Abstract
Background/Objectives: Patients with spina bifida (SB) commonly experience neurogenic bowel dysfunction, characterized by defecation-related symptoms. While anorectal dysfunction and slow transit constipation (STC) have been implicated, the role of colonic motility in SB remains unclear. This study aimed to evaluate colonic motility in SB patients with refractory bowel dysfunction. Methods: This retrospective cohort study included SB patients who failed the repeated optimization of a bowel regimen including stimulant laxatives and subsequently underwent anorectal manometry (ARM), colonic transit time (CTT) studies, or colonic manometry (CM). Diagnostic findings were analyzed alongside treatment outcomes. Results: A total of 13 patients with myelomeningocele were included; one declined further treatment, and 12 underwent treatment optimization, with four achieving bowel continence. Of the five patients who proceeded with advanced motility testing, two had abnormal ARM findings, one of three had abnormal CTT results, and all five had normal CM findings. Conclusions: These findings suggest that anorectal dysfunction or STC may play a larger role in refractory bowel symptoms, while colonic motility appears to be preserved, and this highlights the importance of maximizing conservative therapies, particularly with stimulant laxatives, before pursuing invasive tests or surgical interventions for bowel dysfunction in this population.
1. Introduction
Spina bifida (SB) is a congenital malformation of the spinal cord that affects multiple organ systems and can lead to significant long-term disabilities, even after surgical repair. It occurs due to the failure of neural tube closure during the third to fourth week of gestation, resulting in open neural tube defects [1]. Depending on the level of involvement, SB is associated with various functional impairments, including cognitive deficits, paralysis and sensory loss in the lower limbs, and abnormal bladder and bowel function. Constipation and/or fecal incontinence affect up to 86% of patients with SB, likely due to neurogenic bowel dysfunction involving sensory and motor disturbances, which significantly impacts quality of life [2].
Our understanding of the effect of SB on gastrointestinal motility physiology remains limited. Some studies have reported abnormalities in colonic transit and anal sphincter function in patients with SB, while others have noted anal spasms and prolonged sphincter relaxation during rectal balloon distention in patients with other spinal lesions (e.g., tethered cord, fatty filum, and spinal lipoma) [2,3,4,5]. To our knowledge, there are currently no data on colonic motility in patients with SB. Here, we present our experience with colonic motility assessment in SB patients experiencing refractory bowel dysfunction.
2. Materials and Methods
A retrospective cohort study was conducted at a single institution from September 2019 to June 2022, involving patients with SB and bowel dysfunction refractory to medical therapy. Institutional review board approval was obtained. Patients between 4 and 18 years old with SB and constipation and/or fecal incontinence were included, while those with anorectal malformations, Hirschsprung disease, sacral agenesis, previous spinal injury due to trauma or tumor, or overt secondary causes were excluded.
Data collection included demographics, the lesion level, the SB subtype, mobility, medical and surgical history, key physical exam findings, labs, and previous medical treatment. SB lesion levels were categorized into four groups based on nerve innervation: (1) T8 and above, (2) T9–L2, (3) L3–S1, and (4) S2 or lower. Mobility was classified as ambulatory, semi-ambulatory, or wheelchair-bound. The symptoms of bowel dysfunction prior to treatment optimization were categorized as constipation, fecal incontinence, or both.
Referred patients were encouraged to complete a treatment optimization and evaluation process following the facility’s pathway, as illustrated in Supplemental Figures S1 and S2. Medically refractory bowel dysfunction was defined as the failure to respond to optimized conventional therapy (routine use of maximum doses of oral or enteral stimulants combined with one or more other pharmacologic therapies) for at least eight weeks. Those who failed to respond underwent more advanced testing, including colonic transit time (CTT), anorectal manometry (ARM), and/or colonic manometry (CM). Data on surgical procedures performed following testing, if applicable, were also collected.
