Severe Asthma in School-Age Children: An Updated Appraisal on Biological Options and Challenges in This Age Group
Abstract
1. Introduction
Managing Severe Asthma in School-Age Children: Key Challenges
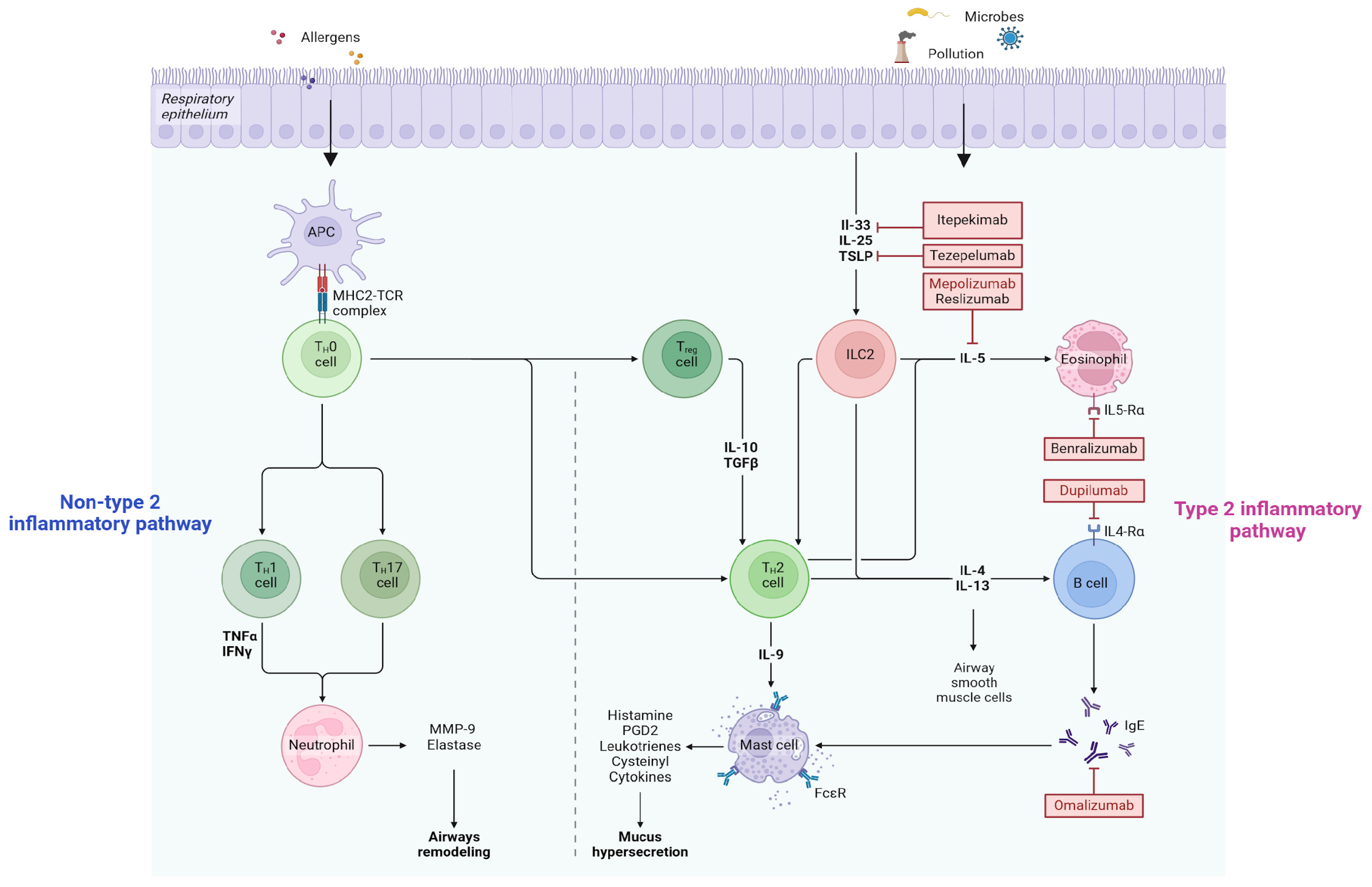
2. Approved Biological Therapies in School-Age Children
2.1. Dupilumab
2.2. Mepolizumab
2.3. Omalizumab
3. Discussion
- Adherence to treatment: Pediatric patients often face unique challenges regarding compliance with biologic therapies, which are typically injectable. Younger children might find the injections uncomfortable or distressing, and their understanding of the importance of consistent treatment may be limited [71]. This highlights the need for strong caregiver involvement and educational strategies to support adherence and ensure the therapy is administered correctly and consistently.
- Safety considerations: Although the safety profiles of these biological therapies are generally reassuring, offering a strong foundation for their use, with serious side effects being rare, safety remains a primary concern in younger populations. In children, the risk of adverse effects must be continuously monitored, as their developing immune systems and unique physiology may react differently to treatment. Vigilance in identifying and managing even rare side effects, such as injection site reactions, is particularly important in this age group.
- Duration of therapy and discontinuation: A significant challenge in pediatric biologic therapy is determining the appropriate length of treatment. Long-term data in children are limited, and questions remain about the optimal timing for discontinuation. Clinicians need to consider what happens when therapy is stopped—whether symptoms will rebound, if disease control will deteriorate, or if treatment can be tapered without compromising outcomes. The existing data on omalizumab show conflicting results [72]. Addressing these questions requires more robust data from longitudinal studies targeting pediatric populations [73].
