Final Fusion Strategies in Early-Onset Scoliosis: Does Implant Density Make a Difference After Magnetically Controlled Growing Rod Treatment?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- Non-walking;
- Congenital EOS;
- Without final fusion after completing the lengthening program;
- With hybrid constructs (HCs), consisting of instrumentation with screws, hooks, and/or universal clamps, after the final fusion.
2.2. Study Design
2.3. Outcomes
2.4. Study Variables and Outcome Measures
2.5. Surgical Procedures
2.6. Statistical Analysis and Ethics
3. Results
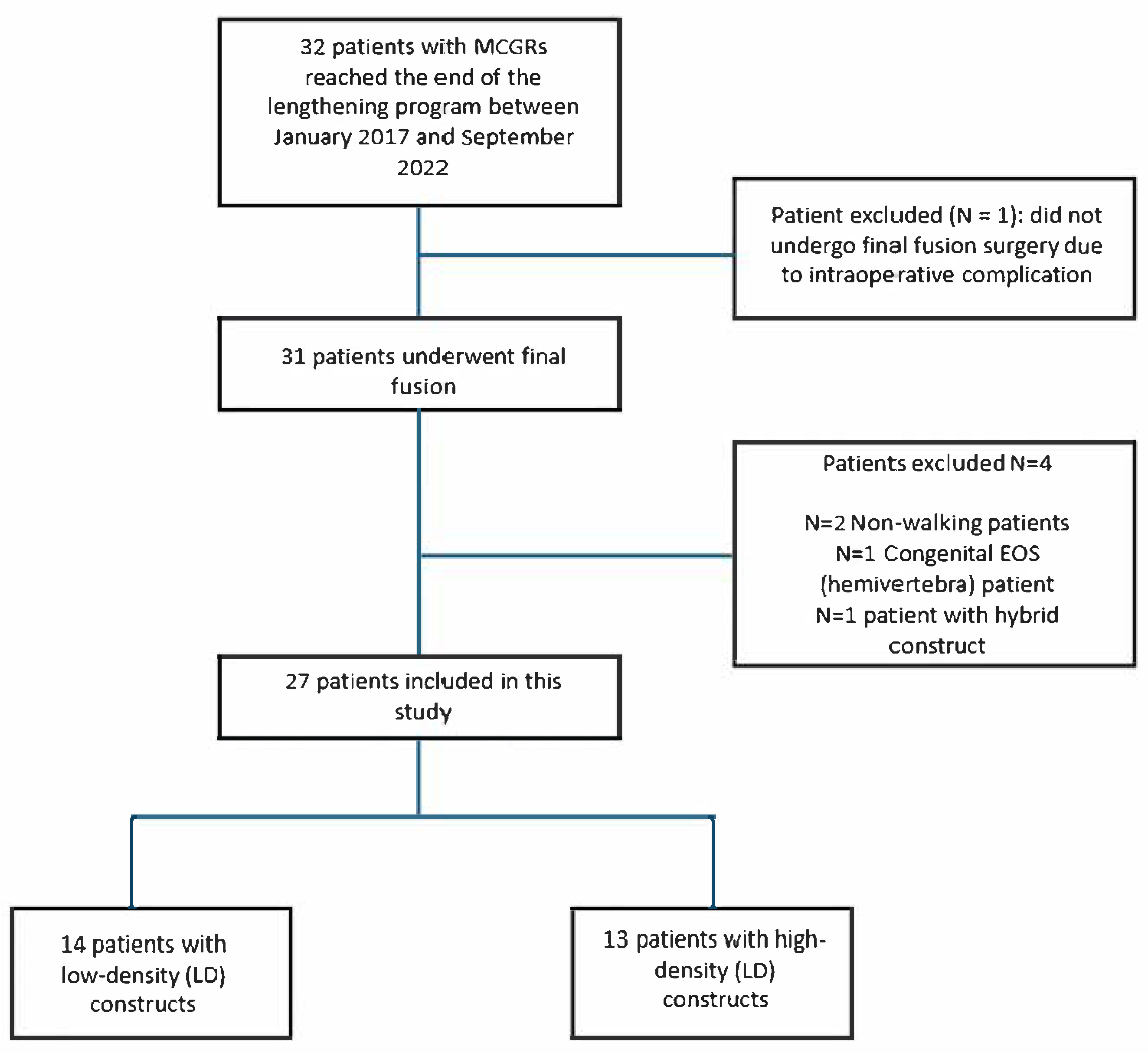
3.1. Patient Selection
3.2. Patient Population
3.3. Demographic Characteristics
3.4. Fixation and Implant Characteristics
3.5. Coronal and Sagittal Plane Characteristics
3.6. Implant Cost and Surgical Outcomes
3.7. Postoperative Complications
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | Adolescent Idiopathic Scoliosis |
| AP | Anteroposterior |
| AS | All-screw |
| BMI | Body Mass Index |
| CDC | Clavien Dindo Classification |
| CDCS | Centers for Disease Control and Prevention Scale |
| CR | Correction Rate |
| DSI | Delayed Surgical Site iInfection |
| EOS | Early-onset Scoliosis |
| FUP | Follow-up |
| HD | High-density |
| HC | Hybrid Constructs |
| Hb | Hemoglobin |
| ICU | Intensive Care Unit |
| LD | Low-density |
| LIV | Lower Instrumented Vertebra |
| LL | Lumbar Lordosis |
| LOS | Length of Hospital Stay |
| MCGR | Magnetically Controlled Growing Rods |
| MEPs | Motor-evoked Potentials |
| PCOs | Posterior Column Osteotomies |
| PROMs | Patient-Reported Outcome Measures |
| SEPs | Sensory-evoked Potentials |
| SSI | Superficial Site Infection |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| TGR | Traditional Growing Rods |
| TK | Thoracic Kyphosis |
| UIV | Upper Instrumented Vertebra |
References
- Jenks, M.; Craig, J.; Higgins, J.; Willits, I.; Barata, T.; Wood, H.; Kimpton, C.; Sims, A. The MAGEC System for Spinal Lengthening in Children with Scoliosis: A NICE Medical Technology Guidance. Appl. Health Econ. Health Policy 2014, 12, 587–599. [Google Scholar] [CrossRef]
- Vialle, R.; Thévenin-Lemoine, C.; Mary, P. Neuromuscular Scoliosis. Orthop. Traumatol. Surg. Res. 2013, 99, S124–S139. [Google Scholar] [CrossRef]
- Mehta, J.; Shah, S.; Hothi, H.; Tognini, M.; Gardner, A.; Johnston, C.E.; Murphy, R.; Thompson, G.; Sponseller, P.; Emans, J.; et al. Outcome of Distraction-Based Growing Rods at Graduation: A Comparison of Traditional Growing Rods and Magnetically Controlled Growing Rods. Spine Deform. 2025, 13, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Thakar, C.; Kieser, D.C.; Mardare, M.; Haleem, S.; Fairbank, J.; Nnadi, C. Systematic Review of the Complications Associated with Magnetically Controlled Growing Rods for the Treatment of Early Onset Scoliosis. Eur. Spine J. 2018, 27, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Filan, J.; Bowey, A.; Joyce, T. An Analysis of the Food and Drug Administration Manufacturer and User Facility Device Experience Database for MAGnetic Expansion Control Spinal Rods. Ther. Innov. Regul. Sci. 2025, 59, 31–40. [Google Scholar] [CrossRef]