2.1. Gastrointestinal Motility Studies
2.1.1. Colon Transit Time (CTT)
The simplified method was used to assess colonic transit, utilizing a gelatin capsule containing 24 radio-opaque single-pattern markers. Patients with fecal impaction underwent a bowel cleanout prior to capsule ingestion. Patients were instructed to discontinue stimulant laxatives or anti-diarrheal agents 72 h before the study and to avoid all laxatives, enemas, or suppositories for five days following capsule ingestion. On day 0, the capsule was taken orally, and an abdominal X-ray was performed on day 5. An abnormal CTT was defined as the retention of ≥6 markers [6,7,8].
2.1.2. Anorectal Manometry (ARM)
Preparation included an age-dependent single dose of a glycerin or bisacodyl suppository three hours before the study. Studies were performed with a high-resolution solid-state catheter, following previously published protocols [9]. The variables recorded included the anal resting pressure (mmHg), squeeze pressure (mmHg), and presence and quality of the rectal–anal inhibitory reflex (RAIR), defined as present when a drop of ≥15% in the resting pressure was observed; prolonged RAIR and anal spasms during sustained rectal balloon inflation were also assessed. Normative data published by Kumar et al. were used for comparison [9,10].
2.1.3. Colon Manometry (CM)
Patients underwent bowel preparation the day before the procedure. The procedure was performed as per previously published guidelines [9]. Key parameters recorded included the gastric–colonic response to a meal (GCS) and the presence of high-amplitude propagating contractions (HAPCs). A normal CM study was defined as the presence of both GCS and HAPCs [9].
- GCS: Visual observation of increased post-prandial colonic activity after meal challenge compared to fasting recording.
- HAPCs: Contractions lasting ≥10 s and propagating more than 30 cm with an amplitude of ≥60 mmHg, defined as normal when migrating from the cecum and reaching the recto-sigmoid junction.
2.2. Statistical Analysis
We used descriptive statistics to summarize the basic demographic and clinical characteristics of the study population. We summarize continuous variables using medians and interquartile ranges (IQR) and categorical variables as proportions.
3. Results
A total of 13 patients were included, all with myelomeningocele, with a median (IQR) age of 7 (5–11) years old; nine (69%) were male and four (31%) were female. Three out of these 13 (23.1%) had lesions located at T9–L2, seven (53.8%) at L3–S1, one (7.7%) at S2 and below, and two (15.4%) had an unknown level of lesion. In terms of their bowel dysfunction type before optimization, eight (61.5%) of the patients presented with constipation, one (7.7%) had fecal incontinence, three (23%) had both, and information was not available in one (7.7%) patient. None of them were receiving the optimal enteral bowel regimen at the first visit; nine of them (69%) were not receiving any type of enteral bowel regimen.
Figure 1 outlines the process for patients receiving therapy and undergoing motility studies. Of the 13 patients, one declined further treatment and the remaining 12 patients underwent treatment optimization; seven completed it, while five did not, with a total of four (33%) successful cases (all presented with constipation and remained well controlled until the end of the study). Among these four patients, two had lesions at T9–L2, one at S2 or lower, and one had an unknown lesion level. Of the three patients with refractory bowel dysfunction post-optimization, one had a lesion at T9–L2, and two had lesions at L3–S1. Ultimately, five patients who either failed medical interventions or declined optimization proceeded with more advanced testing.
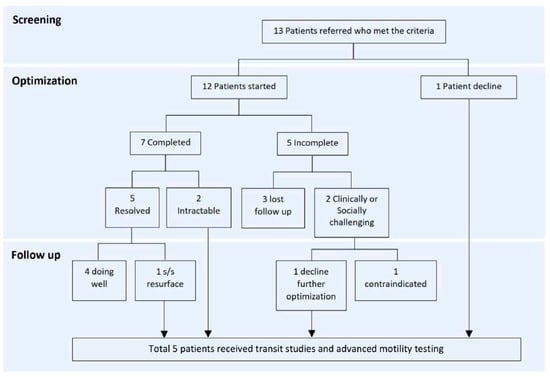
Figure 1.
Treatment optimization process.
Detailed demographic characteristics and results, for those with motility testing, are described in Table 1. In summary, two (40%) presented with constipation, one (20%) with incontinence, and two (40%) with both. All five patients underwent ARM and CM, and three (60%) patients received all testing (CTT, ARM, and CM).

Table 1.