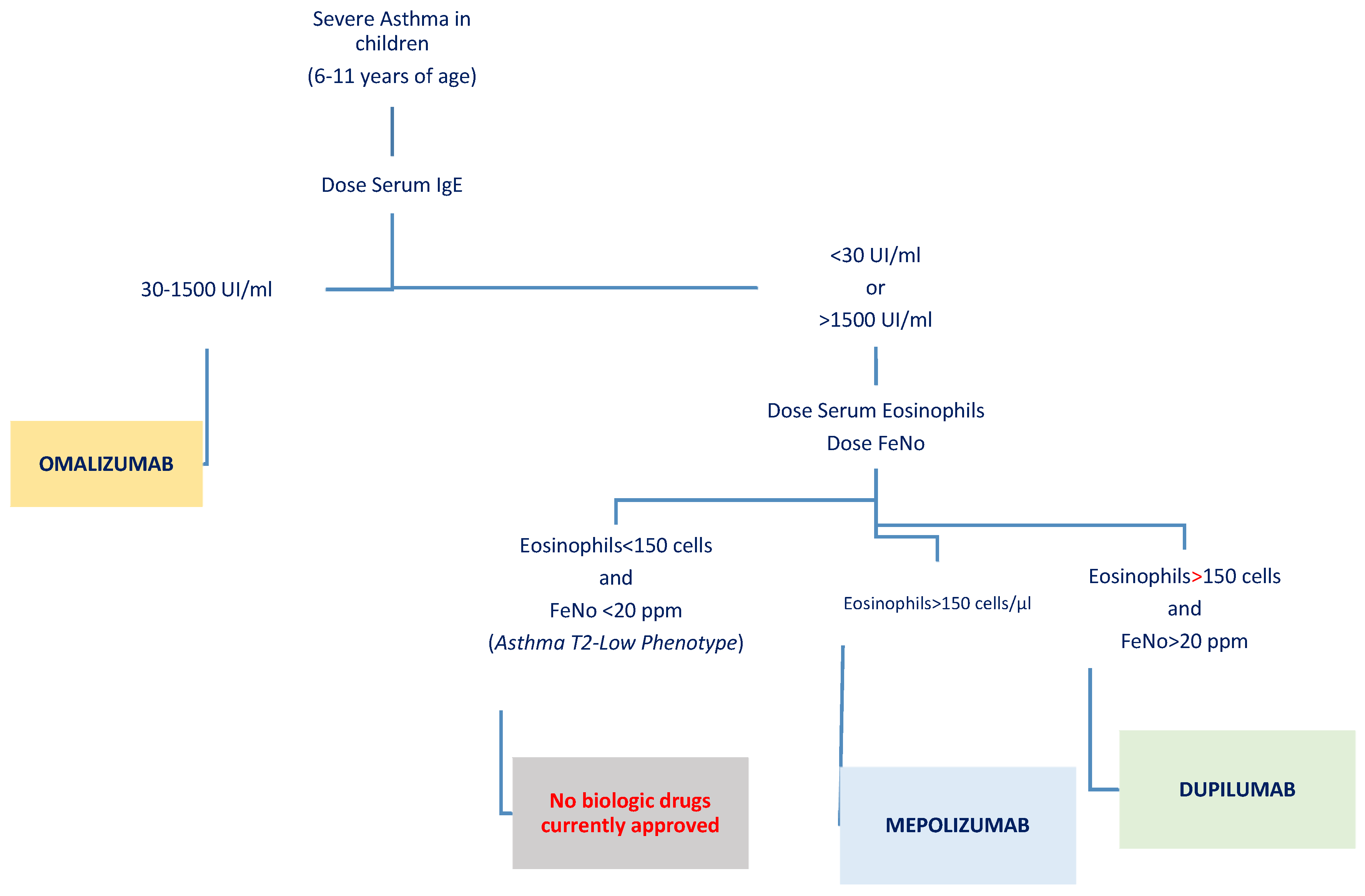
- Identification of biomarkers: Choosing the right biological therapy for each patient requires a detailed understanding of their disease phenotype and the use of reliable biomarkers (Figure 2). Personalized treatment strategies, which take into account individual biomarkers and comorbid conditions, can significantly enhance the likelihood of achieving optimal therapeutic outcomes. Yet, several challenges persist, including the lack of robust head-to-head studies comparing these biologics and the fact that responses to treatment can vary widely across patients. While markers for identifying the T2-high phenotype are well established, IL-4, IL-5, IL-13, IgE, eosinophilia, and high FeNO levels, evidence for T2-low biomarkers remains limited [74,75].
- Impact on healthcare sustainability: A balance exists between the advantages and costs of biological therapies for children with severe asthma. These treatments markedly reduced hospital admissions, emergency room visits, and reliance on oral corticosteroids, enhancing asthma control and addressing comorbidities. However, they also significantly increased overall healthcare expenses. This trade-off highlights the importance of weighing clinical benefits against financial impacts and emphasizes the need for thoughtful, individualized use of biologics to ensure healthcare sustainability [77].
