Hypo- and Hypernatremia in Extremely Low Birth Weight Infants in the First 10 Days of Life: A Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Structured Literature Review
2.2. Data Handling
3. Results
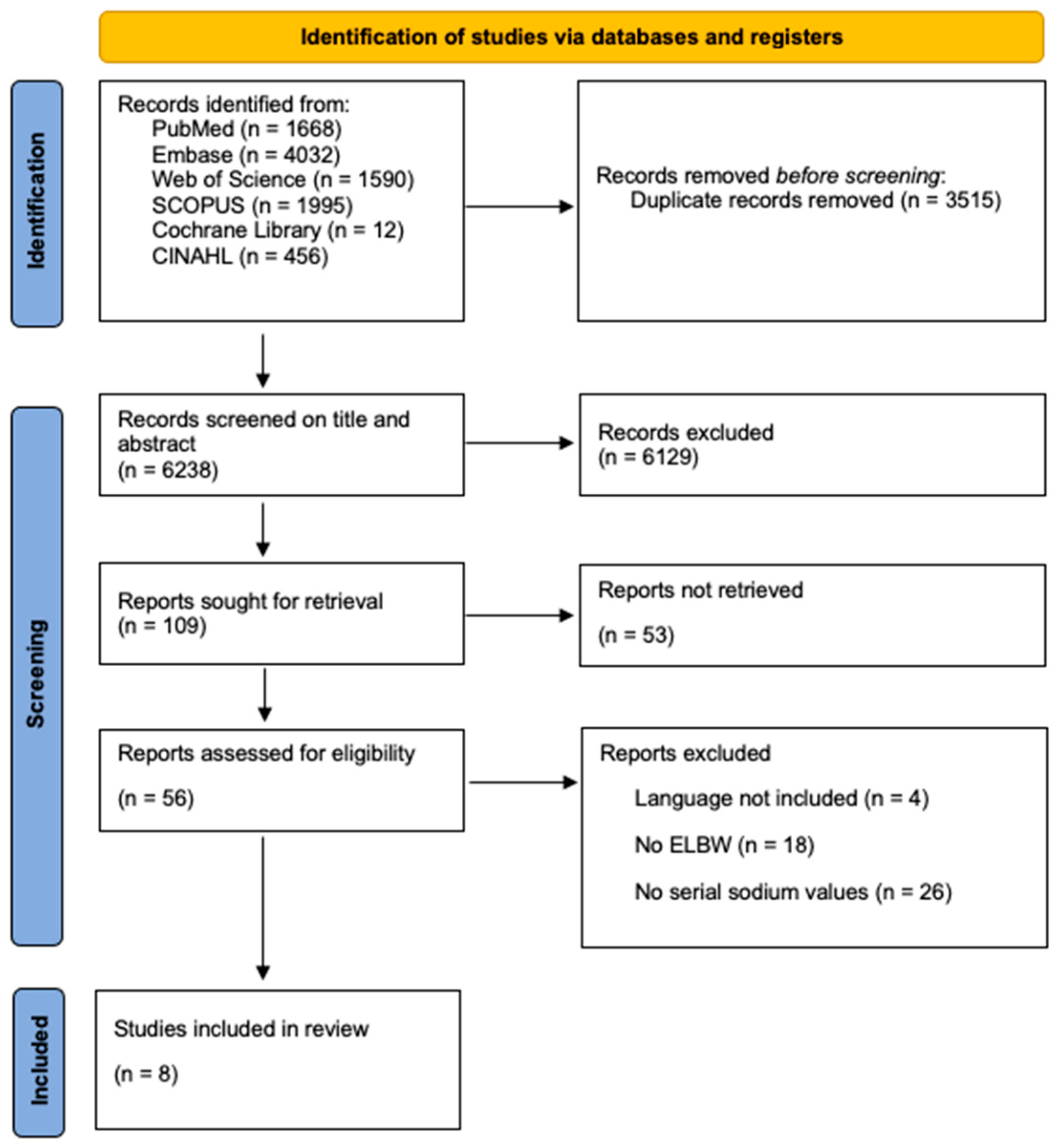
3.1. Structured Literature Review
3.2. Study Characteristics
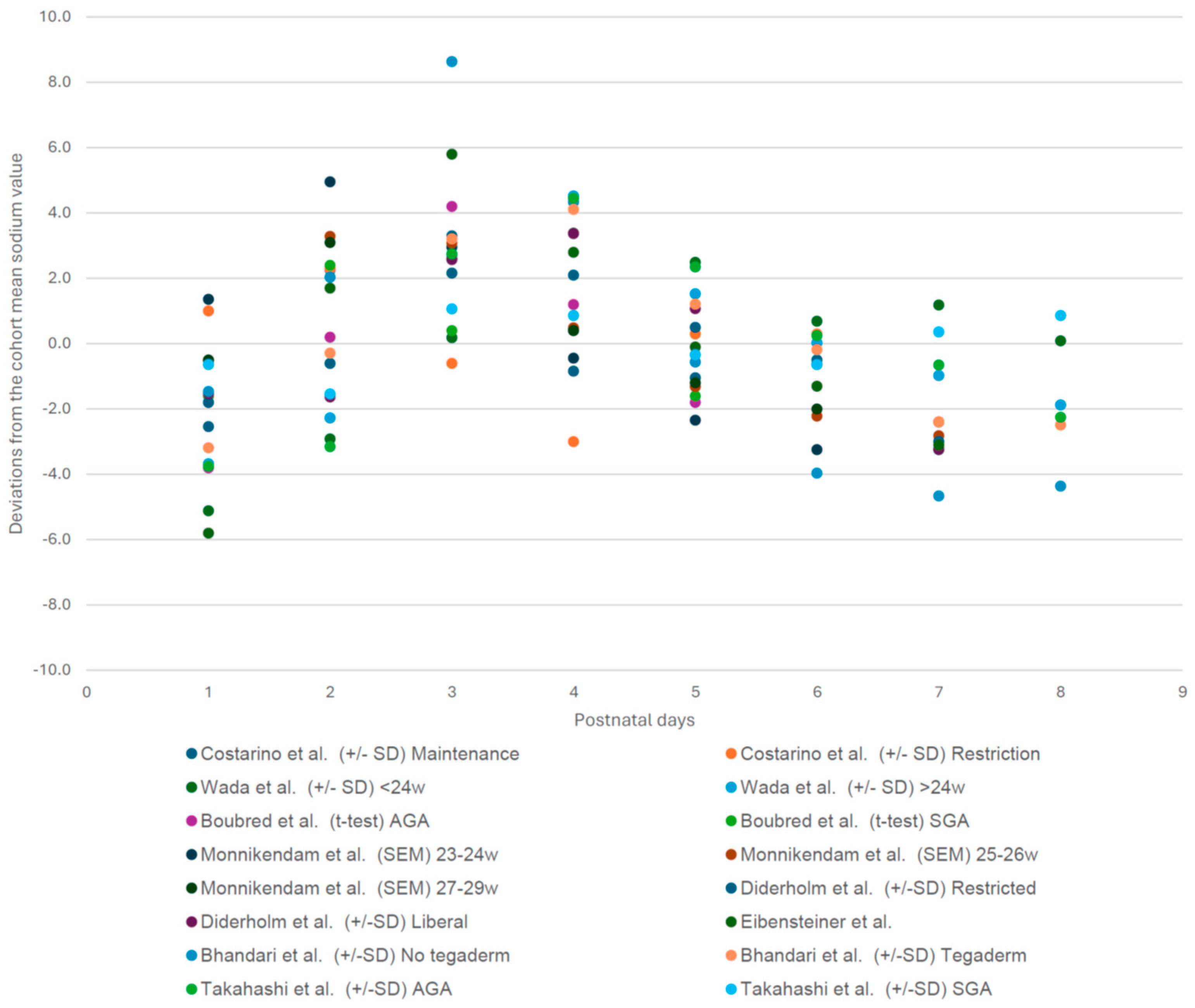
3.3. Cohort Specific Sodium Deviation Pattern of Each Postnatal Day (1–8) Compared to the Cohort Mean Sodium Value [1,3,7,17,18,19,20,21]
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wada, M.; Kusuda, S.; Takahashi, N.; Nishida, H. Fluid and electrolyte balance in extremely preterm infants < 24 weeks of gestation in the first week of life. Pediatr. Int. 2008, 50, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Stritzke, A.; Thomas, S.; Amin, H.; Fusch, C.; Lodha, A. Renal consequences of preterm birth. Mol. Cell. Pediatr. 2017, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Monnikendam, C.S.; Mu, T.S.; Aden, J.K.; Lefkowitz, W.; Carr, N.R.; Aune, C.N.; Ahmad, K.A. Dysnatremia in extremely low birth weight infants is associated with multiple adverse outcomes. J. Perinatol. 2019, 39, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Gawlowski, Z.; Aladangady, N.; Coen, P.G. Hypernatraemia in preterm infants born at less than 27 weeks gestation. J. Paediatr. Child Health 2006, 42, 771–774. [Google Scholar] [CrossRef]
- Segar, J.L. A physiological approach to fluid and electrolyte management of the preterm infant: Review. J. Neonatal Perinat. Med. 2020, 13, 11–19. [Google Scholar] [CrossRef]
- Jochum, F.; Moltu, S.J.; Senterre, T.; Nomayo, A.; Goulet, O.; Iacobelli, S.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Fluid and electrolytes. Clin. Nutr. 2018, 37, 2344–2353. [Google Scholar] [CrossRef]
- Costarino, A.T.; Ruskey, J.A.; Corcoran, L.; Polin, R.A.; Baumgart, S. Sodium restriction versus daily maintenance replacement in very low birth weight premature neonates: A randomized, blind therapeutic trial. J. Pediatr. 1992, 120, 99–106. [Google Scholar] [CrossRef]