- Chehrassan, M.; Shakeri, M.; Nikouei, F.; Yaqubnejad, M.; Mahabadi, E.A.; Ghandhari, H. Achievements and Complications Related to Final Fusion Surgery in Early Onset Scoliosis at the End of “Traditional Dual Growing Rod Mission”. Musculoskelet. Surg. 2024, 108, 333–337. [Google Scholar] [CrossRef]
- Abdelaal, A.; Munigangaiah, S.; Trivedi, J.; Davidson, N. Magnetically Controlled Growing Rods in the Treatment of Early Onset Scoliosis: A Single Centre Experience of 44 Patients with Mean Follow-up of 4.1 Years. Bone Jt. Open 2020, 1, 405–414. [Google Scholar] [CrossRef] [PubMed]
- De Salvatore, S.; Oggiano, L.; Sessa, S.; Curri, C.; Fumo, C.; Costici, P.F.; Ruzzini, L. Patients Treated by Magnetic Growing Rods for Early-Onset Scoliosis Reach the Expected Average Growth. Spine Deform. 2024, 12, 843–851. [Google Scholar] [CrossRef]
- Gurel, R.; Elbaz, E.; Sigal, A.; Gigi, R.; Otremski, H.; Lebel, D.E.; Ovadia, D. Magnetically Controlled Growing Rods Graduation: Lessons From a Single-Center Series of 48 Patients. J. Pediatr. Orthop. 2024, 44, e157–e162. [Google Scholar] [CrossRef]
- Larson, A.N.; Aubin, C.-E.; Polly, D.W.; Ledonio, C.G.T.; Lonner, B.S.; Shah, S.A.; Richards, B.S.; Erickson, M.A.; Emans, J.B.; Weinstein, S.L.; et al. Are More Screws Better? A Systematic Review of Anchor Density and Curve Correction in Adolescent Idiopathic Scoliosis. Spine Deform. 2013, 1, 237–247. [Google Scholar] [CrossRef]
- Larson, A.N.; Polly, D.W.; Ackerman, S.J.; Ledonio, C.G.T.; Lonner, B.S.; Shah, S.A.; Emans, J.B.; Richards, B.S.; Minimize Implants Maximize Outcomes Study Group. What Would Be the Annual Cost Savings If Fewer Screws Were Used in Adolescent Idiopathic Scoliosis Treatment in the US? J. Neurosurg. Spine 2016, 24, 116–123. [Google Scholar] [CrossRef]
- Roach, J.W.; Mehlman, C.T.; Sanders, J.O. Does the Outcome of Adolescent Idiopathic Scoliosis Surgery Justify the Rising Cost of the Procedures? J. Pediatr. Orthop. 2011, 31, S77–S80. [Google Scholar] [CrossRef]
- Wang, F.; Xu, X.-M.; Lu, Y.; Wei, X.-Z.; Zhu, X.-D.; Li, M. Comparative Analysis of Interval, Skipped, and Key-Vertebral Pedicle Screw Strategies for Correction in Patients With Lenke Type 1 Adolescent Idiopathic Scoliosis. Medicine 2016, 95, e3021. [Google Scholar] [CrossRef]
