Nephrogenic Diabetes Insipidus Affecting Three Males in Two Generations—Case Report and Review of the Literature
Abstract
1. Introduction
2. Material and Methods
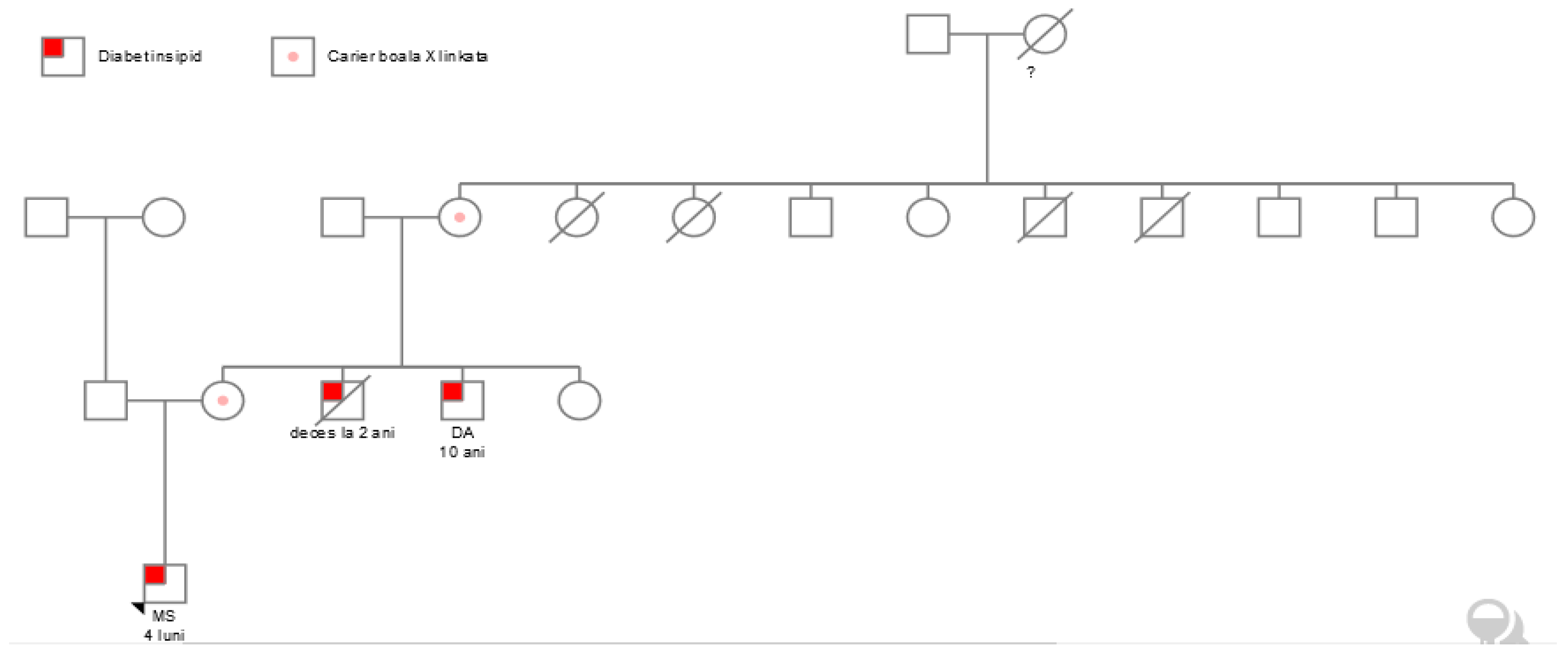
3. Results
3.1. Polyuria and Polydipsia with Normal Development
3.2. Failure to Thrive in an Infant with Polyuria
3.3. Genetic Testing
3.4. Treatment and Evolution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NDI | Nephrogenic diabetes insipidus |
| CDI | Central diabetes insipidus |
| AQP2 | Aquaporin-2 water channel |
| AVPR2 | Vasopressin V2 receptor |
References
- Moeller, H.B.; Rittig, S.; Fenton, R.A. Nephrogenic Diabetes Insipidus: Essential Insights into the Molecular Background and Potential Therapies for Treatment. Endocr. Rev. 2013, 34, 278–301. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ashton, E.; Iancu, D.; Arthus, M.-F.; Hayes, W.; Van’t Hoff, W.; Kleta, R.; Bichet, D.G.; Bockenhauer, D. Long-term outcome in inherited nephrogenic diabetes insipidus. Clin. Kidney J. 2019, 12, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Duicu, C.; Pitea, A.M.; Săsăran, O.M.; Cozea, I.; Man, L.; Bănescu, C. Nephrogenic diabetes insipidus in children (Review). Exp. Ther. Med. 2021, 22, 1–6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bockenhauer, D.; Bichet, D.G. Urinary concentration: Different ways to open and close the tap. Pediatr. Nephrol. 2013, 29, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandri-Silva, C.; Carpenter, M.; Ayoob, R.; Barcia, J.; Chishti, A.; Constantinescu, A.; Dell, K.M.; Goodwin, J.; Hashmat, S.; Iragorri, S.; et al. Diagnosis, Treatment, and Outcomes in Children with Congenital Nephrogenic Diabetes Insipidus: A Pediatric Nephrology Research Consortium Study. Front. Pediatr. 2020, 7, 550. [Google Scholar] [CrossRef] [PubMed]
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Francioli, L.C.; Goodrich, J.K.; Collins, R.L.; Kanai, M.; Wang, Q.; Alföldi, J.; Watts, N.A.; Vittal, C.; Gauthier, L.D.; et al. A genomic mutational constraint map using variation in 76,156 human genomes. Nature 2024, 625, 92–100. [Google Scholar] [CrossRef]
- Li, C.; Zhi, D.; Wang, K.; Liu, X. MetaRNN: Differentiating rare pathogenic and rare benign missense SNVs and InDels using deep learning. Genome Med. 2022, 14, 1–14. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2017, 46, D1062–D1067. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ma, L.; Li, L.; Luo, J.; Sun, T. Case Report: A Case of Congenital Nephrogenic Diabetes Insipidus Caused by Thr273Met Mutation in Arginine Vasopressin Receptor 2. Front. Pediatr. 2021, 9, 707452. [Google Scholar] [CrossRef] [PubMed]
- Amberger, J.S.; Hamosh, A. Searching Online Mendelian Inheritance in Man (OMIM): A Knowledgebase of Human Genes and Genetic Phenotypes. Curr. Protoc. Bioinform. 2017, 58, 1.2.1–1.2.12. [Google Scholar] [CrossRef]
- Hureaux, M.; Vargas-Poussou, R. Genetic basis of nephrogenic diabetes insipidus. Mol. Cell. Endocrinol. 2022, 560, 111825. [Google Scholar] [CrossRef]