- Allegaert, K.; Hildebrand, H.; Singh, K.; Turner, M.A. The publication quality of laboratory values in clinical studies in neonates. Pediatr. Res. 2023, 94, 96–98. [Google Scholar] [CrossRef]
- van Donge, T.; Allegaert, K.; Gotta, V.; Smits, A.; Levtchenko, E.; Mekahli, D.; Anker, J.v.D.; Pfister, M. Characterizing dynamics of serum creatinine and creatinine clearance in extremely low birth weight neonates during the first 6 weeks of life. Pediatr. Nephrol. 2021, 36, 649–659. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, 4–10. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment Scale Case Control Studies; Ottawa Hospital Research Institute: Ottawa, ON, USA, 2011. [Google Scholar]
- Rohatgi, A. WebPlotDigitizer. 2022. Available online: https://automeris.io/WebPlotDigitizer (accessed on 30 January 2024).
- SAS Institute Inc. SAS Software, version 9.4; SAS Institute Inc.: Cary, NC, USA, 2014. [Google Scholar]
- Boubred, F.; Herlenius, E.; Bartocci, M.; Jonsson, B.; Vanpée, M. Extremely preterm infants who are small for gestational age have a high risk of early hypophosphatemia and hypokalemia. Acta Paediatr. 2015, 104, 1077–1083. [Google Scholar] [CrossRef]
- Diderholm, B.; Normann, E.; Ahlsson, F.; Sindelar, R.; Ågren, J. The Impact of Restricted versus Liberal Early Fluid Volumes on Plasma Sodium, Weight Change, and Short-Term Outcomes in Extremely Preterm Infants. Nutrients 2022, 14, 795. [Google Scholar] [CrossRef]
- Eibensteiner, F.; Laml-Wallner, G.; Thanhaeuser, M.; Ristl, R.; Ely, S.; Jilma, B.; Berger, A.; Haiden, N. ELBW infants receive inadvertent sodium load above the recommended intake. Pediatr. Res. 2020, 88, 412–420. [Google Scholar] [CrossRef]
- Bhandari, V.; Brodsky, N.; Porat, R. Improved outcome of extremely low birth weight infants with Tegaderm® application to skin. J. Perinatol. 2005, 25, 276–281. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, N.; Hoshi, J.; Nishida, H. Water balance, electrolytes and acid-base balance in extremely premature infants. Pediatr. Int. 1994, 36, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Ågren, J.; Segar, J.L.; Söderström, F.; Bell, E.F. Fluid management considerations in extremely preterm infants born at 22–24 weeks of gestation. Semin. Perinatol. 2022, 46, 151541. [Google Scholar] [CrossRef]