- Luo, M.; Shen, M.; Wang, W.; Xia, L. Comparison of Consecutive, Interval, and Skipped Pedicle Screw Techniques in Moderate Lenke Type 1 Adolescent Idiopathic Scoliosis. World Neurosurg. 2017, 98, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, W.; Shen, M.; Luo, X.; Xia, L. Does Higher Screw Density Improve Radiographic and Clinical Outcomes in Adolescent Idiopathic Scoliosis? A Systematic Review and Pooled Analysis. J. Neurosurg. Pediatr. 2017, 19, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, S.; Trefzer, R.; Renkawitz, T.; Baumann, L.; Pepke, W. Evaluation of the Lengthening of the Magnetically Controlled Growing Rods in Juvenile and Early-Onset Scoliosis. Orthop. Traumatol. Surg. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Hickey, B.A.; Towriss, C.; Baxter, G.; Yasso, S.; James, S.; Jones, A.; Howes, J.; Davies, P.; Ahuja, S. Early Experience of MAGEC Magnetic Growing Rods in the Treatment of Early Onset Scoliosis. Eur. Spine J. 2014, 23 (Suppl. 1), S61–S65. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, H.; Luo, M.; Wang, W.; Li, N.; Wang, L.; Xia, L. Comparison of Low Density and High Density Pedicle Screw Instrumentation in Lenke 1 Adolescent Idiopathic Scoliosis. BMC Musculoskelet. Disord. 2017, 18, 336. [Google Scholar] [CrossRef]
- Gotfryd, A.O.; Avanzi, O. Randomized Clinical Study on Surgical Techniques With Different Pedicle Screw Densities in the Treatment of Adolescent Idiopathic Scoliosis Types Lenke 1A and 1B. Spine Deform. 2013, 1, 272–279. [Google Scholar] [CrossRef]
- Langlais, T.; Bouy, A.; Eloy, G.; Mainard, N.; Skalli, W.; Vergari, C.; Vialle, R. Sagittal Plane Assessment of Manual Concave Rod Bending for Posterior Correction in Adolescents with Idiopathic Thoracic Scoliosis (Lenke 1 and 3). Orthop. Traumatol. Surg. Res. 2023, 109, 103654. [Google Scholar] [CrossRef]
- Moreno Mateo, F.; Bovonratwet, P.; Peiró García, A. Early-Onset Scoliosis. Curr. Opin. Pediatr. 2024, 36, 105–111. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L.; Zhao, L.; Wang, Y.; Chen, M.; Wang, S.; Lv, Z.; Luo, Y. Coronal Balance vs. Sagittal Profile in Adolescent Idiopathic Scoliosis, Are They Correlated? Front. Pediatr. 2020, 7, 523. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.A.; Ge, D.H.; Gomez, J.A. The Importance of Sagittal Alignment in Patients with Adolescent Idiopathic Scoliosis and Early Onset Scoliosis: A Review on Preoperative versus Postoperative Changes. Semin. Spine Surg. 2021, 33, 100909. [Google Scholar] [CrossRef]
- Clavien, P.A.; Sanabria, J.R.; Strasberg, S.M. Proposed Classification of Complications of Surgery with Examples of Utility in Cholecystectomy. Surgery 1992, 111, 518–526. [Google Scholar]
- Ponte, A.; Orlando, G.; Siccardi, G.L. The True Ponte Osteotomy: By the One Who Developed It. Spine Deform. 2018, 6, 2–11. [Google Scholar] [CrossRef]
