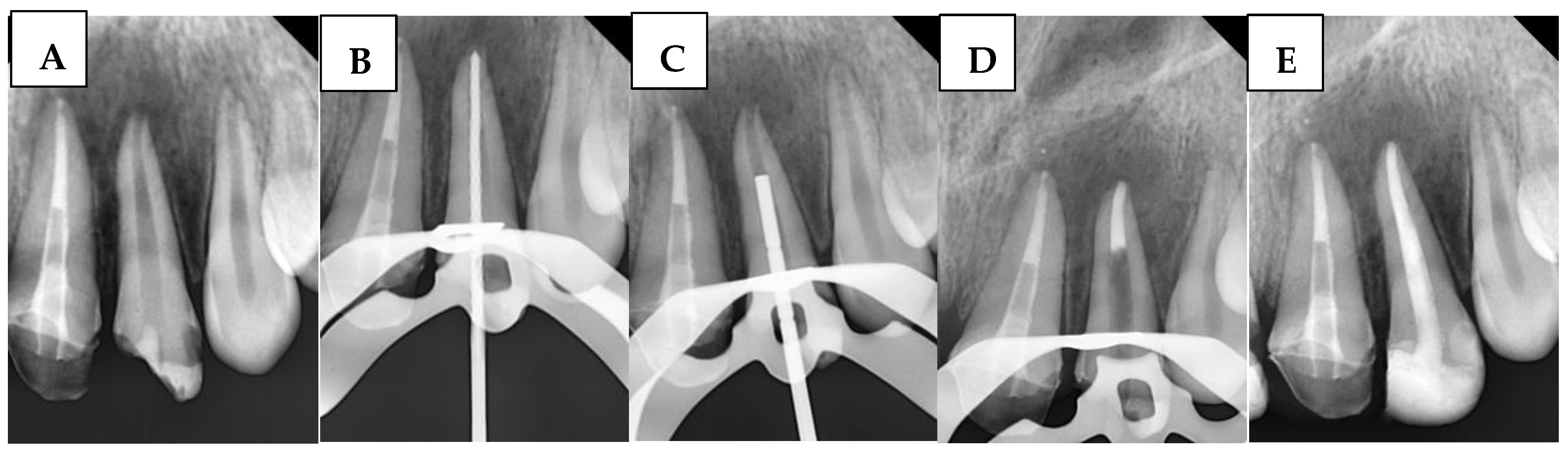
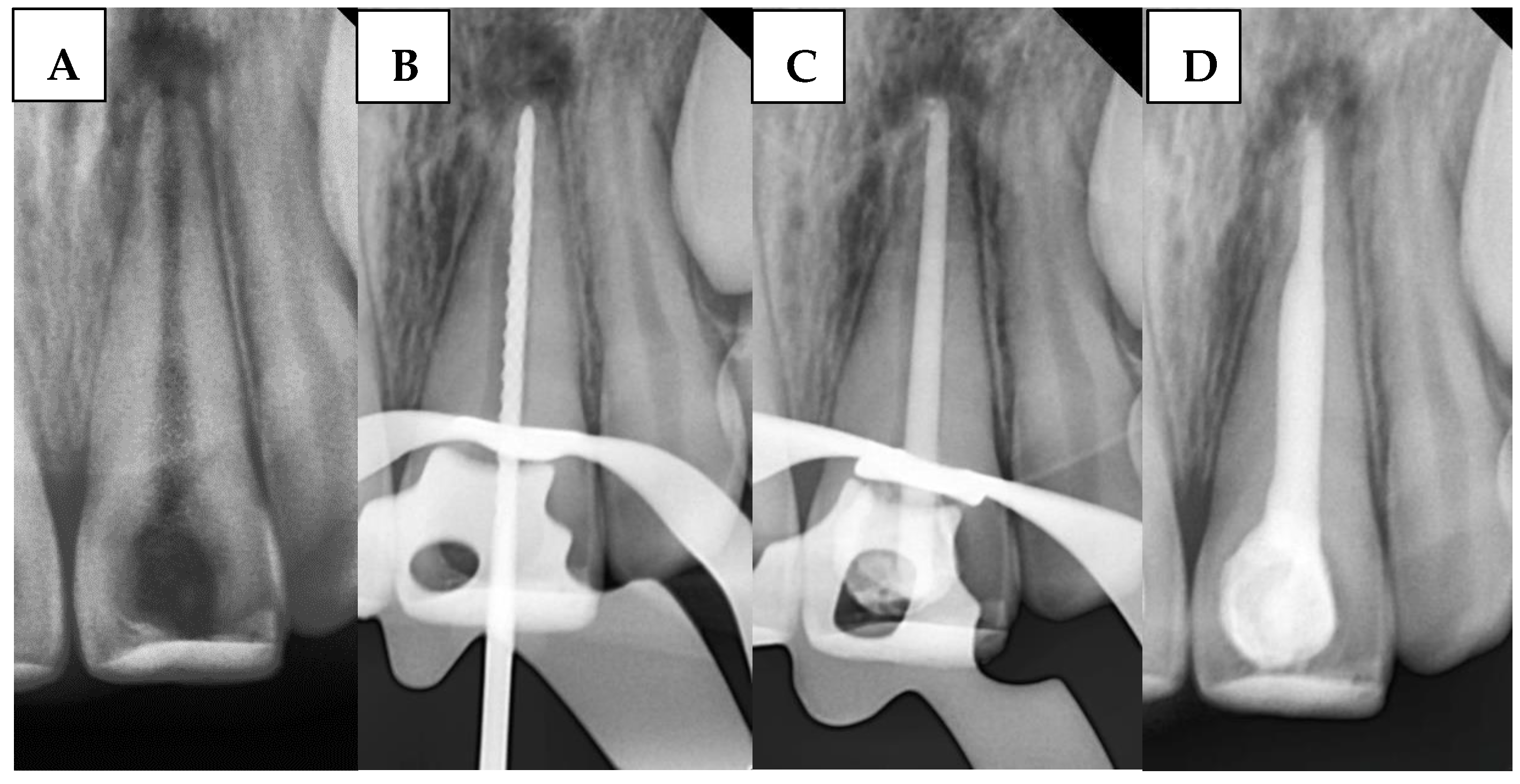
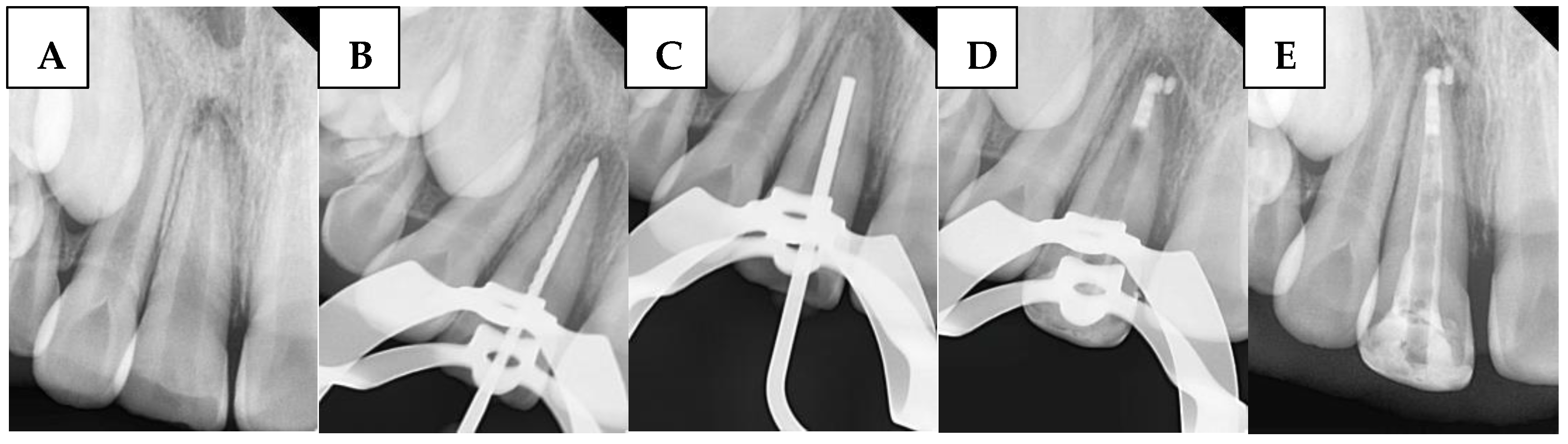
1. Introduction
Dental trauma remains a prevalent issue among children and young adults, with an estimated 25% of school-age children and 33% of adults experiencing trauma to permanent teeth [
1]. While these injuries can generally be managed, immature permanent teeth are especially susceptible to complications if treatment is delayed or inadequate, leading to pulp necrosis [
2]. Addressing these challenges in immature anterior teeth requires a nuanced approach that balances esthetics, function, and effective management of the root canal system [
3]. Notably, the child’s behavior may influence the immediate management of the immature teeth and the long-term treatment outcomes. This fact underscores the importance of considering the child’s behavior as an additional challenge in the endodontic management of immature teeth [
4,
5].
Apical barrier treatment with calcium silicate cement apical plugs has proven to be an effective treatment for necrotic immature permanent teeth, particularly when root development aligns with stages 2 to 4 of Cvek’s classification [
6].
