Orofacial Myofunctional Therapy: Investigating a Novel Therapeutic Approach for Pediatric Obstructive Sleep Apnea in Children with and Without Down Syndrome—A Study Protocol
Abstract
1. Introduction
1.1. Epidemiology and Therapeutic Challenges in Pediatric OSA
1.2. Orofacial Myofunctional Disorders and Therapy in OSA
2. Materials and Methods
2.1. Participants
2.1.1. Non-Syndromic Children
2.1.2. Children with Down Syndrome
2.2. Sample Size
2.3. Design
2.4. ENT Screening
2.5. Demographic and Medical Questionnaire
2.6. Assessment Protocol
2.6.1. Orofacial Myofunctional Assessment
2.6.2. Oromyofunctional Postures, Mobility, and Functions
2.6.3. Tongue and Lip Strength and Endurance
2.7. Sleep Assessment
2.7.1. Screening for Pediatric Sleep Disorders
2.7.2. OSA Severity and Symptoms
2.7.3. Quality of Life Assessment
2.8. Intervention
2.8.1. Oromyofunctional Therapy
2.8.2. Therapy Provider and Treatment Fidelity Checks
2.9. Statistical Analysis
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Exercise 1 Goal: 1,2 Timing NS: 1 w DS: 1–2 w | Smelling and humming | Become aware of the role of the nose by humming and smelling. | 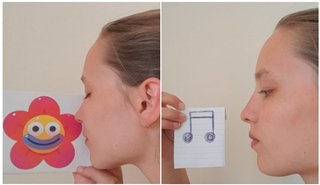 |
| Exercise 1 Goal: 1,2 Timing NS: 2–10 w DS: 4–20 w | Breathing through nose | Breathe in and out through your nose while your mouth is closed. (Gradually increase duration and awareness) | 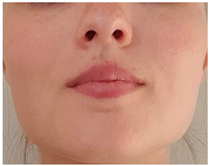 |
| Exercise 3 Goal: 1,2 Timing NS: 1 w DS: 1-2 w | Breathing through one nostril | Close your right nostril and breathe through your left for 1 min. Then, switch: Close your left nostril and breathe through your right for 1 min. (3×) | 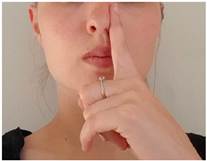 |
| Exercise 4 Goal: 1,2 Timing NS: 2–3 w DS: 4–6 w | Lippo | Put a plastic disk (Lippo) between your lips while performing another activity (e.g., drawing, coloring). (Gradually increase duration) |  |
| Exercise 5 Goal: 1,2 Timing NS: 1–10 w DS: 1–20 w | Button | Put a button, to which a string is attached, behind the lips and pull the string in a forward direction with increasing force for 10 s (15×). | 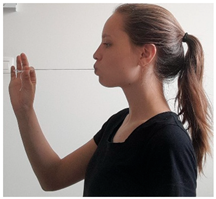 |
| Exercise 6 Goal: 1,2 Timing NS: 3–10 w DS: 6–20 w | Tape | Taping around the lips with stretching kinesiology tape (day and night training). | 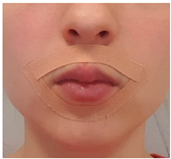 |
| Exercise 7 Goal: 3 Timing NS: 1–2 w DS: 1–4 w | Tongue tapping: alveolar ridge | With your mouth open, tap with the tip of your tongue against the alveolar ridge repeatedly, keeping your mandible fixed. (15×) | 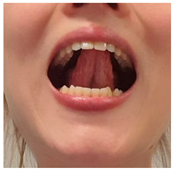 |
| Exercise 8 Goal: 3,4 Timing NS: 3–10 w DS: 6–20 w | Tongue pressure: alveolar ridge | With your mouth open, press the tip of your tongue against the alveolar ridge for 5 s, keeping your mandible fixed. (15×) | |
| Exercise 9 Goal: 3 Timing NS: 1–2 w DS: 1–4 w | Tongue tapping: up/down/left/right | Protrude your tongue to the maximum extension alternately: (1) upwards (2) downwards, (3) to the left, (4) to the right. (15×) | 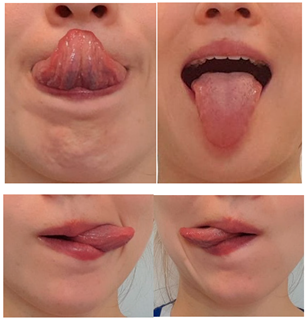 |
| Exercise 10 Goal: 3,4 Timing NS: 3–10 w DS: 6–20 w | Maximum tongue protrusion | Protrude your tongue to the maximum extension and hold it like that for 5 s: (1) upwards, (2) downwards, (3) to the left, (4) to the right (5×). | |
| Exercise 11 Goal: 3–4 Timing NS: 1–3 w DS: 1–6 w | Tongue clicking | With your mouth open, place your tongue tip on the alveolar ridge and suck your tongue against your palate. Quickly move your tongue downwards, making a clicking sound. (30×) | 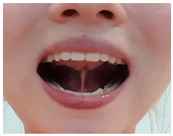 |