- The ‘modifying disease’ effect and prevention impact: Recent findings underscore the potential of biologics to not only manage symptoms of allergic diseases but also prevent their progression, a concept integral to the atopic march [35]. The atopic march describes the sequential development of allergic conditions, beginning with AD in infancy and advancing to food allergies, asthma, and allergic rhinitis in later childhood or adolescence. Dupilumab has shown promising results in reducing the onset of new allergic comorbidities in individuals with severe early-onset AD [78]. Meta-analyses of clinical trials suggest that early intervention with dupilumab significantly decreases the risk of additional allergic diseases by modulating the immune response during a critical window of immune system plasticity [34]. This preventative effect is particularly pronounced in younger patients, where the immune system remains more adaptable, allowing biologics to effectively recalibrate inflammatory pathways before the full establishment of chronic allergic patterns. For instance, reductions of up to 37% in the incidence of new allergies and even greater effects when targeting IgE-mediated conditions suggest the potential for profound impacts on long-term disease outcomes. Omalizumab has also broadened its indications to include severe food allergies, marking a critical step forward in the prevention of severe reactions [79,80]. This effect not only lowers the risk of life-threatening reactions but also alleviates the psychological burden of food allergies, improving the quality of life of children and their families [81]. The ability of omalizumab to modulate immune responses highlights its role in potentially preventing the progression of allergic conditions by reducing the severity and frequency of sensitizations over time. The concept of prevention through biologics extends beyond individual conditions to the broader atopic march. However, achieving this preventative potential requires a better understanding of optimal intervention windows and patient selection criteria [82]. Current data suggest that biologics offer a promising avenue for reshaping the management of allergic diseases, not only by controlling symptoms but by fundamentally altering the immune landscape to reduce the development of future allergies. Further research is essential to refine these strategies, ensuring biologics achieve their full potential as preventative tools while balancing efficacy, cost, and long-term sustainability.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICS | Inhaled Corticosteroid |
| GERD | Gastroesophageal Reflux Disease |
| OSA | Obstructive Sleep Apnea |
| PEF | Peak Expiratory Flow |
| LABA | Long-Acting Beta2-Agonist |
| GINA | Global Initiative for Asthma |
| FeNO | Fractional Exhaled Nitric Oxide |
| T2 | Type 2 |
| IL | Interleukin |
| IgE | Immunoglobulin E |
| FDA | Food and Drug Administration |
| EMA | European Medicines Agency |
| AIFA | Agenzia Italiana Del Farmaco (Italian Medicines Agency) |
| Q4W | Every Four Weeks |
| Q2W | Every Two Weeks |
| ACQ-7 | Asthma Control Questionnaire 7 |
| FEV1 | Forced Expiratory Volume in 1 Second |
| FVC | Forced Vital Capacity |
| EF25–75% | Forced Expiratory Flow 25–75% |
| Th2 | T-helper 2 (Cells) |
| T2-high | Type 2 High (Phenotype) |
| T2-low | Type 2 Low (Phenotype) |
| IL-5 | Interleukin-5 |
| RCT | Randomized Controlled Trial |
| C-ACT | Childhood Asthma Control Test |
| OFC | Oral Food Challenge |
| NOAEL | No Observed Adverse Effect Level |
| BDP | Beclometasone Dipropionate |
| PK | Pharmacokinetics |
| PD | Pharmacodynamics |
| TSLP | Thymic Stromal Lymphopoietin |
| IL-13 | Interleukin-13 |
| JAK | Janus Kinase |
References
- The Global Asthma Report 2018; GlobalAsthma Network: Auckland, New Zealand, 2018; Available online: https://globalasthmareport.org/2018/index.html (accessed on 26 December 2024).
- To, T.; Stanojevic, S.; Moores, G.; Gershon, A.S.; Bateman, E.D.; Cruz, A.A.; Boulet, L.P. Global asthma prevalence in adults: Findings from the cross-sectional world health survey. BMC Public Health 2012, 12, 204. [Google Scholar] [CrossRef]
- Fuhlbrigge, A.L.; Jackson, B.; Wright, R. Gender and asthma. Immunol. Allergy Clin. N. Am. 2002, 22, 753–789. [Google Scholar] [CrossRef]
- GINA 2024 Stategy Report. Available online: https://ginasthma.org/wp-content/uploads/2024/05/GINA-2024-Strategy-Report-24_05_22_WMS.pdf (accessed on 26 December 2024).
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Porcaro, F.; Ullmann, N.; Di Marco, A.; Allegorico, A.; Cherchi, C.; Paglietti, M.G.; Cutrera, R. Severe asthma guidelines in children and adolescents: A practical document for physicians. Pediatr. Pulmonol. 2023, 58, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Hedlin, G.; Bush, A.; Lødrup Carlsen, K.; Wennergren, G.; De Benedictis, F.M.; Melén, E.; Paton, J.; Wilson, N.; Carlsen, K.-H. Problematic severe asthma in children, not one problem but many: A GA2LEN initiative. Eur. Respir. J. 2010, 36, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, M.; Kantar, A.; Masini, B.; Nuzzi, G.; Ragazzo, V.; Peroni, D. Structural and functional development in airways throughout childhood: Children are not small adults. Pediatr. Pulmonol. 2021, 56, 240–251. [Google Scholar] [CrossRef]
- Busse, P.J.; Mathur, S.K. Age-related changes in immune function: Effect on airway inflammation. J. Allergy Clin. Immunol. 2010, 126, 690–701. [Google Scholar] [CrossRef]
- Manti, S.; Magri, P.; De Silvestri, A.; De Filippo, M.; Votto, M.; Marseglia, G.L.; Licari, A. Epidemiology of severe asthma in children: A systematic review and meta-analysis. Eur. Respir. Rev. 2024, 33, 240095. [Google Scholar] [CrossRef] [PubMed]
- Bacharier, L.B.; Jackson, D.J. Biologics in the treatment of asthma in children and adolescents. J. Allergy Clin. Immunol. 2023, 151, 581–589. [Google Scholar] [CrossRef]
- Andrenacci, B.; De Filippo, M.; Votto, M.; Prevedoni Gorone, M.S.; De Amici, M.; La Grutta, S.; Marseglia, G.L.; Licari, A. Severe pediatric asthma endotypes: Current limits and future perspectives. Expert Rev. Respir. Med. 2023, 17, 675–690. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, Y.E.; Rutjes, N.W.; Golebski, K.; Şahin, H.; Hashimoto, S.; Maitland-van der Zee, A.H.; Vijverberg, S.J. Developments in the Management of Severe Asthma in Children and Adolescents: Focus on Dupilumab and Tezepelumab. Paediatr. Drugs 2023, 25, 677–693. [Google Scholar] [CrossRef] [PubMed]
- Dinardo, G.; Indolfi, C.; Klain, A.; Bencivenga, C.L.; Ferrara, S.; Decimo, F.; Miraglia del Giudice, M. Overview on the treatment of severe bronchial asthma. Glob. Pediatr. 2024, 7, 100117. [Google Scholar] [CrossRef]
- Nopsopon, T.; Brown, A.; Hahn, G.; Rank, M.; Huybrechts, K.F.; Akenroye, A. Temporal variation in the effectiveness of biologics in asthma: Effect modification by changing patient characteristics. Respir. Med. 2024, 234, 107802. [Google Scholar] [CrossRef] [PubMed]
- Indolfi, C.; Dinardo, G.; Klain, A.; Contieri, M.; Umano, G.R.; Decimo, A.; Ciprandi, G.; Del Giudice, M.M. Time effect of dupilumab to treat severe uncontrolled asthma in adolescents: A pilot study. Allergol. Immunopathol. 2023, 51, 12–18. [Google Scholar] [CrossRef]
- Dinardo, G.; Indolfi, C.; Klain, A.; Decimo, F.; Miraglia Del Giudice, M. Treatment of severe asthma: Fast action of dupilumab in the pediatric setting. Minerva Pediatr. 2023, 75, 312–313. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, G.L.; Licari, A.; Tosca, M.A.; Miraglia Del Giudice, M.; Indolfi, C.; Ciprandi, G. An Updated Reappraisal of Dupilumab in Children and Adolescents with Severe Asthma. Children 2024, 11, 843. [Google Scholar] [CrossRef]
- Press Release: FDA Expands Approval of Dupixent® (Dupilumab) to Include Children Aged 6 to 11 YEARS with Moderate-to-Severe Asthma. Available online: https://www.sanofi.com/en/media-room/press-releases/2021/2021-10-20-21-30-00-2317854 (accessed on 26 December 2024).
