossRef]
- Ferreira, C.E.; Fonseca, A.M.; Silva, I.D.; Girão, M.J.; Sartori, M.G.; Castro, R.A. The relationship between the Trp 64 Arg polymorphism of the beta 3-adrenoceptor gene and idiopathic overactive bladder. Am. J. Obstet. Gynecol. 2011, 205, 82.e10–82.e14. [Google Scholar] [CrossRef]
- Heikkilä, K.; Ebrahim, S.; Lawlor, D.A. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J. Epidemiol. Community Health 2007, 61, 824–833. [Google Scholar] [CrossRef]
- Hsiao, S.M.; Lin, H.H.; Kuo, H.C. The role of serum C-reactive protein in women with lower urinary tract symptoms. Int. Urogynecol. J. 2012, 23, 935–940. [Google Scholar] [CrossRef]
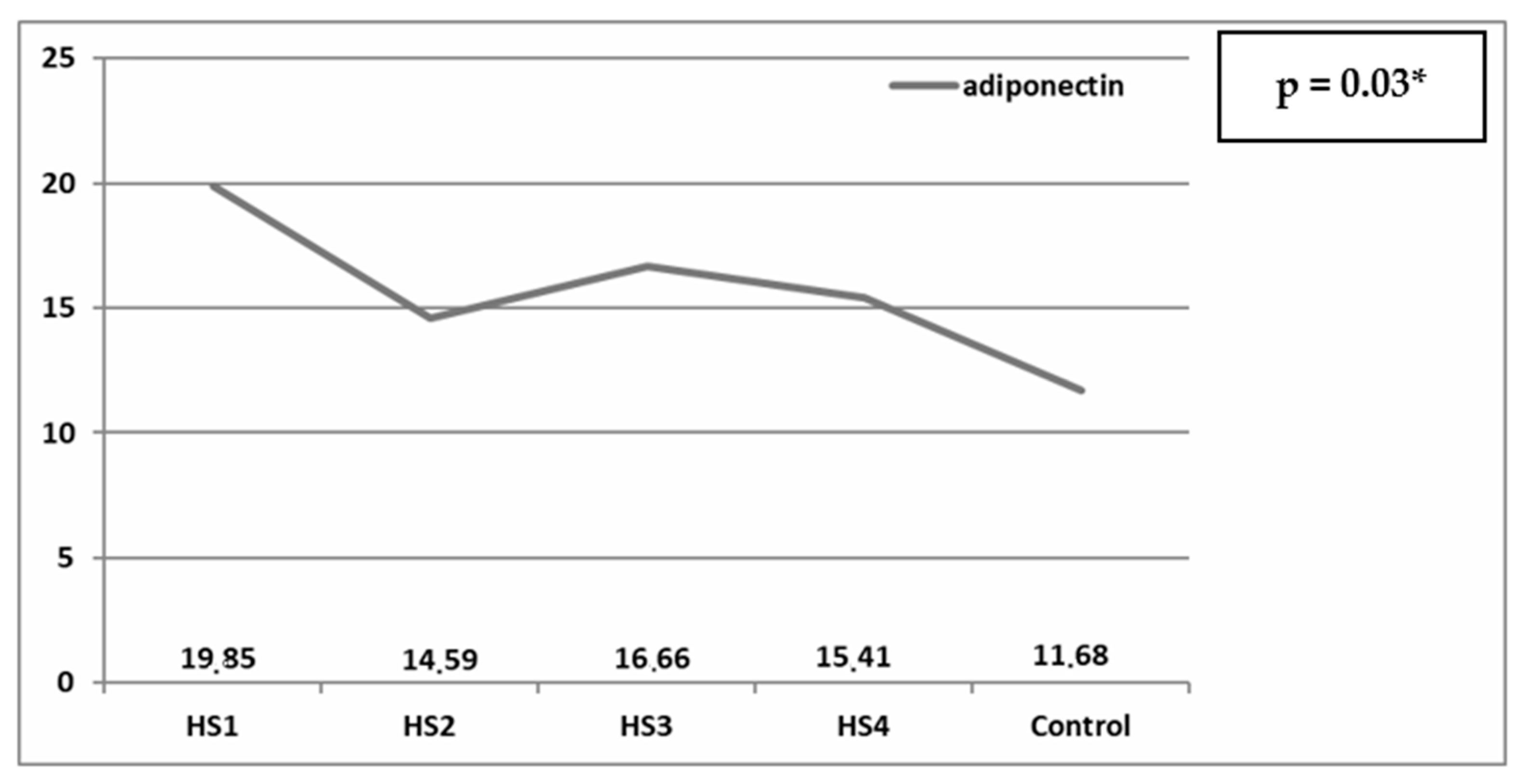
| Score | Name | Criteria |
|---|---|---|
| HS1 | Non-walker | Wheelchair-dependent |
| HS2 | Exercise walker | Walking only in therapeutic situations |
| HS3 | Household walker | Using braces or crutches for indoors and using wheelchair outdoors |
| HS4 | Community walker | Ambulating outdoors with or without braces but using wheelchair |
| Variable | NB Group | Control Group | p Value |
|---|---|---|---|
| Median (minimum–maximum) | |||
| Age (years) | 9.0 (0.75–17.7) | 8.29 (1.42–17.75) | 0.94 |
| Height (cm) | 130 (7–165) | 127 (78–179) | 0.49 |
| Body mass (kg) | 28 (7.4–84.8) | 26.5 (9.15–79) | 0.71 |
| BMI (kg/m2) | 16.89 (10.33–31.5) | 16.4 (13.5–29.4) | 0.99 |
| Systolic blood pressure (mmHg) | 107.45 (85–141) | 93.1 (73–120) | 0.99 |
| Diastolic blood pressure (mmHg) | 67.6 (50–101) | 57.9 (41–70) | 0.52 |
| Triglycerides (mg%) | 87.7 (30–302) | 90.6 (48–243) | 0.98 |
| Total cholesterol (mg%) | 181 (112–229) | 168.5 (106–203) | 0.09 |
| HDL (mg%) | 55 (40–83) | 51.5 (39–64) | 0.89 |
| LDL (mg%) | 102 (67–160) | 100.5 (26–151) | 0.36 |
| Creatinine (mg/dL) | 0.39 (0.15–0.8) | 0.45 (0.18–0.76) | 0.9 |
| Urea (mg/dL) | 25.5 (11–45) | 27 (22–40) | 0.89 |
| Adiponectin (ug/mL) | 16.7 (0.25–30) | 11.4 (5.96–18.09) | 0.001 * |
| hsCRP (mg/L) | 9.09 (0.55–334.47) | 4.07 (0.36–18.21) | 0.035 * |