| Exercise 12 Goal: 3–4 Timing NS: 3–10 w DS: 5–20 w | Tongue suction | With your mouth open, place your tongue tip on the alveolar ridge and suck your tongue against your palate. Hold it like that for 5 s. (15×) | |
| Exercise 13 Goal: 1–2–3 Timing NS: 5–10 w DS: 6–20 w | Correct tongue posture | Close your mouth. Place your tongue tip on the alveolar ridge and suck your tongue against your palate. Breathe in and out through your nose. (Gradually increase duration and awareness) | 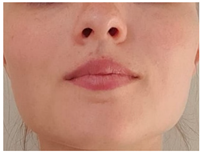 |
| Exercise 13 Goal: 4 Timing NS: 3–10 w DS: 6–20 w | Tongue sliding | With your mouth open, place your tongue tip on the alveolar ridge and slide backward toward the soft palate. Keep your tongue in the posterior position for 5 s, then slide back forward. (5×) | 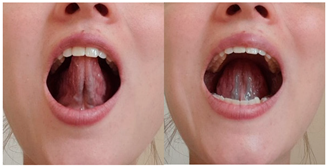 |
| Exercise 14 Goal: 4 Timing NS: 3–10 w DS: 6–20 w | Expiratory effort: straw | Place the straw in your mouth, sealing your lips around it. Blow intensely through the straw for 5 s. (15×) | 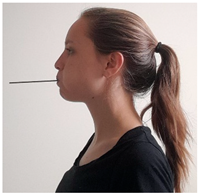 |
| Exercise 15 Goal: 4 Timing NS: 4–5 w DS: 7–8 w | Straw phonation | Place the straw in your mouth, sealing your lips around it. Blow through the straw while producing a sustained /u/ vowel for 5 s. (15×) | |
| Exercise 16 Goal: 4 Timing NS: 6–10 w DS: 9–20 w | Expiratory effort: straw in water | Place the straw in your mouth, sealing your lips around it. Submerge the end of the straw five centimeters into the water. Blow intensely through the straw for 5 s. (15×) |  |
| Exercise 17 Goal: 4 Timing NS: 6–10 w DS: 9–20 w | Straw phonation in water | Place the straw in your mouth, sealing your lips around it. Submerge the end of the straw five centimeters into the water. Blow through the straw while producing a sustained /u/ vowel for 5 s. (15×) | |
| Exercise 18 Goal: 4 Timing NS: 1–2 w DS: 1–5 w | Expiratory effort: balloon | Breath in through your nose, breathe out while blowing the balloon. (15×) If the child is unable to blow a balloon, place the straw in your mouth, sealing your lips around it. Breathe out while blowing the balloon. (15×) |  |
| Exercise 19 Goal: 4 Timing NS: 1–2 w DS: 1–5 w | Expiratory effort: blower ball | Place the blower in your mouth, sealing your lips around it. Blow intensely through the blower for 5 s, the ball goes up. (15×) |  |
References
- DelRosso, L.M. Epidemiology and Diagnosis of Pediatric Obstructive Sleep Apnea. Curr. Probl. Pediatr. Adolesc. Health Care 2016, 46, 2–6. [Google Scholar] [CrossRef] [PubMed]
- von Lukowicz, M.; Herzog, N.; Ruthardt, S.; Quante, M.; Iven, G.; Poets, C.F. Effect of a 1-week intense myofunctional training on obstructive sleep apnoea in children with Down syndrome. Arch. Dis. Child. 2019, 104, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.H.; How, C.H.; Chan, Y.H.; Teoh, O.H. Approach to the snoring child. Singap. Med. J. 2020, 61, 170–175. [Google Scholar] [CrossRef]
- Huang, Y.S.; Guilleminault, C. Pediatric Obstructive Sleep Apnea: Where Do We Stand? Adv. Oto-Rhino-Laryngol. 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Alsufyani, N.; Isaac, A.; Witmans, M.; Major, P.; El-Hakim, H. Predictors of failure of DISE-directed adenotonsillectomy in children with sleep disordered breathing. J. Otolaryngol. Head Neck Surg. 2017, 46, 37. [Google Scholar] [CrossRef]
- Lee, C.F.; Lee, C.H.; Hsueh, W.Y.; Lin, M.T.; Kang, K.T. Prevalence of Obstructive Sleep Apnea in Children With Down Syndrome: A Meta-Analysis. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2018, 14, 867–875. [Google Scholar] [CrossRef]