Demographics and results of motility testing (N = 5).
For ARM, all patients had normal resting pressure and a positive RAIR response. One patient (patient 3) had prolonged anal sphincter relaxation during RAIR, and one out of the three had low squeeze pressure (patient 2). Of the three CTT tests, two of them were normal, and one was abnormal. All had normal CM results. Patient 3 underwent an antegrade continence enema (ACE) procedure.
4. Discussion
In our cohort of children with SB, all of whom had myelomeningocele, we observed normal CM findings in all patients, regardless of the bowel dysfunction type or the results of CTT and ARM. To our knowledge, this is the first early case series to describe CM findings in patients with SB and refractory bowel dysfunction.
Colonic motility is governed by the intricate interaction of neuromuscular components, with the Interstitial Cells of Cajal (ICC) playing a critical role in regulating motor patterns such HAPCs. The enteric nervous system, located entirely within the gastrointestinal tract, operates autonomously but is modulated by the extrinsic nervous system [11]. CM is the gold standard in assessing colonic neuromuscular function, although studies on colonic motility in children with spinal cord injury are scarce. Maartji et al. reported the predictive value of CM and contrast enema before cecostomy placement in children with defecation disorders, which included six patients with spinal abnormalities [12]. Among these, two exhibited HAPCs limited to the proximal colon, while one had the complete absence of HAPCs. Notably, the study did not specify the exact spinal abnormalities and used a catheter with a limited number of widely spaced sensors (eight recording sites spaced 10–15 cm apart), which may have reduced the sensitivity in detecting CM parameters compared to high-resolution manometry. Interestingly, a recent study on tethered cord syndrome (TCS) found that the CM results in children with TCS after detethering surgery were similar to those in a matched cohort with functional constipation. While both TCS and SB, specifically myelomeningocele, involve spinal cord injury, their pathophysiology differs. TCS leads to excessive traction, impairing spinal cord perfusion and metabolism, which can improve following detethering [13]. In contrast, myelomeningocele involves an initial failure of neural tube closure followed by ongoing prenatal injury, leading to progressive neurological deterioration. Our findings could suggest that, while the extrinsic nervous system is affected in SB, the intrinsic enteric nervous system could possibly be maintained in patients with myelomeningocele, resulting in normal CM findings. No colonic manometry results for other neurogenic bowel conditions are available in the literature. However, since the extrinsic nervous supply is typically affected while the intrinsic enteric nervous system remains intact regardless of the etiology, our findings may be generalizable to most neurogenic bowel conditions.
Contrary to reports in the literature suggesting that SB patients typically exhibit lower resting pressures on ARM, all patients in our study demonstrated normal resting pressures. Notably, in patient 3, we identified abnormal prolonged sphincter relaxation, aligning with prior studies that have reported significantly longer durations of the RAIR in children with myelomeningocele [3]. Patient 5 exhibited abnormal CTT, a frequent finding in SB. These tests assess different aspects of bowel function. CTT reflects the overall transit time and can be influenced by multiple factors. While colonic motility is one factor, other variables, such as extrinsic nervous regulation, the anorectal complex (continence and defecation regulation), sensory issues in SB, developmental maturity, central muscle tone, mobility, and behavior, also play significant roles. Given that SB patients often display normal CM results but present with abnormal ARM or CTT findings, these observations suggest that anorectal dysfunction or STC may play a more important role in defecation symptoms than colonic dysmotility in this population.
The evaluation and treatment approaches for SB patients are currently limited, with recent studies proposing stepwise approaches, including conservative therapy, oral laxatives, rectal therapy, trans-anal irrigation, and the ACE procedure [14]. Despite the advocacy for stimulant laxatives to improve colonic transit and facilitate recto-sigmoid emptying, only one out of twelve patients in our study was receiving stimulant laxative therapy before optimization. Interestingly, four patients who presented with constipation-type bowel dysfunction successfully achieved continence after optimizing their regimens with stimulant laxatives. The underutilization of stimulant laxatives may be explained by the scope of practice of primary providers in this patient population, where concerns about potential side effects and dependency on stimulants may have discouraged their use. However, a recent study on functional constipation demonstrated that bisacodyl is safe, well tolerated, and can be successfully discontinued in the majority without dependency in patients with functional constipation [15]. Increased education on the safety and long-term use of stimulant laxatives, especially in first-line providers, could enhance the treatment of bowel dysfunction in SB patients, potentially reducing the reliance on invasive testing and surgical interventions.