| Underweight (<3 pc) | Normal (3–85 pc) | Overweight (85–97 pc) | Obese (>97 pc) | Total Number | |
|---|---|---|---|---|---|
| Number of patients (% of group) | |||||
| HS1 | 9 (26.47%) | 13 (38.24%) | 5 (14.7%) | 7 (20.59%) | 34 |
| HS2 | 1 (8.33%) | 6 (50.00%) | 2 (16.67%) | 3 (25.00%) | 12 |
| HS3 | 1 (11.11%) | 5 (55.56%) | 1 (11.11%) | 2 (22.22%) | 9 |
| HS4 | 1 (8.33%) | 9 (75.00%) | 2 (16.67%) | 0 (0.00%) | 12 |
| Total number | 12 (17.91%) | 33 (49.25%) | 10 (14.92%) | 12 (17.91%) | 67 |
| Control group | 0 (0.00%) | 16 (80.00%) | 3 (15.00%) | 1 (5.00%) | 20 |
| Adiponectin | hsCRP | |
|---|---|---|
| Median (minimum–maximum) | ||
| Underweight | 23.94 (19.85–29.9) | 5.09 (0.14–52.6) |
| Normal | 16.56 (8.18–28.85) | 7.41 (0.05–16.97) |
| Overweight | 7.29 (1.3–108.7) | 14.57 (6.58–17.16) |
| Obese | 15.54 (0.25–22.58) | 9.09 (1.3–64.83) |
| CVD Risk | Low | Average | High |
|---|---|---|---|
| Number of patients (%) | 6 (9%) | 15 (22%) | 46 (69%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagińska-Chyży, J.; Szymańska, A.; Korzeniecka-Kozerska, A. Role of Combined Use of Adiponectin and hsCRP in Cardiovascular Risk in Pediatric Neurogenic Bladder. Children 2025, 12, 748. https://doi.org/10.3390/children12060748
Bagińska-Chyży J, Szymańska A, Korzeniecka-Kozerska A. Role of Combined Use of Adiponectin and hsCRP in Cardiovascular Risk in Pediatric Neurogenic Bladder. Children. 2025; 12(6):748. https://doi.org/10.3390/children12060748
Chicago/Turabian StyleBagińska-Chyży, Joanna, Alicja Szymańska, and Agata Korzeniecka-Kozerska. 2025. "Role of Combined Use of Adiponectin and hsCRP in Cardiovascular Risk in Pediatric Neurogenic Bladder" Children 12, no. 6: 748. https://doi.org/10.3390/children12060748
APA StyleBagińska-Chyży, J., Szymańska, A., & Korzeniecka-Kozerska, A. (2025). Role of Combined Use of Adiponectin and hsCRP in Cardiovascular Risk in Pediatric Neurogenic Bladder. Children, 12(6), 748. https://doi.org/10.3390/children12060748