- Marcus, C.L.; McColley, S.A.; Carroll, J.L.; Loughlin, G.M.; Smith, P.L.; Schwartz, A.R. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J. Appl. Physiol. (1985). 1994, 77, 918–924. [Google Scholar] [CrossRef]
- Olczak-Kowalczyk, D.; Korporowicz, E.; Gozdowski, D.; Lecka-Ambroziak, A.; Szalecki, M. Oral findings in children and adolescents with Prader-Willi syndrome. Clin. Oral Investig. 2019, 23, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.M.; Jensen, E.L.; Simon, S.L.; Friedman, N.R. Correlates of Pediatric CPAP Adherence. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2016, 12, 879–884. [Google Scholar] [CrossRef]
- Guilleminault, C.; Huang, Y.S.; Monteyrol, P.J.; Sato, R.; Quo, S.; Lin, C.H. Critical role of myofascial reeducation in pediatric sleep-disordered breathing. Sleep Med. 2013, 14, 518–525. [Google Scholar] [CrossRef]
- Gounden, M.R.; Chawla, J.K. Management of residual OSA post adenotonsillectomy in children with Down Syndrome: A systematic review*. Int. J. Pediatr. Otorhinolaryngol. 2022, 152, 110966. [Google Scholar] [CrossRef] [PubMed]
- Propst, E.J.; Amin, R.; Talwar, N.; Zaman, M.; Zweerink, A.; Blaser, S.; Zaarour, C.; Luginbuehl, I.; Karsli, C.; Aziza, A.; et al. Midline posterior glossectomy and lingual tonsillectomy in obese and nonobese children with down syndrome: Biomarkers for success. Laryngoscope 2017, 127, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Ulualp, S. Outcomes of Tongue Base Reduction and Lingual Tonsillectomy for Residual Pediatric Obstructive Sleep Apnea after Adenotonsillectomy. Int. Arch. Otorhinolaryngol. 2019, 23, e415–e421. [Google Scholar] [CrossRef]
- Skirko, J.R.; Jensen, E.L.; Friedman, N.R. Lingual tonsillectomy in children with Down syndrome: Is it safe? Int. J. Pediatr. Otorhinolaryngol. 2018, 105, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Guilleminault, C.; Chiu, H.Y.; Sullivan, S.S. Mouth breathing, nasal disuse, and pediatric sleep-disordered breathing. Sleep Breath.=Schlaf Atm. 2015, 19, 1257–1264. [Google Scholar] [CrossRef]
- Katz, E.S.; White, D.P. Genioglossus activity in children with obstructive sleep apnea during wakefulness and sleep onset. Am. J. Respir. Crit. Care Med. 2003, 168, 664–670. [Google Scholar] [CrossRef]
- Fitzpatrick, M.F.; McLean, H.; Urton, A.M.; Tan, A.; O’Donnell, D.; Driver, H.S. Effect of nasal or oral breathing route on upper airway resistance during sleep. Eur. Respir. J. 2003, 22, 827–832. [Google Scholar] [CrossRef]
- Izu, S.C.; Itamoto, C.H.; Pradella-Hallinan, M.; Pizarro, G.U.; Tufik, S.; Pignatari, S.; Fujita, R.R. Obstructive sleep apnea syndrome (OSAS) in mouth breathing children. Braz. J. Otorhinolaryngol. 2010, 76, 552–556. [Google Scholar] [CrossRef]
- Tagaya, M.; Nakata, S.; Yasuma, F.; Miyazaki, S.; Sasaki, F.; Morinaga, M.; Suzuki, K.; Otake, H.; Nakashima, T. Relationship between adenoid size and severity of obstructive sleep apnea in preschool children. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1827–1830. [Google Scholar] [CrossRef]
- Verse, T.; Pirsig, W. Impact of impaired nasal breathing on sleep-disordered breathing. Sleep Breath.=Schlaf Atm. 2003, 7, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Choi, J.H.; Kim, K.W.; Kim, T.H.; Lee, S.H.; Lee, H.M.; Shin, C.; Lee, K.Y.; Lee, S.H. The impacts of open-mouth breathing on upper airway space in obstructive sleep apnea: 3-D MDCT analysis. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. (EUFOS) Affil. Ger. Soc. Oto-Rhino-Laryngol. —Head Neck Surg. 2011, 268, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Douglas, N.J.; White, D.P.; Weil, J.V.; Zwillich, C.W. Effect of breathing route on ventilation and ventilatory drive. Respir. Physiol. 1983, 51, 209–218. [Google Scholar] [CrossRef]