A prospective study by Daeze et al. demonstrated that patients with SB who have both normal CTT and normal anal resting pressure are likely to experience spontaneous fecal continence [5]. We observed a similar trend, as patient 1 responded well to the initial optimization, with recurrence suspected due to non-compliance. CM has shown promise in predicting and guiding surgical treatment for patients with refractory functional constipation, both initially and in assessing post-surgical improvements in colonic motility to guide decisions on ACE weaning and ostomy closure [16,17]. However, its utility in managing neurogenic bowels in children with SB is unclear. As CM often tends to be normal in these patients’ post-optimization, and none had ACE procedures based solely on the CM results, it may not influence decision-making as it does in functional constipation. Therefore, treatment decisions for SB, such as ACE or ostomy placement, are more likely driven by clinical factors aimed at improving quality of life due to refractoriness to medical treatment, rather than the CM results. This approach mirrors that used for functional constipation unresponsive to conventional therapy. It is possible that CM could become abnormal over time after prolonged treatment failure and progressive colonic distension, following similar clinical progression as refractory functional constipation. Additionally, patients with sacral-level lesions (L3–S1) may have a higher optimization failure rate compared to those with higher thoracic/lumbar lesions (T9–L2), suggesting a potential correlation between the lesion level and refractoriness. Future studies stratifying patients according to the level of lesions could provide further insights, refining the use of advanced motility testing and therapeutic interventions.
Our study has several limitations, including its retrospective design and small sample size. Only the severe form—myelomeningocele—was included, which we believe provides more insight, especially given the normal results; however, this may limit its generalizability. The universally established normal values for motility parameters in ARM and CM, particularly in children, are lacking and remain an ongoing concern among providers. However, efforts have been made to standardize the interpretation and define what is considered normal at present. The sustainability of the optimization outcome is limited to the duration of the study. Additionally, in congruence with any retrospective study design, we also had some missing data elements, limiting our ability to evaluate and interpret all data of interest. Despite these limitations, we hope that our initial report of colonic manometry findings in patients with SB and refractory bowel dysfunction provides a framework and encourages future large, multi-center, prospective cohort collaborations to enhance the generalizability and reliability.
5. Conclusions
Bowel dysfunction is nearly universal in patients with SB, with varying degrees in severity. While anorectal dysfunction and STC are frequently observed, our early findings suggest that colonic manometry evaluations are often normal. Optimizing conservative therapy, particularly the use of stimulants for severe constipation, is crucial before considering invasive tertiary motility evaluations like CM. However, since the CM results tend to be normal in these patients, treatment decisions are more likely guided by clinical judgment rather than test results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/children12020184/s1, Figure S1: Facility clinical decision-making pathway; Figure S2: Facility treatment optimization and level of treatment pathway.
Author Contributions
A.Y.C.S.: Contributed to the conception and design of the study, participated in data collection, interpreted data, drafted and revised the manuscript. B.D.: Assisted in conducting the study and data collection and reviewed/revised the manuscript. T.P.: Assisted in conducting the study and data collection and reviewed/revised the manuscript. P.B.: Contributed to data analysis and interpretation and revised the manuscript for intellectual content. L.R.: Provided guidance on study design, contributed to data interpretation, and critically reviewed and revised the manuscript. D.P.: Oversaw study planning and execution, contributed to manuscript revisions and data interpretation, and critically reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Institutional Review Board of Saint Louis University (protocol code 31588; date of approval 23 December 2020).
Informed Consent Statement
Patient consent was waived under the exempt category 4—secondary data analysis, meeting criterion (iii), for which consent is not required as per IRB guidelines. The specific criteria are as follows: the research involves only information collection and analysis involving the investigator’s use of identifiable health information when that use is regulated under [HIPAA], for the purposes of “health care operations” or “research” as those terms are defined [by HIPAA] or for “public health activities and purposes” as described under [HIPAA].