- Press Release: Dupixent® (Dupilumab) Approved by European Commission for Children Aged 6 to 11 Years with Severe Asthma with Type 2 Inflammation. Available online: https://www.sanofi.com/en/media-room/press-releases/2022/2022-04-07-05-00-00-2418107 (accessed on 26 December 2024).
- Regime di Rimborsabilita’ e Prezzo, a Seguito di Nuove Indicazioni Terapeutiche, Riclassificazione e Rinegoziazione del Medicinale per Uso Umano «Dupixent» AIFA. Available online: https://www.aifa.gov.it/documents/20142/961234/Determina_630-2024_Dupixent.pdf (accessed on 26 December 2024).
- Dupixent EMA. Available online: https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf (accessed on 26 December 2024).
- Bacharier, L.B.; Maspero, J.F.; Katelaris, C.H.; Fiocchi, A.G.; Gagnon, R.; de Mir, I.; Jain, N.; Sher, L.D.; Mao, X.; Liu, D.; et al. Liberty Asthma VOYAGE Investigators Dupilumab in Children with Uncontrolled Moderate-to-Severe Asthma. N. Engl. J. Med. 2021, 385, 2230–2240. [Google Scholar] [CrossRef]
- Jackson, D.J.; Hamelmann, E.; Roberts, G.; Bacharier, L.B.; Xia, C.; Gall, R.; Ledanois, O.; Coleman, A.; Tawo, K.; Jacob-Nara, J.A.; et al. Dupilumab Efficacy and Safety in Children With Moderate-to-Severe Asthma and High Blood Eosinophils: A Post Hoc Analysis of VOYAGE. J. Allergy Clin. Immunol. Pract. 2024. advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, T.W.; Murphy, K.R.; Hamelmann, E.; Ross, K.R.; Gupta, A.; Fiocchi, A.; Xia, C.; Gall, R.; Ledanois, O.; Radwan, A.; et al. Impact of Lung Function on Asthma Exacerbation Rates in Children Treated with Dupilumab: The VOYAGE Study. J. Asthma Allergy 2024, 17, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Bacharier, L.B.; Phipatanakul, W.; Sher, L.; Domingo, C.; Papadopoulos, N.; Modena, B.; Li, N.; Xia, C.; Kamal, M.A.; et al. Dupilumab pharmacokinetics and effect on type 2 biomarkers in children with moderate-to-severe asthma. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2023, 131, 44–51.e4. [Google Scholar] [CrossRef] [PubMed]
- Maspero, J.F.; Antila, M.A.; Deschildre, A.; Bacharier, L.B.; Altincatal, A.; Laws, E.; Mortensen, E.; Radwan, A.; Jacob-Nara, J.A.; Deniz, Y.; et al. Dupilumab Efficacy in Children With Type 2 Asthma Receiving High/Medium-Dose ICS (VOYAGE). J. Allergy Clin. Immunol. Pract. 2024. advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Maspero, J.F.; Fiocchi, A.G.; Deschildre, A.; Bacharier, L.B.; Altincatal, A.; Laws, E.; Lederer, D.J.; Akinlade, B.; Hardin, M. Dupilumab improves pediatric type 2 asthma outcomes independent of patient baseline characteristics. J. Allergy Clin. Immunol. Pract. 2024, 12, 3135–3138.e2. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.; Szefler, S.J.; Bacharier, L.B.; Maspero, J.F.; Domingo, C.; Daizadeh, N.; Lederer, D.J.; Hardin, M.; Jacob-Nara, J.A.; Deniz, Y.; et al. Dupilumab efficacy in children with uncontrolled, moderate-to-severe asthma with and without an allergic phenotype. Eur. Respir. J. 2021, 58 (Suppl. 65), OA2568. [Google Scholar] [CrossRef]
- Bacharier, L.B.; Guilbert, T.W.; Katelaris, C.H.; Deschildre, A.; Phipatanakul, W.; Liu, D.; Altincatal, A.; Mannent, L.P.; Amin, N.; Laws, E.; et al. Dupilumab Improves Lung Function Parameters in Pediatric Type 2 Asthma: VOYAGE Study. J. Allergy Clin. Immunol. Pract. 2024, 12, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Bacharier, L.B.; Maspero, J.F.; Katelaris, C.H.; Fiocchi, A.G.; Gagnon, R.; de Mir, I.; Guilbert, T.W.; Jackson, D.J.; Staudinger, H.W.; Laws, E.; et al. Assessment of long-term safety and efficacy of dupilumab in children with asthma (LIBERTY ASTHMA EXCURSION): An open-label extension study. Lancet Respir. Med. 2024, 12, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Phipatanakul, W.; Vogelberg, C.; Bacharier, L.B.; Dell, S.; Altincatal, A.; Gall, R.; Ledanois, O.; Sacks, H.; Jacob-Nara, J.A.; Deniz, Y.; et al. Dupilumab 200 mg was efficacious in children (6–11 years) with moderate-to-severe asthma for up to 2 years: EXCURSION open-label extension study. Pediatr. Pulmonol. 2024, 59, 2976–2983. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, G.; Fiocchi, A.; Marseglia, G.; Miraglia Del Giudice, M.; Cutrera, R.; Bitonti, R.; Fanelli, F.; Stassaldi, A.; Nicolosi, G.; Furneri, G. Type 2 asthma paediatric patients eligible for dupilumab: An Italian biomarker-based analysis. World Allergy Organ. J. 2024, 17, 100933. [Google Scholar] [CrossRef]