- Haight, J.S.; Djupesland, P.G. Nitric oxide (NO) and obstructive sleep apnea (OSA). Sleep Breath.=Schlaf Atm. 2003, 7, 53–62. [Google Scholar] [CrossRef]
- Evangelisti, M.; Martella, S.; Barreto, M.; Villa, M.P. Tongue strength evaluation in children with and without sleep disordered breathing. Eur. Respir. J. 2017, 50, PA3334. [Google Scholar] [CrossRef]
- Coceani, L. Oral structures and sleep disorders: A literature review. Int. J. Orofac. Myol. Off. Publ. Int. Assoc. Orofac. Myol. 2003, 29, 15–28. [Google Scholar] [CrossRef]
- Remmers, J.E.; deGroot, W.J.; Sauerland, E.K.; Anch, A.M. Pathogenesis of upper airway occlusion during sleep. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 44, 931–938. [Google Scholar] [CrossRef]
- Sumathy, G.; Sathyapriya, B.; Chandrakala, B.; Sinha, P. Obstructive Sleep Apnea and Genioglossus. 2020, Volume 7, pp. 1675–1680. Available online: https://www.academia.edu/71200763/Obstructive_Sleep_Apnea_and_Genioglossus (accessed on 28 February 2025).
- Kanezaki, M.; Ogawa, T.; Izumi, T. Tongue Protrusion Strength in Arousal State Is Predictive of the Airway Patency in Obstructive Sleep Apnea. Tohoku J. Exp. Med. 2015, 236, 241–245. [Google Scholar] [CrossRef]
- Camacho, M.; Certal, V.; Abdullatif, J.; Zaghi, S.; Ruoff, C.M.; Capasso, R.; Kushida, C.A. Myofunctional Therapy to Treat Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. Sleep 2015, 38, 669–675. [Google Scholar] [CrossRef]
- Van Dyck, C.; Dekeyser, A.; Vantricht, E.; Manders, E.; Goeleven, A.; Fieuws, S.; Willems, G. The effect of orofacial myofunctional treatment in children with anterior open bite and tongue dysfunction: A pilot study. Eur. J. Orthod. 2016, 38, 227–234. [Google Scholar] [CrossRef]
- da Silva, A.S.; Bianchini, E.M.G.; Thuler, E.R.; Liu, S.Y.C.; Yui, M.S.; Kayamori, F.; dos Santos Junior, V.; Rabelo, F.A.W. Upper Airway Morphofunctional Changes During Oropharyngeal Exercises for Sleep-Disordered Breathing. Orthod. Craniofacial Res. 2025. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.P.; Brasili, L.; Ferretti, A.; Vitelli, O.; Rabasco, J.; Mazzotta, A.R.; Pietropaoli, N.; Martella, S. Oropharyngeal exercises to reduce symptoms of OSA after AT. Sleep Breath.=Schlaf Atm. 2015, 19, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.P.; Evangelisti, M.; Martella, S.; Barreto, M.; Del Pozzo, M. Can myofunctional therapy increase tongue tone and reduce symptoms in children with sleep-disordered breathing? Sleep Breath.=Schlaf Atm. 2017, 21, 1025–1032. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Kaneshiro, K.; Camacho, M. Effect of myofunctional therapy on children with obstructive sleep apnea: A meta-analysis. Sleep Med. 2020, 75, 210–217. [Google Scholar] [CrossRef]
- Gastelum, E.; Cummins, M.; Singh, A.; Montoya, M.; Urbano, G.L.; Tablizo, M.A. Treatment Considerations for Obstructive Sleep Apnea in Pediatric Down Syndrome. Children 2021, 8, 1074. [Google Scholar] [CrossRef]
- Saccomanno, S.; Martini, C.; D’Alatri, L.; Farina, S.; Grippaudo, C. A specific protocol of myo-functional therapy in children with Down syndrome. A pilot study. Eur. J. Paediatr. Dent. 2018, 19, 243–246. [Google Scholar] [CrossRef]