- Durrani, N.U.R.; Imam, A.A.; Soni, N. Hypernatremia in Newborns: A Practical Approach to Management. Biomed. Hub 2022, 7, 55–69. [Google Scholar] [CrossRef]
- Stritzke, A.I.; Ismail, R.; Rose, M.S.; Lyon, A.W.; Fenton, T.R. Cord-Blood Derived Chemistry Reference Values in Preterm Infants for Sodium, Chloride, Potassium, Glucose, and Creatinine. Am. J. Perinatol. 2024, 41, 722–729. [Google Scholar] [CrossRef]
| Author | Study Design | Groups | Number of Neonates Included | Weeks of Gestation at Birth | Birth Weight | Follow Up Period | Exclusion Criteria |
|---|---|---|---|---|---|---|---|
| Costarino et al. [7] | Randomized controlled trial | Sodium restriction | 9 | 27 ± 1 weeks | 870 ± 120 g | 5 days | Renal malformation, renal arterial or venous thrombosis, Apgar score of <5 at 5 min. |
| No sodium restriction | 8 | 27 ± 1 weeks | 820 ± 130 g | ||||
| Wada et al. [1] | Retrospective case–control | Cases | 17 | 22.9 ± 0.6 weeks | 493.4 ± 66.2 g | 7 days | Congenital anomalies, severe infections or symptomatic persistent ductus arteriosus. |
| Controls | 72 | 25.6 ± 0.7 weeks | 777.8 ± 99.1 g | ||||
| Boubred et al. [17] | Retrospective cohort study | AGA | 36 | 25.1 ± 0.9 weeks | 837 ± 146 g | 5 days | On the national growth curves for children. Congenital malformations or severe illness requiring medication that could influence the electrolyte balance, such as insulin, NSAIDs, diuretics. Infants who died during the study period. Lacking laboratory variables on days 1–4. |
| SGA | 12 | 25.9 ± 1.1 weeks | 665 ± 109 g | ||||
| Monnikendam et al. [3] | Retrospective cohort study | 12,428 | N/A | N/A | 7 days | Significant congenital anomalies, death/transfer within 7 days of birth or missing daily sodium in the first 7 days of life leaving. | |
| Diderholm et al. [18] | Retrospective cohort study | Restricted fluid intake | 63 | 25.2 ± 1.2 weeks | 744 ± 192 g | 5 days | Died or transferred to other units within the first week of life. |
| Liberal fluid intake | 112 | 25.1 ± 1.1 weeks | 718 ± 156 g | ||||