- Geba, G.P.; Li, D.; Xu, M.; Mohammadi, K.; Attre, R.; Ardeleanu, M.; Musser, B. Attenuating the atopic march: Meta-analysis of the dupilumab atopic dermatitis database for incident allergic events. J. Allergy Clin. Immunol. 2023, 151, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. In search of the Holy Grail in atopic dermatitis: Will dupilumab become the first disease-modifying atopic dermatitis drug? J. Allergy Clin. Immunol. 2023, 151, 694–696. [Google Scholar] [CrossRef]
- Giovannini, M.; Mori, F.; Barni, S.; de Martino, M.; Novembre, E. Omalizumab and mepolizumab in the landscape of biological therapy for severe asthma in children: How to choose? Ital. J. Pediatr. 2019, 45, 151. [Google Scholar] [CrossRef]
- Indolfi, C.; Dinardo, G.; Klain, A.; Decimo, F.; Miraglia Del Giudice, M. Treatment of Severe Asthma: Case Report of Fast Action of Mepolizumab in a Patient with Recent SARS-CoV-2 Infection. Life 2024, 14, 1063. [Google Scholar] [CrossRef] [PubMed]
- Nucala Access Data FDA. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125526Orig1s021,761122Orig1s011Corrected_lbl.pdf (accessed on 26 December 2024).
- van Dijk, Y.E.; Brandsen, M.A.; Hashimoto, S.; Rutjes, N.W.; Golebskiet, K.; Vermeulen, F.; Terheggen-Lagro, S.W.J.; van Ewijk, B.E.; der Zee, A.H.; Vijverberg, S.J. Factors influencing the initiation of biologic therapy in children with severe asthma: Results of the pediatric asthma noninvasive diagnostic approaches (PANDA) study. Pediatr. Pulmonol. 2024, 59, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.; Chan, R.; Brown, N.; Howarth, P.; Gilson, M.; Price, R.G.; Maspero, J. Long-term safety of mepolizumab for up to ∼10 years in patients with severe asthma: Open-label extension study. Ann. Med. 2024, 56, 2417184. [Google Scholar] [CrossRef]
- Morris, T.S.; Autry, E.B.; Kuhn, R.J. The Role of Biologics in the Management of Asthma in the Pediatric Patient. J. Pediatr. Pharmacol. Ther. 2021, 26, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Pouliquen, I.; Austin, D.; Price, R.G.; Kempsford, R.; Steinfeld, J.; Bradford, E.S.; Yancey, S.W. Subcutaneous mepolizumab in children aged 6 to 11 years with severe eosinophilic asthma. Pediatr. Pulmonol. 2019, 54, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Ikeda, M.; Geng, B.; Azmi, J.; Price, R.G.; Bradford, E.S.; Yancey, S.W.; Steinfeld, J. Long-term safety and pharmacodynamics of mepolizumab in children with severe asthma with an eosinophilic phenotype. J. Allergy Clin. Immunol. 2019, 144, 1336–1342.e7. [Google Scholar] [CrossRef]
- Jackson, D.J.; Bacharier, L.B.; Gergen, P.J.; Gagalis, L.; Calatroni, A.; Wellford, S.; Gill, M.A.; Stokes, J.; Liu, A.H.; Gruchalla, R.S.; et al. Mepolizumab for urban children with exacerbation-prone eosinophilic asthma in the USA (MUPPITS-2): A randomised, double-blind, placebo-controlled, parallel-group trial. Lancet 2022, 400, 502–511. [Google Scholar] [CrossRef]
- Maglione, M.; Borrelli, M.; Dorato, A.; Cimbalo, C.; Del Giudice, L.A.; Santamaria, F. Mepolizumab in Severe Pediatric Asthma: Certainties and Doubts through a Single-Center Experience and Review of the Literature. Children 2024, 11, 895. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.E.; Knight, J.; Liu, Q.; Shelar, A.; Stewart, E.; Wang, X.; Yan, X.; Sanders, J.; Visness, C.; Gill, M.; et al. Activated sputum eosinophils associated with exacerbations in children on mepolizumab. J. Allergy Clin. Immunol. 2024, 154, 297–307.e13. [Google Scholar] [CrossRef] [PubMed]
- FDA Drug Safety Communication: FDA Approves Label Changes for Asthma Drug Xolair (Omalizumab), Including Describing Slightly Higher Risk of Heart and Brain Adverse Events. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-approves-label-changes-asthma-drug-xolair-omalizumab-including#:~:text=FDA%20approved%20Xolair%20in%202003,asthma%20medicines%20called%20inhaled%20corticosteroids (accessed on 26 December 2024).