- Sutherland, K.; Weichard, A.J.; Davey, M.J.; Horne, R.S.C.; Cistulli, P.A.; Nixon, G.M. Craniofacial photography and association with sleep-disordered breathing severity in children. Sleep Breath. 2020, 24, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, P.; Vaisakh, R.; Thomson, S.; Sajna, M.P..; Varsha, V. The hereditary pattern of gummy smile—A cross sectional study. Int. J. Oral Health Dent. 2021, 7, 40–42. [Google Scholar] [CrossRef]
- Katz, S.L.; Blinder, H.; Naik, T.; Barrowman, N.; Narang, I. Does neck circumference predict obstructive sleep apnea in children with obesity? Sleep Med. 2021, 78, 88–93. [Google Scholar] [CrossRef]
- Katz, S.L.; Vaccani, J.P.; Clarke, J.; Hoey, L.; Colley, R.C.; Barrowman, N.J. Creation of a reference dataset of neck sizes in children: Standardizing a potential new tool for prediction of obesity-associated diseases? BMC Pediatr. 2014, 14, 159. [Google Scholar] [CrossRef]
- Robitschek, J.; Dresner, H.; Hilger, P. Utility of a Systematic Approach to Teaching Photographic Nasal Analysis to Otolaryngology Residents. JAMA Facial Plast. Surg. 2017, 19, 459–462. [Google Scholar] [CrossRef]
- Mark, K.; Wax, M. Primary Care Otolaryngology; American Academy of Otolaryngology—Head and Neck Surgery Foundation: Alexandria, VA, USA, 2011; p. 139. [Google Scholar]
- Berg, L.M.; Ankjell, T.K.S.; Sun, Y.Q.; Trovik, T.A.; Sjögren, A.; Rikardsen, O.G.; Moen, K.; Hellem, S.; Bugten, V. Friedman Score in Relation to Compliance and Treatment Response in Nonsevere Obstructive Sleep Apnea. Int. J. Otolaryngol. 2020, 2020, 6459276. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, G.K.; Rokkam, V.R. Macroglossia; StatPearls Publishing: Petersburg, FL, USA, 2023. [Google Scholar]
- Rajain, T.; Tsomu, K.; Saini, N.; Namdev, R. Lingual Frenuloplasty for Ankyloglossia in Children: A Case Series. Contemp. Clin. Dent. 2021, 12, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.V.; Schroeder, J.W.; Gang, Z.; Sheldon, S.H. Mallampati score and pediatric obstructive sleep apnea. J. Clin. Sleep Med. 2014, 10, 985–990. [Google Scholar] [CrossRef]
- Felício, C.M.; Ferreira, C.L. Protocol of orofacial myofunctional evaluation with scores. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 367–375. [Google Scholar] [CrossRef]
- Van Nuffelen, G.; Van den Steen, L.; Vanderveken, O.; Specenier, P.; Van Laer, C.; Van Rompaey, D.; Guns, C.; Mariën, S.; Peeters, M.; Van de Heyning, P.; et al. Study protocol for a randomized controlled trial: Tongue strengthening exercises in head and neck cancer patients, does exercise load matter? Trials 2015, 16, 395. [Google Scholar] [CrossRef] [PubMed]
- Mezzofranco, L.; Agostini, L.; Boutarbouche, A.; Melato, S.; Zalunardo, F.; Franco, A.; Gracco, A. Sleep Habits and Disorders in School-Aged Children: A Cross-Sectional Study Based on Parental Questionnaires. Children 2025, 12, 489. [Google Scholar] [CrossRef]
- Bruni, O.; Ottaviano, S.; Guidetti, V.; Romoli, M.; Innocenzi, M.; Cortesi, F.; Giannotti, F. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J. Sleep Res. 1996, 5, 251–261. [Google Scholar] [CrossRef]
- Primozic, J.; Farcnik, F.; Perinetti, G.; Richmond, S.; Ovsenik, M. The association of tongue posture with the dentoalveolar maxillary and mandibular morphology in Class III malocclusion: A controlled study. Eur. J. Orthod. 2013, 35, 388–393. [Google Scholar] [CrossRef]
- de Felício, C.M.; da Silva Dias, F.V.; Folha, G.A.; de Almeida, L.A.; de Souza, J.F.; Anselmo-Lima, W.T.; Trawitzki, L.V.; Valera, F.C. Orofacial motor functions in pediatric obstructive sleep apnea and implications for myofunctional therapy. Int. J. Pediatr. Otorhinolaryngol. 2016, 90, 5–11. [Google Scholar] [CrossRef]