| Eibensteiner et al. [19] | Retrospective cohort study | 94 included, 90 analyzed | Mean: 24 + 6/7 weeks | 718 g | 14 days | Death within 24 h of birth, acute kidney injury (serum creatinine > 1.5 mg/dL), syndrome of inappropriate ADH secretion, adrenogenital syndrome, diabetes insipidus. | |
| Bhandari et al. [20] | Retrospective case–control | Pre-Tegaderm | 39 | 26.1 ± 1.9 weeks | 756 ± 158 g | 7 days | N/A |
| Tegaderm | 30 | 26.3 ± 1.8 | 802 ± 160 g | ||||
| Takahashi et al. [21] | Retrospective cohort study | AGA | 72 | 25.9 ± 1.7 weeks | 787.35 ± 148.5 g | 7 days | Died, had PDA, intestinal obstruction and severe infection. |
| SGA | 28 | 28.9 ± 2.6 weeks | 762.2 ± 156.3 g |
| Author | Cutoff for Hyponatremia | Cutoff for Hypernatremia | Incidence of Hyponatremia | Incidence of Hypernatremia | Peak Sodium | Incubator Settings | Fluid Regimen | |
|---|---|---|---|---|---|---|---|---|
| Costarino et al. [7] | Sodium restriction | <130 mEq/L | >150 mEq/L | 22.22% | 0% | Day 2 | Radiant warmers + transparent plastic blankets. | 3–4 mEq/kg/day of sodium, volume of fluid determined by physician. |
| No sodium restriction | 0% | 25% | Day 2 | Maintenance sodium supplementation, volume of fluid determined by physician. | ||||
| Wada et al. [1] | Cases | <130 mEq/L | >150 mEq/L | 17.70% | 29% | Day 4 | Closed incubator, near 100% humidity day 0–3, then highly humidified environment >80%. | Solution of 7.5% glucose mixed with calcium gluconate in the ratio of 19:1 (or 18:2) at 50 mL/kg per day. |
| Controls | 26.40% | 9.70% | Day 4 | |||||
| Boubred et al. [17] | AGA | N/A | N/A | N/A | N/A | Day 3 | N/A | Day 1: total parenteral nutrition provided glucose (6 g/kg/day), protein (2 g/kg/day) and lipid (1 g/kg/day. Progressively increased until they reached 4 g/kg/day protein and 3 g/kg/day lipids by the end of the first week. |
| SGA | N/A | N/A | Day 2 | |||||
| Monnikendam et al. [3] | 125–134 mEq/L, <125 mEq/L for severe | 145–154 mEq/L, >154 mEq/L for severe | <135: 35.1% | 145–154: 65.4%, >154 21.7% | Day 2 | N/A | N/A | |