- Schwab, F.; Blondel, B.; Chay, E.; Demakakos, J.; Lenke, L.; Tropiano, P.; Ames, C.; Smith, J.S.; Shaffrey, C.I.; Glassman, S.; et al. The Comprehensive Anatomical Spinal Osteotomy Classification. Neurosurgery 2014, 74, 112–120; discussion 120. [Google Scholar] [CrossRef]
- Menapace, B.; Jain, V.; Sturm, P. Autofusion in Early-Onset Scoliosis Growing Constructs: Occurrence, Risk Factors, and Impacts. Spine Deform. 2024, 12, 1155–1163. [Google Scholar] [CrossRef]
- Mainard, N.; Saghbini, E.; Pesenti, S.; Gouron, R.; Ilharreborde, B.; Lefevre, Y.; Haumont, T.; Sales de Gauzy, J.; Canavese, F. Is Posterior Vertebral Arthrodesis at the End of the Electromagnetic Rod Lengthening Program Necessary for All Patients? Comparative Analysis of Sixty Six Patients Who Underwent Definitive Spinal Arthrodesis and Twenty Four Patients with in Situ Lengthening Rods. Int. Orthop. 2024, 48, 1599–1609. [Google Scholar] [CrossRef]
- Aoun, M.; Daher, M.; Bizdikian, A.-J.; Kreichati, G.; Kharrat, K.; Sebaaly, A. Implant Density in Adolescent Idiopathic Scoliosis: A Meta-Analysis of Clinical and Radiological Outcomes. Spine Deform. 2024, 12, 909–921. [Google Scholar] [CrossRef]
| Preoperative Data | Details |
|---|---|
| Sex | Male/female (M/F) |
| Age at surgery | Age (years) |
| BMI | Measured in kg/m2 |
| Etiology of scoliosis | Type (AIS, NMS, SS) |
| Curve characteristic | Thoracic/double/lumbar |
| Main curve side | Right/left convex |
| Lumbar modifier | A/B/C |
| Follow-up | Follow-up time (months) between MCGR and final fusion and after final fusion |
| Radiographic data | |
| Main curve | Preoperatively, postoperatively, and at last follow-up (Cobb angle) |
| Percentage of curve correction | CR (%) for main curve |
| Frontal balance | The angle between the line connecting C7-S1 with respect to the vertical. Preoperatively, postoperatively, and at last follow-up. |
| Shoulder balance | The angle of the bi-coracoid line to the Horizontal. Preoperatively, postoperatively, and at last follow-up |
| Pelvis balance | The angle between the perpendicular to the line connecting the iliac wings and the line connecting T1-S1. Preoperatively, postoperatively, and at last follow-up |
| SVA | Measures the overall balance of the spine and corresponds to the horizontal distance between the plumb line of C7 and the posterosuperior corner of S1. Preoperatively, postoperatively, and at last follow-up |
| CL | Cobb angle between C2 and C7. Preoperatively, postoperatively, and at last follow-up |
| TK | Cobb angle between T5 and T12. Preoperatively, postoperatively, and at last follow-up |
| LL | Cobb angle between L1 and S1. Preoperatively, postoperatively, and at last follow-up |
| T1-T12 distance | Distance between the upper plate of T1 to upper plate of T12. Preoperatively, postoperatively, and at last follow-up |