- Chong, W.; Li, H.; Wang, J. Therapeutic efficacy of omalizumab in children with moderate-to-severe allergic asthma combined with chronic sinusitis. Front. Allergy 2023, 4, 1236798. [Google Scholar] [CrossRef]
- Castagnoli, R.; Brambilla, I.; Giovannini, M.; Marseglia, G.L.; Licari, A. New approaches in childhood asthma treatment. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 319–326. [Google Scholar] [CrossRef]
- Berger, W.; Gupta, N.; McAlary, M.; Fowler-Taylor, A. Evaluation of long-term safety of the anti-IgE antibody, omalizumab, in children with allergic asthma. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2003, 91, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Chipps, B.E.; Lanier, B.; Milgrom, H.; Deschildre, A.; Hedlin, G.; Szefler, S.J.; Kattan, M.; Kianifard, F.; Ortiz, B.; Haselkorn, T.; et al. Omalizumab in children with uncontrolled allergic asthma: Review of clinical trial and real-world experience. J. Allergy Clin. Immunol. 2017, 139, 1431–1444. [Google Scholar] [CrossRef] [PubMed]
- Fenu, G.; La Tessa, A.; Calogero, C.; Lombardi, E. Severe pediatric asthma therapy: Omalizumab-A systematic review and meta-analysis of efficacy and safety profile. Front. Pediatr. 2023, 10, 1033511. [Google Scholar] [CrossRef] [PubMed]
- Approvazione Xolair AIFA. Available online: https://torino.fimmg.org/files/news_0642.pdf (accessed on 26 December 2024).
- US Food and Drug Administration. Xolair FDA Prescribing Information. 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/103976s5225lbl.pdf (accessed on 26 December 2024).
- Milgrom, H.; Berger, W.; Nayak, A.; Gupta, N.; Pollard, S.; McAlary, M.; Taylor, A.F.; Rohane, P. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics 2001, 108, E36. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Morgan, W.J.; Gergen, P.J.; Mitchell, H.E.; Gern, J.E.; Liu, A.H.; Gruchalla, R.S.; Kattan, M.; Teach, S.J.; Pongracic, J.A.; et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N. Engl. J. Med. 2011, 364, 1005–1015. [Google Scholar] [CrossRef]
- Lanier, B.; Bridges, T.; Kulus, M.; Taylor, A.F.; Berhane, I.; Vidaurre, C.F. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J. Allergy Clin. Immunol. 2009, 124, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Kulus, M.; Hébert, J.; Garcia, E.; Fowler Taylor, A.; Fernandez Vidaurre, C.; Blogg, M. Omalizumab in children with inadequately controlled severe allergic (IgE-mediated) asthma. Curr. Med. Res. Opin. 2010, 26, 1285–1293. [Google Scholar] [CrossRef]
- Brodlie, M.; McKean, M.C.; Moss, S.; Spencer, D.A. The oral corticosteroid-sparing effect of omalizumab in children with severe asthma. Arch. Dis. Child. 2012, 97, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Silkoff, P.E.; Romero, F.A.; Gupta, N.; Townley, R.G.; Milgrom, H. Exhaled nitric oxide in children with asthma receiving Xolair (omalizumab), a monoclonal anti-immunoglobulin E antibody. Pediatrics 2004, 113, e308–e312. [Google Scholar] [CrossRef] [PubMed]
- Lemanske RFJr Nayak, A.; McAlary, M.; Everhard, F.; Fowler-Taylor, A.; Gupta, N. Omalizumab improves asthma-related quality of life in children with allergic asthma. Pediatrics 2002, 110, e55. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.; Gross, G.; van Bavel, J.; Lee, T.; Windom, H.; Everhard, F.; Fowler-Taylor, A.; Liu, J.; Gupta, N. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J. Allergy Clin. Immunol. 2003, 111, 278–284. [Google Scholar] [CrossRef]
- Brannick, S.; McDonald, M.; Greally, P.; Elnazir, B.; Ahmareen, O. Omalizumab for the treatment of severe allergic asthma in children: A tale of two. Clin. Case Rep. 2022, 10, e6255. [Google Scholar] [CrossRef] [PubMed]
- Giubergia, V.; Ramírez Farías, M.J.; Pérez, V.; Crespi, N.; Castaños, C. Clinical impact of omalizumab treatment in children with severe asthma: Report of a local experience. Impacto clínico del tratamiento con omalizumab en niños con asma grave: Reporte de una experiencia local. Arch. Argent. Pediatr. 2019, 117, e115–e120. [Google Scholar] [CrossRef] [PubMed]
- Morales-Múnera, O.; Pedraza, Á.; Niño-Serna, L. Omalizumab en niños con asma no controlada: Estudio en la vida real realizado en Colombia [Omalizumab in children with uncontrolled asthma: A real-life study carried out in Colombia]. Rev. Alerg. Mex. 2018, 65, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Folqué, M.M.; Lozano, J.; Riggioni, C.; Piquer, M.; Álvaro, M.; Machinena, A.; Giner, M.T.; Domínguez, O.; Jiménez-Feijoo, R.M.; Dias da Costa, M.; et al. ‘Real-life’ experience in asthmatic children treated with omalizumab up to six-years follow-up. Allergol. Immunopathol. 2019, 47, 336–341. [Google Scholar] [CrossRef]
- FDA Approves First Medication to Help Reduce Allergic Reactions to Multiple Foods After Accidental Exposure. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-medication-help-reduce-allergic-reactions-multiple-foods-after-accidental. (accessed on 26 December 2024).