- Chervin, R.D.; Hedger, K.; Dillon, J.E.; Pituch, K.J. Pediatric sleep questionnaire (PSQ): Validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000, 1, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Kallus, A. Myofunctionele-Oefenordner: Materiaal voor Diagnostiek en Therapie van Myofunctionele Problemen Vanaf Jonge Leeftijd; K2 Publishers: Bodegraven, The Netherlands, 2004. [Google Scholar]
- Verma, R.K.; Johnson, J.J.; Goyal, M.; Banumathy, N.; Goswami, U.; Panda, N.K. Oropharyngeal exercises in the treatment of obstructive sleep apnoea: Our experience. Sleep Breath.=Schlaf Atm. 2016, 20, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, K.C.; Drager, L.F.; Genta, P.R.; Marcondes, B.F.; Lorenzi-Filho, G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2009, 179, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Oh, E.G.; Choi, M.; Choi, S.J.; Joo, E.Y.; Lee, H.; Kim, H.Y. Development and evaluation of myofunctional therapy support program (MTSP) based on self-efficacy theory for patients with obstructive sleep apnea. Sleep Breath.=Schlaf Atm. 2020, 24, 1051–1058. [Google Scholar] [CrossRef]
- Jansonius-Schultheiss, K.V.C.L.; Beyaert, E. Afwijkende Mondegewoonten: Inleiding, Onderzoek en Behandeling; Acco Leuven/Den Haag, 2009; Available online: https://www.bookmatch.nl/studieboeken/9789033424380-Afwijkende-mondgewoonten (accessed on 16 April 2025).
| Inspection of the Face |
|---|
| Facial morphological patterns [36] Gummy smile [37] Neck circumference at the level of cricothyroid cartilage [38,39] |
| Inspection of the nose |
| External examination: deformities, symmetry, size, and patency of nares, frontal and dorsal profile [40] Anterior rhinoscopy: septal deviations, mucosa, turbinate hypertrophy [40,41] |
| Inspection of the face |
| Tonsils: grading according to Friedman’s tonsil classification [42] Teeth: type of malocclusion (Angle’s classification: class I–III) Tongue: macroglossia, ankyloglossia, functionality [43,44] Mallampati score [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verbeke, J.; Meerschman, I.; Dhondt, K.; De Leenheer, E.; Willekens, J.; Van Lierde, K.; Claeys, S. Orofacial Myofunctional Therapy: Investigating a Novel Therapeutic Approach for Pediatric Obstructive Sleep Apnea in Children with and Without Down Syndrome—A Study Protocol. Children 2025, 12, 737. https://doi.org/10.3390/children12060737
Verbeke J, Meerschman I, Dhondt K, De Leenheer E, Willekens J, Van Lierde K, Claeys S. Orofacial Myofunctional Therapy: Investigating a Novel Therapeutic Approach for Pediatric Obstructive Sleep Apnea in Children with and Without Down Syndrome—A Study Protocol. Children. 2025; 12(6):737. https://doi.org/10.3390/children12060737
Chicago/Turabian StyleVerbeke, Jolien, Iris Meerschman, Karlien Dhondt, Els De Leenheer, Julie Willekens, Kristiane Van Lierde, and Sofie Claeys. 2025. "Orofacial Myofunctional Therapy: Investigating a Novel Therapeutic Approach for Pediatric Obstructive Sleep Apnea in Children with and Without Down Syndrome—A Study Protocol" Children 12, no. 6: 737. https://doi.org/10.3390/children12060737
APA StyleVerbeke, J., Meerschman, I., Dhondt, K., De Leenheer, E., Willekens, J., Van Lierde, K., & Claeys, S. (2025). Orofacial Myofunctional Therapy: Investigating a Novel Therapeutic Approach for Pediatric Obstructive Sleep Apnea in Children with and Without Down Syndrome—A Study Protocol. Children, 12(6), 737. https://doi.org/10.3390/children12060737