| T1-S1 distance | Distance between the upper plate of T1 to upper plate of S1. Preoperatively, postoperatively, and at last follow-up |
| SS | The angle of the sacral plateau to the horizontal. Preoperatively, postoperatively, and at last follow-up |
| Postoperative Data | |
| UIV | UIV level (e.g., T2, T3, etc.) |
| LIV | LIV level (e.g., L3, L4, etc.) |
| Number of levels fused | Total levels fused (e.g., 10, 12, etc.) |
| Number of screws in construct | Total number of screws (n) |
| Density of the implant | Screw density (high density (HD) or low density (LD)) |
| Cost of the implant | Calculated by adding the cost of the screws, rods, and cross-links in Euros (EUR) |
| Posterior column osteotomies performed | Number of cases with at least one posterior column osteotomy |
| Postoperative clinical data | |
| Duration of surgery | Total time of surgery (Minutes) |
| Blood loss | Total bleeding during surgery (mL) |
| Loss of Hb | Total points of Hb lost postoperatively (g/dL) |
| Complications | Type and number of perioperative complications (n, %) according to CDSC and type and number of late-onset complications (n, %) |
| LOS | Total duration of recovery (days) |
| Variable | HD (N = 13) (%) | LD (N = 14) (%) |
|---|---|---|
| Sex | ||
| M | 3 (23.1) | 4 (28.5) |
| F | 10 (76.9) | 10 (71.5) |
| Etiology | ||
| Idiopathic | 9 (69.2) | 4 (28.5) |
| Syndromic | 2 (15.4) | 8 (57.1) |
| Prader–Willi Syndrome | 3 (21.4) | |
| Sotos Syndrome | 2 (14.2) | |
| Cornelia De Lange Syndrome | 1 (7.7) | |
| Robinow Syndrome | 1 (7.1) | |
| Coffin Syndrome | 1 (7.1) | |
| DiGeorge Syndrome | 1 (7.1) | |
| Larsen Syndrome | 1 (7.7) | |
| Neuromuscular | 2 (15.4) | 2 (14.2) |
| Perinatal Asphyxia | 2 (15.4) | |
| Congenital Myopathy | 1 (7.1) | |
| IIH | 1 (7.1) | |
| Curve Characteristic | ||
| Thoracic | 10 (76.9) | 7 (50) |
| Double | 2 (15.4) | 6 (42.8) |
| Lumbar | 1 (7.7) | 1 (7.1) |
| Main Curve Side | ||
| Right Convex | 9 (69.2) | 8 (57.1) |
| Left Convex | 4 (30.6) | 6 (42.8) |
| Lumbar Modifier | ||
| A | 7 (53.8) | 7 (50) |
| B | 4 (30.6) | 4 (28.5) |
| C | 2 (15.4) | 3 (21.4) |
| Parameters | HD (N = 13) | LD (N = 14) | p |
|---|---|---|---|
| Age (years, mean, SD, range) | 14.2 (±1.2) (13–17) | 14.5 (±1.6) (11–17) | 0.483 |
| Follow-up time between MCGR and final fusion (months, mean, SD, range) | 60.1 (±25) (31–128) | 66.5 (±29.6) (24–125) | 0.748 |
| Follow-up time after final fusion (months, mean, SD, range) | 43.6 (±23.7) (24–96) | 33.6 (±12.4) (24–60) | 0.575 |
| BMI (kg/m2, mean, SD, range) | 21.3 (±3.5) (15.3–26.5) | 22.5 (±7.5) (12.4–36.5) | 0.572 |
| Parameters | HD (N = 13) (%) | LD (N = 14) (%) | p |
|---|---|---|---|
| Number of pedicle screws (n, mean, SD, range) | 22.7 (±4.5) (15–30) | 16.1 (±3.9) (7–22) | 0.001 |