- Dinardo, G.; Cafarotti, A.; Fierro, V.; Artesani, M.C.; Indolfi, C.; Miraglia Del Giudice, M.; Fiocchi, A. Role of biologics in severe food allergy. Curr. Opin. Allergy Clin. Immunol. 2024, 24, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Muraro, A.; Nurmatov, U.; Arasi, S.; Stevanovic, K.; Anagnostou, A.; Bonaguro, R.; Chinthrajah, S.; Lack, G.; Fiocchi, A.; et al. GA2LEN ANACARE consensus statement: Potential of omalizumab in food allergy management. Clin. Transl. Allergy 2024, 14, e70002. [Google Scholar] [CrossRef] [PubMed]
- Arasi, S.; Cafarotti, A.; Galletta, F.; Panetta, V.; Riccardi, C.; Calandrelli, V.; Fierro, V.; Dahdah, L.; Artesani, M.C.; Valluzzi, R.; et al. Omalizumab reduces anaphylactic reactions and allows food introduction in food-allergic in children with severe asthma: An observational study. Allergy 2024. [Google Scholar] [CrossRef] [PubMed]
- Racine, N.M.; Riddell, R.R.; Khan, M.; Calic, M.; Taddio, A.; Tablon, P. Systematic Review: Predisposing, Precipitating, Perpetuating, and Present Factors Predicting Anticipatory Distress to Painful Medical Procedures in Children. J. Pediatr. Psychol. 2016, 41, 159–181. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Bourdin, A.; Taillé, C.; Kamar, D.; Thonnelier, C.; Lajoinie, A.; Rigault, A.; Deschildre, A.; Molimard, M. Real-life omalizumab exposure and discontinuation in a large nationwide population-based study of paediatric and adult asthma patients. Eur. Respir. J. 2022, 60, 2103130. [Google Scholar] [CrossRef]
- Nieto, A.; El-Sayed, Z.A.; Gómez, R.M.; Hossny, E.; Jiu-Yao, W.; Kalayci, Ö.; Morais-Almeida, M.; Phipatanakul, W.; Pitrez, P.M.; Pozo Beltrán, C.F.; et al. Unanswered questions on the use of biologics in pediatric asthma. World Allergy Organ. J. 2023, 16, 100837. [Google Scholar] [CrossRef] [PubMed]
- Indolfi, C.; Klain, A.; Dinardo, G.; Bencivenga, C.L.; D’Addio, E.; Ferrara, S.; Decimo, F.; Ciprandi, G.; Miraglia del Giudice, M. Contemporary biomarkers in severe asthma management. J. Biol. Regul. Homeost. Agents 2024, 38, 3569–3581. [Google Scholar] [CrossRef]
- Mahmood, L.; Keskin, S.; Jefferson, A.A. Precision care in the treatment of pediatric asthma. Curr. Opin. Pediatr. 2024, 36, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Lessmann, A.; Curto, E.; Mateus Medina, E.F.; Palones, E.; Belda Soler, A.; Sánchez Maza, S.; Soto-Retes, L.; Plaza, V. Characteristics of Induced-Sputum Inflammatory Phenotypes in Adults with Asthma: Predictors of Bronchial Eosinophilia. J. Asthma Allergy 2023, 16, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Monzio Compagnoni, M.; Conflitti, C.; Capuano, V.; Bonaiti, G.; Franchi, M.; Vimercati, C.; Biondi, A.; Luppi, F.; Corrao, G.; Faverio, P. Healthcare costs and resources utilization in children with difficult-to-control asthma treated with biologic therapies: A population-based cohort study. Pediatr. Pulmonol. 2024, 59, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. Disease modification in inflammatory skin disorders: Opportunities and challenges. Nat. Rev. Drug Discov. 2023, 22, 935. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.A.; Togias, A.; Sicherer, S.H.; Shreffler, W.G.; Kim, E.H.; Jones, S.M.; Leung, D.Y.M.; Vickery, B.P.; Bird, J.A.; Spergel, J.M.; et al. Omalizumab for the Treatment of Multiple Food Allergies. N. Engl. J. Med. 2024, 390, 889–899. [Google Scholar] [CrossRef] [PubMed]
- En, E.H.S.; Wei, J.C.C. Omalizumab for the Treatment of Multiple Food Allergies. N. Engl. J. Med. 2024, 390, 1936. [Google Scholar] [CrossRef]
- Indolfi, C.; Klain, A.; Dinardo, G.; Grella, C.; Perrotta, A.; Decimo, F.; Miraglia del Giudice, M. The dawn of a new era for biological drugs in allergology. Ital. J. Pediatr. Allergy Immunol. 2024, 38, 14–16. [Google Scholar] [CrossRef]
- Comberiati, P.; Muraro, A. Omalizumab: A definitive cure for food allergies? Ital. J. Pediatr. Allergy Immunol. 2024, 38, 36–37. [Google Scholar] [CrossRef]
- Nopsopon, T.; Barrett, N.A.; Phipatanakul, W.; Laidlaw, T.M.; Weiss, S.T.; Akenroye, A. Lung function trajectories in a cohort of patients with moderate-to-severe asthma on mepolizumab, omalizumab, or dupilumab. Allergy 2024, 79, 1195–1207. [Google Scholar] [CrossRef]
- Biener, L.; Mümmler, C.; Hinze, C.A.; Suhling, H.; Korn, S.; Fisser, C.; Biener, A.; Pizarro, C.; Lenoir, A.; Hackl, C.; et al. Real-World Data on Tezepelumab in Patients With Severe Asthma in Germany. J. Allergy Clin. Immunol. Pract. 2024, 12, 2399–2407.e5. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Hoyte, F.C.L.; Lindsley, A.W.; Ambrose, C.S.; Spahn, J.D.; Roseti, S.L.; Cook, B.; Griffiths, J.M.; Hellqvist, Å.; Martin, N.; et al. Tezepelumab reduces exacerbations across all seasons in patients with severe, uncontrolled asthma (NAVIGATOR). Ann. Allergy Asthma Immunol. 2023, 131, 587–597.e3. [Google Scholar] [CrossRef] [PubMed]
- Pharmacokinetics, Pharmacodynamics and Safety of Tezepelumab in Children with Asthma. Available online: https://publications.ersnet.org/content/erj/64/suppl68/pa3562. (accessed on 26 December 2024).