- Bichet, D.G. V2R mutations and nephrogenic diabetes insipidus. Prog. Mol. Biol. Transl. Sci. 2009, 89, 15–29. [Google Scholar] [CrossRef]
- Monnens, L.; Jonkman, A.; Thomas, C. Response to indomethacin and hydrochlorothiazide in nephrogenic diabetes insipidus. Clin. Sci. 1984, 66, 709–715. [Google Scholar] [CrossRef]
- Boussemart, T.; Nsota, J.; Martin–Coignard, D.; Champion, G. Nephrogenic diabetes insipidus: Treat with caution. Pediatr. Nephrol. 2009, 24, 1761–1763. [Google Scholar] [CrossRef]
- Ma, L.; Wu, D.; Wang, X.; Yang, Y. A Case of Congenital Nephrogenic Diabetes Insipidus Caused by Thr108Met Variant of Aquaporin 2. Front. Pediatr. 2020, 8, 15. [Google Scholar] [CrossRef]
- Levtchenko, E.; Ariceta, G.; Flores, O.A.; Bichet, D.G.; Bockenhauer, D.; Emma, F.; Hoorn, E.J.; Koster-Kamphuis, L.; Nijenhuis, T.; Trepiccione, F.; et al. International expert consensus statement on the diagnosis and management of congenital nephrogenic diabetes insipidus (arginine vasopressin resistance). Nat. Rev. Nephrol. 2024, 21, 83–96. [Google Scholar] [CrossRef] [PubMed]
- César, K.R.; Magaldi, A.J. Thiazide induces water absorption in the inner medullary collecting duct of normal and Brattleboro rats. Am. J. Physiol. Physiol. 1999, 277, F756–F760. [Google Scholar] [CrossRef] [PubMed]
- Loffing, J. Paradoxical Antidiuretic Effect of Thiazides in Diabetes Insipidus. J. Am. Soc. Nephrol. 2004, 15, 2948–2950. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wesche, D.; Deen, P.M.T.; Knoers, N.V.A.M. Congenital nephrogenic diabetes insipidus: The current state of affairs. Pediatr. Nephrol. 2012, 27, 2183–2204. [Google Scholar] [CrossRef]
- Tuncdemir, B.E.; Mergen, H.; Ozer, E.S. Evaluation of pharmacochaperone-mediated rescue of mutant V2 receptor proteins. Eur. J. Pharmacol. 2019, 865, 172803. [Google Scholar] [CrossRef]
- Prosperi, F.; Suzumoto, Y.; Marzuillo, P.; Costanzo, V.; Jelen, S.; Iervolino, A.; Guarino, S.; La Manna, A.; Del Giudice, E.M.; Perna, A.F.; et al. Characterization of five novel vasopressin V2 receptor mutants causing nephrogenic diabetes insipidus reveals a role of tolvaptan for M272R-V2R mutation. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Cyranoski, D.; Reardon, S. Embryo editing sparks epic debate. Nature 2015, 520, 593–594. [Google Scholar] [CrossRef]
- Robben, J.H.; Sze, M.; Knoers, N.V.; Deen, P.M. Functional rescue of vasopressin v2 receptor mutants in mdck cells by pharmacochaperones: Relevance to therapy of nephrogenic diabetes insipidus. Am. J. Physiol. Ren. Physiol. 2007, 292, F253–F260. [Google Scholar] [CrossRef] [PubMed]
- Szalai, L.; Sziráki, A.; Erdélyi, L.S.; Kovács, K.B.; Tóth, M.; Tóth, A.D.; Turu, G.; Bonnet, D.; Mouillac, B.; Hunyady, L.; et al. Functional Rescue of a Nephrogenic Diabetes InsipidusCausing Mutation in the V2 Vasopressin Receptor by Specific Antagonist and Agonist Pharmacochaper-ones. Front. Pharmacol. 2022, 13, 811836. [Google Scholar] [CrossRef]
- Milano, S.; Carmosino, M.; Gerbino, A.; Svelto, M.; Procino, G. Hereditary Nephrogenic Diabetes Insipidus: Pathophysiology and Possible Treatment. An Update. Int. J. Mol. Sci. 2017, 18, 2385. [Google Scholar] [CrossRef] [PubMed]