Mineral Trioxide Aggregate (MTA) has served as the gold standard for apical plugs in apexification treatments, demonstrating high radiographic success rates and yielding good clinical and histological outcomes [
7,
8]. However, MTA has limitations, including tooth discoloration, a prolonged setting time, and handling challenges [
9].
Recent advancements have led to the introduction of new calcium silicate-based materials with enhanced composition, handling characteristics, viscosity, and faster setting times, including Biodentine, BioAggregate, and BioCeramics [
9].
BioCeramics, a more recent class of hydraulic calcium silicate cements [
10], can effectively penetrate dentinal tubules, creating a tight seal while promoting biocompatibility and minimizing inflammation [
11,
12]. These materials also exhibit antibacterial properties, offering a less invasive treatment option that reduces the need for multiple visits [
13,
14,
15,
16]. These materials are available in different viscosities, including TotalFill BioCeramic Sealer, Root Repair Material Paste, Root Repair Material Putty, and Fast Putty [
17]. The viscosity raises the question of how these materials are utilized in apical barrier treatments and how the application method of the apical plug influences clinical treatment outcomes.
Several factors have been identified as influencing postoperative pain in closed apices teeth, including preoperative pain, obturation technique, the extent of apical enlargement, and the extrusion of obturation materials [
18]. Moreover, it is well established that the use of calcium silicate–based sealers results in less postoperative pain compared to other types of sealers in mature teeth with closed apices [
19]. However, the impact of these factors on permanent teeth with open apices remains unknown. Moreover, the use of calcium silicate–based sealers is not yet considered a common practice in the management of immature teeth with open apices, according to recently published literature [
9].
In the case of immature permanent teeth indicated for apical barrier treatment, additional apical enlargement is typically not performed. Instead, a conservative preparation of the coronal and middle thirds is carried out to eliminate the reverse taper, thereby facilitating access to the apical third with instruments and irrigation solutions [
7]. However, the impact of apex size on the intensity of postoperative pain has not been previously evaluated in studies involving immature teeth.
Notably, the extrusion of obturation materials has been commonly reported in numerous case reports during the treatment of open apices with apical barriers [
20,
21]. However, no precise studies have been found that correlate the extent of apical width with the extrusion of biomaterials. Moreover, the extrusion of obturation materials has been correlated with postoperative pain and obturation techniques in several recent systematic reviews focusing on endodontic treatment of closed apical teeth [
22,
23]. However, no studies have specifically addressed these aspects in teeth with open apices.
Children are more susceptible to dental fatigue during prolonged treatment periods associated with traumatic dental injuries [
24]. However, the impact of apical barrier treatment visit duration on clinical outcomes remains largely unexplored. Additionally, it is unclear whether this factor is influenced by the obturation technique used or by patient- and tooth-specific factors, such as those proposed previously [
25].
This trial directly compared three distinct application methods: the Bioceramic Putty Apical Plugs (BPAP) method, the Single Cone Gutta-percha with BioCeramic Sealer (SBS) method, and the BioCeramic Putty and Sealer Mixture (BPSM) method, and also investigated patient-related factors such as behavior, apical size, and preoperative pain, which may impact treatment outcomes. The null hypothesis (A) stated that the three apical barrier methods were equivalent in terms of postoperative pain. The null hypothesis (B) assumed that the methods would be comparable in terms of the incidence of bioceramic extrusion. Finally, the null hypothesis (C) proposed that the methods would not differ in terms of procedure duration.
3. Results
3.1. Descriptive Demographic Characteristics of the Study Sample
The study sample consisted of 99 immature permanent maxillary incisors with pulp necrosis, comprising 59 maxillary incisors in boys and 40 in girls. The overall number of participants was 40 boys and 29 girls.