- Wedner, H.J.; Fujisawa, T.; Guilbert, T.W.; Ikeda, M.; Mehta, V.; Tam, J.S.; Lukka, P.B.; Asimus, S.; Durżyński, T.; Johnston, J.; et al. Benralizumab in children with severe eosinophilic asthma: Pharmacokinetics and long-term safety (TATE study). Pediatr. Allergy Immunol. 2024, 35, e14092. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Ruddy, M.K.; Pavord, I.D.; Israel, E.; Rabe, K.F.; Ford, L.B.; Maspero, J.F.; Abdulai, R.M.; Hu, C.C.; Martincova, R.; et al. Efficacy and Safety of Itepekimab in Patients with Moderate-to-Severe Asthma. N. Engl. J. Med. 2021, 385, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Georas, S.N.; Donohue, P.; Connolly, M.; Wechsler, M.E. JAK inhibitors for asthma. J. Allergy Clin. Immunol. 2021, 148, 953–963. [Google Scholar] [CrossRef] [PubMed]
| Biologic Therapy | Mechanism of Action | Indications | Dosage and Administration | Key Studies and Findings | Safety Profile |
|---|---|---|---|---|---|
| Dupilumab | Targets IL-4Rα to inhibit IL-4 and IL-13 signaling, reducing Th2 inflammation. | Moderate-to-severe asthma with T2 inflammation (elevated eosinophils or FeNO); also approved for AD and CRSwNP. | Subcutaneous injection based on weight: <30 kg: 300 mg Q4W; 30–60 kg: 200 mg Q2W or 300 mg Q4W; >60 kg: 200 mg Q2W. | VOYAGE study: 59.3% reduction in annual exacerbation rate; improved FEV1 (+5.2 points over placebo); reduced FeNO and ACQ-7 scores. Long-term efficacy and safety confirmed in LIBERTY Asthma EXCURSION. Early intervention may modify the atopic march. | Common: Viral URTIs, injection site reactions. Rare: Serious adverse events comparable to placebo. |
| Mepolizumab | Anti-IL-5 antibody inhibiting eosinophil maturation and survival. | Severe eosinophilic asthma with elevated FeNO or eosinophils (>150/µL at assessment or >300/µL in 12 months). | Subcutaneous injection every 4 weeks: <40 kg: 40 mg; ≥40 kg: 100 mg. | Gupta et al.’s study: Reduced annualized exacerbations (0.96 vs. 1.30 in placebo); significant eosinophil reduction (>80%); improvements in ACQ-7 and C-ACT scores but no significant FEV1 changes. Jackson et al.’s research: Reduced fall exacerbation peak; some pathways independent of IL-5 identified. | Common: Headache, injection site reactions. Rare: Serious adverse events in 6%, unrelated to treatment. |
| Omalizumab | Anti-IgE antibody reducing cell-bound IgE and downregulating IgE receptors. | Moderate-to-severe allergic asthma (positive allergy tests, IgE 30–1500 IU/mL); also licensed for chronic idiopathic urticaria and food allergies. | Dosed by IgE and weight: 0.016 mg/kg per IU of IgE/4 weeks; subcutaneous injection every 2–4 weeks (150–375 mg US; up to 600 mg EU). | Landmark and Milgrom RCT: 100% median reduction in ICS dose; 55% discontinued ICSs. Significant reduction in exacerbations (0.42 vs. 2.72 episodes). Arasi et al.: Improved food tolerance and reduced anaphylactic episodes in children with comorbid food allergies. Potential as an adjunct to oral immunotherapy. | Common: Headache, URTIs, injection site reactions. Rare: Anaphylaxis; patients should be monitored after administration. |
| Drug | Target | Main Indications | Studies and Results | Dosage | Side Effects | Approval Status |
|---|---|---|---|---|---|---|
| Tezepelumab | TSLP (Thymic Stromal Lymphopoietin) | Severe asthma with non-specifically T2 inflammation | PATHWAY and NAVIGATOR studies: significant reduction in asthma exacerbations (up to 71% in NAVIGATOR) regardless of type 2 biomarkers (eosinophils or FeNO). Improvements in lung function (FEV1), asthma control, and quality of life. Efficacy demonstrated even in patients with low levels of eosinophils or FeNO. | 210 mg administered subcutaneously every 4 weeks. | Upper respiratory infections, injection site reactions, headache. | FDA-approved in 2021 for adults and adolescents; pediatric approval under review. |
| Benralizumab | IL-5Rα | Severe eosinophilic asthma | SIROCCO, CALIMA, and BORA studies: reduction in asthma exacerbations (up to 51–70%); reduction in eosinophil levels to nearly zero. Significant improvements in lung function and quality of life. | 30 mg subcutaneous every 4 weeks for 3 doses, then every 8 weeks. | Injection site reactions, headache, upper respiratory tract viral infections. | FDA- and EMA-approved for patients aged >12; pediatric use under review. |
| Itepekimab | IL-33 | Severe asthma (both eosinophilic and non-eosinophilic) | ECLIPSE study: significant reduction in asthma exacerbations in patients with high IL-33 levels. Improvements in lung function (FEV1) and symptoms. Effective both in the presence and absence of elevated T2 biomarkers (e.g., eosinophils). | Under study, variable dosages administered every 4 weeks. | Injection site reactions, fever, flu-like symptoms. | In clinical trial phase. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indolfi, C.; Klain, A.; Capuano, M.C.; Colosimo, S.; Rapillo, R.; Miraglia del Giudice, M. Severe Asthma in School-Age Children: An Updated Appraisal on Biological Options and Challenges in This Age Group. Children 2025, 12, 167. https://doi.org/10.3390/children12020167
Indolfi C, Klain A, Capuano MC, Colosimo S, Rapillo R, Miraglia del Giudice M. Severe Asthma in School-Age Children: An Updated Appraisal on Biological Options and Challenges in This Age Group. Children. 2025; 12(2):167. https://doi.org/10.3390/children12020167
Chicago/Turabian StyleIndolfi, Cristiana, Angela Klain, Maria Cristina Capuano, Simone Colosimo, Renata Rapillo, and Michele Miraglia del Giudice. 2025. "Severe Asthma in School-Age Children: An Updated Appraisal on Biological Options and Challenges in This Age Group" Children 12, no. 2: 167. https://doi.org/10.3390/children12020167
APA StyleIndolfi, C., Klain, A., Capuano, M. C., Colosimo, S., Rapillo, R., & Miraglia del Giudice, M. (2025). Severe Asthma in School-Age Children: An Updated Appraisal on Biological Options and Challenges in This Age Group. Children, 12(2), 167. https://doi.org/10.3390/children12020167