| Number of levels fused (n, mean, SD, range) | 16.5 (±3) (11–21) | 18.9 (±1.8) (15–21) | 0.036 |
| Number of pedicle screws per level (n, mean, SD, range) | 1.38 (±0.2) (1.1–18) | 0.8 (±0.2) (0.4–1.1) | <0.001 |
| Variable | HD (N = 13) (%) | LD (N = 14) (%) |
|---|---|---|
| PCO (n, %) | 6 (46.1) | 1 (7.1) |
| UIV (n, %) | ||
| T2 | 8 (61.5) | 11 (78.5) |
| T3 | 4 (30.6) | 2 (14.2) |
| T4 | 1 (7.7) | 1 (7.1) |
| LIV (n, %) | ||
| L2 | 3 (23.1) | 2 (14.2) |
| L3 | 6 (46.1) | 4 (28.6) |
| L4 | 4 (30.8) | 8 (57.1) |
| Variable | End of Lengthening (t1, Mean, SD, Range) | After Final Fusion (t2, Mean, SD, Range) | Last Follow-Up (t3, Mean, SD, Range) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HD | LD | p | HD | LD | p | HD | LD | p | |
| Main Curve (°) | 44.6 (±10.2) (25.5–65.9) | 46.3 (±14.2) (23–73) | 0.787 | 30.7 (±11.7) (9.6–49.1) | 36 (±13.4) (8.4–62.4) | 0.368 | 32.6 (±10.7) (15.2–50.2) | 39.9 (±13.6) (14.2–64.7) | 0.126 |
| Frontal Balance (°) | 2.4 (±2.1) (0.7–9.1) | 4.2 (±4.3) (0.9–14.1) | 0.435 | 4.3 (±5.4) (0.3–19.9) | 4.7 (±4.5) (0.5–14.8) | 0.465 | 2.9 (±2.9) (0.2–10.3) | 3.2 (±4.7) (0.3–17.6) | 0.412 |
| Shoulder Balance (°) | 2.8 (±1.9) (0.4–7.6) | 5.4 (±5) (0.1–17.6) | 0.254 | 3.8 (±2.4) (0.4–8.5) | 4.6 (±4.5) (0.1–14.7) | 0.984 | 5.1 (±4.4) (1.3–17.7) | 4.3 (±5.1) (0.1–19.8) | 0.368 |
| Pelvis Balance (°) | 2.7 (±2.1) (0.3–8.5) | 4.2 (±3.5) (0.1–12.8) | 0.207 | 3.5 (±4.1) (0.5–16) | 3.8 (±2.9) (0.9–9.6) | 0.412 | 2.8 (±2.8) (0.1–11.1) | 3.2 (±2.8) (0.1–8.6) | 0.849 |
| Variable | End of Lengthening (t1, Mean, SD, Range) | After Final Fusion (t2, Mean, SD, Range) | Last Follow-Up (t3, Mean, SD, Range) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HD | LD | p | HD | LD | p | HD | LD | p | |
| SVA (mm) | −13.4 (±54.9) (−111.3–79.6) |
5.9 (±42.6) (−86.4–78.9) | 0.453 | 7.6 (±62.1) (−81.2–169.8) |
2.6 (±31) (−50.2–67.7) | 0.984 |
3.8 (±52.3) (−79.3–88.6) | 24.8 (±41.3) (−26.8–99.5) | 0.509 |
| LL (°) | 56.2 (±14.2) (38.5–82.3) | 42.8 (±14.9) (22.9–74.9) | 0.023 | 45.8 (±16.6) (23.9–76.7) | 46.7 (±14.1) (26.6–82) | 0.825 | 42.4 (±20.8) (2.2–71.2) | 46.9 (±18) (15.4–82.1) | 0.718 |
| TK (°) | 23.4 (±13.8) (8.3–62.2) | 16.5 (±18.9) (−23.7–55.2) | 0.197 | 16.3 (±7.6) (8.6–38.7) | 10.9 (±14.4) (−14.1–48.9) | 0.021 | 15.8 (±9.5) (2.7–32.4) | 13.7 (±18.4) (−13.1–62.7) | 0.293 |
| CL (°) | 9.4 (±28.2) (−25–83.2) | −0.1 (±14.2) (−21.8–27) | 0.541 |
7.4 (±25.6) (−28.8–67.9) | −7.2 (±22.7) (−31.6–45.3) | 0.767 |
9.5 (±25.1) (−24.9–62.7) |
−0.3 (±21) (−33.5–51.1) | 0.509 |
| SS (°) | 38.1 (±9.5) (23.1–52.6) | 32.8 (±10.5) (17.7–54.4) | 0.254 | 35.1 (±12.1) (17.6–57.1) | 38.1 (±12.3) (19.6–65.4) | 0.509 | 35.9 (±11.3) (19.9–58.2) | 38.2 (±10.9) (18.−61.2) | 0.596 |