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Janik, K.; Manire, M.A.; Smith, G.M.; Krynska, B. Spinal Cord Injury in Myelomeningocele: Prospects for Therapy. Front. Cell. Neurosci. 2020, 14, 201. [Google Scholar] [CrossRef]
- Velde, S.V.; Pratte, L.; Verhelst, H.; Meersschaut, V.; Herregods, N.; Van Winckel, M.; Van Biervliet, S. Colon transit time and anorectal manometry in children and young adults with spina bifida. Int. J. Color. Dis. 2013, 28, 1547–1553. [Google Scholar] [CrossRef]
- Siddiqui, A.; Rosen, R.; Nurko, S. Anorectal manometry may identify children with spinal cord lesions. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, N.; Leroi, A.M. Colonic transit time in patients with myelomeningocele. Neurogastroenterol. Motil. 1997, 9, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Daeze, C.; Van Biervliet, S.; Van Laecke, E.; Van Winckel, M.; De Bruyne, R.; De Guchtenaere, A.; Hoebeke, P.; Vande Velde, S. The predictive value of colon transit time and anorectal manometry in the approach of faecal continence in children with spina bifida. Acta Gastroenterol. Belg. 2018, 81, 277–282. [Google Scholar]
- Yuan, Z.; Cheng, W.; Hou, A.; Wang, W.; Zhang, S.; Liu, D.; Gao, F.; Li, H.; Wang, W. Constipation is associated with spina bifida occulta in children. Clin. Gastroenterol. Hepatol. 2008, 6, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Tipnis, N.A.; El-Chammas, K.I.; Rudolph, C.D.; Werlin, S.L.; Sood, M.R. Do oro-anal transit markers predict which children would benefit from colonic manometry studies? J. Pediatr. Gastroenterol. Nutr. 2012, 54, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Devroede, G.; Bouchoucha, M.; Steiber, W. A Simplified way to access colorectal transit time. Tech. Coloprotocol. 1999, 3, 71–73. [Google Scholar] [CrossRef]
- Rodriguez, L.; Sood, M.; Di Lorenzo, C.; Saps, M. An ANMS-NASPGHAN consensus document on anorectal and colonic manometry in children. Neurogastroenterol. Motil. 2017, 29, e12944. [Google Scholar] [CrossRef]
- Kumar, S.; Ramadan, S.; Gupta, V.; Helmy, S.; Atta, I.; Alkholy, A. Manometric tests of anorectal function in 90 healthy children: A clinical study from Kuwait. J. Pediatr. Surg. 2009, 44, 1786–1790. [Google Scholar] [CrossRef] [PubMed]
- Wattchow, D.A.; Brookes, S.J.; Spencer, N.J.; Heitmann, P.T.; De Giorgio, R.; Costa, M.; Dinning, P.G. From the organ bath to the whole person: A review of human colonic motility. ANZ J. Surg. 2024, 94, 320–326. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, M.M.; Hogan, M.; Caniano, D.A.; Di Lorenzo, C.; Benninga, M.A.; Mousa, H.M. Colonic manometry as predictor of cecostomy success in children with defecation disorders. J. Pediatr. Surg. 2006, 41, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Weisbrod, L.J.; Thorell, W. Tethered Cord Syndrome (TCS); StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ambartsumyan, L.; Rodriguez, L. Bowel management in children with spina bifida. J. Pediatr. Rehabil. Med. 2018, 11, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, S.; Nurko, S.; Rodriguez, L. Long-term Use of Bisacodyl in Pediatric Functional Constipation Refractory to Conventional Therapy. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.; Heinz, N.; Nurko, S. Utility of Colon Manometry in Guiding Therapy and Predicting Need for Surgery in Children With Defecation Disorders. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 232–237. [Google Scholar] [CrossRef]
- Rodriguez, L.; Colliard, K.; Nurko, S.; Flores, A.; Buchmiller, T.L. Diverting Ileostomy in Children With Functional Constipation: A Study Evaluating the Utility of Colon Manometry. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 578–583. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).