- Jean-Alphonse, F.; Perkovska, S.; Frantz, M.C.; Durroux, T.; Mejean, C.; Morin, D.; Loison, S.; Bonnet, D.; Hibert, M.; Mouillac, B.; et al. Biased agonist pharmacochaperones of the avp v2 receptor may treat congenital nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 2009, 20, 2190–2203. [Google Scholar] [CrossRef]
- Bouley, R.; Pastor-Soler, N.; Cohen, O.; McLaughlin, M.; Breton, S.; Brown, D. Stimulation of aqp2 membrane insertion in renal epithelial cells in vitro and in vivo by the cgmp phosphodiesterase inhibitor sildenafil citrate (viagra). Am. J. Physiol. Ren. Physiol. 2005, 288, F1103–F1112. [Google Scholar] [CrossRef]
- Boone, M.; Kortenoeven, M.; Robben, J.H.; Deen, P.M. Effect of the cgmp pathway on aqp2 ex-pression and translocation: Potential implications for nephrogenic diabetes insipidus. Nephrol. Dial. Transpl. 2010, 25, 48–54. [Google Scholar] [CrossRef]

| Time | Weight (kg) | Weight Deficit (%) | Diuresis(mL) | Na (mmol/L) | Urine Osmolality (mOsm/kg) | Plasma Osmolality (mOsm/kg) |
|---|---|---|---|---|---|---|
| 8 AM | 29 | - | - | 153.4 | 54 | 359 |
| 10 AM | 28 | 2.3 | 850 | 154 | 60 | 360 |
| 12 AM | 27.5 | 3.27 | 1300 | 156.7 | 84 | 363.25 |
| 4 h post 120 mcg of Desmopressin | 27.5 | - | - | 160 | 90 | 365 |
| Clinical and Laboratory Examinations | At Admission | After 3 Months of Treatment | Normal Values |
|---|---|---|---|
| Urine density | 1001 | 1008 | 1.008–1.025 |
| Blood osmolality (mOsm/kg) | 335 | 285 | 280–310 |
| Urine osmolality (mOsm/kg) | 85 | 300 | 550–1100 |
| Blood sodium (mmol/L) | 150.8 | 138 | 135–147 |
| Blood chloride (mmol/L). | 114 | 110 | 95–110 |
| Daily volume of liquid intake (mL) | 550 | 750 | - |
| Daily volume of urine output (mL/kg/h) | 7.8 | 2.85 | - |
| Body height (cm). | 65 | 66 | 65 |
| Body weight (kg) | 5 | 5.8 | 7.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stroescu, R.; Chiriţă-Emandi, A.; Puiu, M.; Chisavu, F.; Steflea, R.; Doroş, G.; Gafencu, M. Nephrogenic Diabetes Insipidus Affecting Three Males in Two Generations—Case Report and Review of the Literature. Children 2025, 12, 195. https://doi.org/10.3390/children12020195
Stroescu R, Chiriţă-Emandi A, Puiu M, Chisavu F, Steflea R, Doroş G, Gafencu M. Nephrogenic Diabetes Insipidus Affecting Three Males in Two Generations—Case Report and Review of the Literature. Children. 2025; 12(2):195. https://doi.org/10.3390/children12020195
Chicago/Turabian StyleStroescu, Ramona, Adela Chiriţă-Emandi, Maria Puiu, Flavia Chisavu, Ruxandra Steflea, Gabriela Doroş, and Mihai Gafencu. 2025. "Nephrogenic Diabetes Insipidus Affecting Three Males in Two Generations—Case Report and Review of the Literature" Children 12, no. 2: 195. https://doi.org/10.3390/children12020195
APA StyleStroescu, R., Chiriţă-Emandi, A., Puiu, M., Chisavu, F., Steflea, R., Doroş, G., & Gafencu, M. (2025). Nephrogenic Diabetes Insipidus Affecting Three Males in Two Generations—Case Report and Review of the Literature. Children, 12(2), 195. https://doi.org/10.3390/children12020195