| T1-T12 (mm) | 22.5 (±2.2) (26.2–19.9) | 21.8 (±3.3) (13.9–29.5) | 0.624 | 24.2 (±5.6) (15.5–40.7) | 21.9 (±3.7) (14.7–29.6) | 0.167 | 26.2 (±5.9) (17.8–42.6) | 22.7 (±3.9) (14.3–28.7) | 0.126 |
| T1-S1 (mm) | 39.8 (±3.6) (35.6–46) | 37.5 (±3.8) (27.7–44.7) | 0.214 | 43.3 (±6.7) (31.1–59.1) | 39.1 (±4.3) (29.5–46.9) | 0.039 * | 45.4 (±7) (33.4–62.3) | 39.7 (±5.1) (28.5–47.5) | 0.021 * |
| Parameters | HD (N = 13) | LD (N = 14) | p |
|---|---|---|---|
| Cost of the implant (EUR, mean, SD, range) | 6046.5 (±1146.9) (4085–7910) | 4376.4 (±999.4) (2045–5870) | <0.001 |
| Surgery duration (min, mean, SD, range) | 268.1 (±46.1) (202–336) | 241.2 (±28.1) (195–290) | 0.108 |
| Loss of Hb (g/dL, mean, SD, range) | 1.4 (±0.7) (0.1–2.4) | 1.6 (±0.8) (0.2–3.2) | 0.453 |
| Blood loss (mL, mean, SD, range) | 630.7 (±222.2) (300–1100) | 521.4 (±279.9) (200–1300) | 0.138 |
| LOS (days, mean, SD, range) | 8.3 (±1.8) (6–13) | 8.7 (±1.7) (6–13) | 0.370 |
| Variable | HD (N = 13) (%) | LD (N = 14) (%) |
|---|---|---|
| Total complications (n, %) | 3 (23.1) | 3 (21.4) |
| Perioperative complications (n, %) | 2 (15.4) | 0 |
| Deep SSI | 1 (7.7) | / |
| CSF leak | 1 (7.7) | / |
| CDSC | / | / |
| Grade I | / | / |
| Grade II | / | / |
| Grade IIIa | / | / |
| Grade IIIb | 1 (7.7) | / |
| Grade IVa | / | / |
| Grade IVb | 1 (7.7) | / |
| Grade V | / | / |
| Late-onset complications (n, %) | 1 (7.7) | 3 (21.4) |
| Delayed SSI | / | 1 (7.1) |
| Rod rupture | 1 (7.7) | / |
| DJK | / | 2 (14.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brigato, P.; Oggiano, L.; De Salvatore, S.; Palombi, D.; Sessa, S.; Longo, U.G.; Vescio, A.; Costici, P.F. Final Fusion Strategies in Early-Onset Scoliosis: Does Implant Density Make a Difference After Magnetically Controlled Growing Rod Treatment? Children 2025, 12, 731. https://doi.org/10.3390/children12060731
Brigato P, Oggiano L, De Salvatore S, Palombi D, Sessa S, Longo UG, Vescio A, Costici PF. Final Fusion Strategies in Early-Onset Scoliosis: Does Implant Density Make a Difference After Magnetically Controlled Growing Rod Treatment? Children. 2025; 12(6):731. https://doi.org/10.3390/children12060731
Chicago/Turabian StyleBrigato, Paolo, Leonardo Oggiano, Sergio De Salvatore, Davide Palombi, Sergio Sessa, Umile Giuseppe Longo, Andrea Vescio, and Pier Francesco Costici. 2025. "Final Fusion Strategies in Early-Onset Scoliosis: Does Implant Density Make a Difference After Magnetically Controlled Growing Rod Treatment?" Children 12, no. 6: 731. https://doi.org/10.3390/children12060731
APA StyleBrigato, P., Oggiano, L., De Salvatore, S., Palombi, D., Sessa, S., Longo, U. G., Vescio, A., & Costici, P. F. (2025). Final Fusion Strategies in Early-Onset Scoliosis: Does Implant Density Make a Difference After Magnetically Controlled Growing Rod Treatment? Children, 12(6), 731. https://doi.org/10.3390/children12060731