| Diderholm et al. [18] | Restricted fluid intake | <130 mmol/L | >145 mmol/L moderate, >150 mmol/L severe | 21% | >145: 29%, >150: 10% | Day 3 | First week: humidity of 85%, after: humidity of 50% | Total fluids 95 mL/kg/day (GA 22–24 weeks) and 85 mL/kg/d (GA 25–26 weeks). Increase in fluid with 10 mL/kg/d, to be adjusted at the discretion of the attending neonatologist. |
| Liberal fluid intake | 21% | >145: 36%, >150: 11% | Day 4 | |||||
| Eibensteiner et al. [19] | N/A | >145 mEq/L: mild, >150 mEq/L: severe | N/A | >145: 92.2%, >150: 64.4% | Day 3 | Double-wall incubators with humidity 85–90%, humidity was reduced by 5%/week. | N/A | |
| Bhandari et al. [20] | No Tegaderm | N/A | > 150 mEq/L | N/A | 51% | Day 3 | Initially in radiant warmers, incubators when clinically stable. | N/A |
| Tegaderm | N/A | 17% | Day 4 | |||||
| Takahashi et al. [21] | AGA | <130 mEq/L | >150 mEq/L | 27.59% | 8.62% | Day 4 | Almost full ambient humidity in closed incubator with nebulizer until 4th day of life, 90% humidity without nebulizer after that. | 50–60 mL/kg on first day of life. |
| SGA | 52.94% | 5.88% | Day 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pace, M.; van Sas, S.; Salaets, T.; Laenen, A.; Raaijmakers, A.; Allegaert, K. Hypo- and Hypernatremia in Extremely Low Birth Weight Infants in the First 10 Days of Life: A Review. Children 2025, 12, 231. https://doi.org/10.3390/children12020231
Pace M, van Sas S, Salaets T, Laenen A, Raaijmakers A, Allegaert K. Hypo- and Hypernatremia in Extremely Low Birth Weight Infants in the First 10 Days of Life: A Review. Children. 2025; 12(2):231. https://doi.org/10.3390/children12020231
Chicago/Turabian StylePace, Myrna, Stijn van Sas, Thomas Salaets, Annouschka Laenen, Anke Raaijmakers, and Karel Allegaert. 2025. "Hypo- and Hypernatremia in Extremely Low Birth Weight Infants in the First 10 Days of Life: A Review" Children 12, no. 2: 231. https://doi.org/10.3390/children12020231
APA StylePace, M., van Sas, S., Salaets, T., Laenen, A., Raaijmakers, A., & Allegaert, K. (2025). Hypo- and Hypernatremia in Extremely Low Birth Weight Infants in the First 10 Days of Life: A Review. Children, 12(2), 231. https://doi.org/10.3390/children12020231