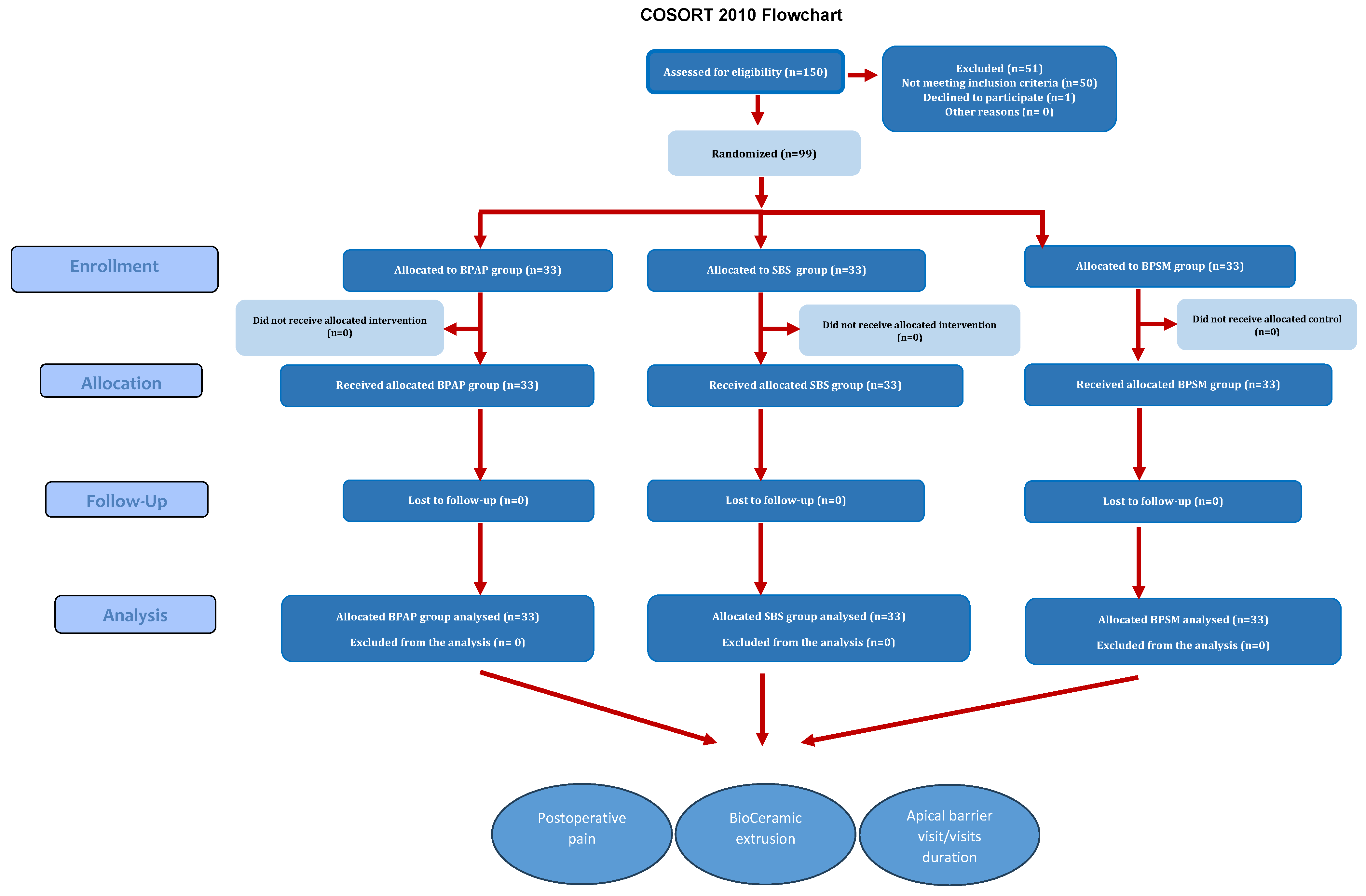
Figure 5 illustrates the flow chart of the study.
Table 1 summarizes the distribution of patients among study groups according to continuous variables: age, working length, apex diameter, preoperative pain level, and pain level before the apical barrier procedure.
Table 2 summarizes the distribution of patients among study groups according to categorical variables: gender, children’s behavior, cause of pulp necrosis, and periapical diagnosis.
As shown in the two previous tables, all uncontrolled variables in the study were randomly distributed across the three groups, with no significant differences in any of the variables (p < 0.05), indicating that randomization was effectively achieved. Nevertheless, these variables will be analyzed separately to assess their potential influence on the study’s primary outcomes, namely postoperative pain, BioCeramic extrusion rate, and the apical barrier visit duration.
3.2. Analysis of Postoperative Pain and Contributing Factors Across Study Groups
Table 3 presents the comparative results of postoperative pain among the study groups in all time intervals.
The Kruskal–Wallis test revealed statistically significant differences in postoperative pain among the three study groups (BPAP, SBS, and BPSM) only on the first day following treatment. Notably, none of the samples in any of the groups reported any postoperative pain two weeks after treatment.
Table 4 presents the results of pairwise comparisons between the study groups on the one-day postoperative pain.
One-day postoperative pain severity among the groups can be summarized as SBS < BPSM < BPAP.
For the one-day postoperative pain, the Spearman test indicated a negative correlation but not significant regarding age (r = −0.146, p = 0.147), positive significant correlation regarding pain before apical barrier procedure level (r = 0.418, p < 0.001), positive but not significant correlation regarding working length (r = 0.173, p = 0.085), positive but not significant correlation regarding apical size (r = 0.035, p = 0.727), and positive significant correlation regarding apical barrier visit duration (r = 0.498, p < 0.001). Moreover, the Mann–Whitney test indicated no effects regarding gender (boys/girls) (p = 0.361), no effect regarding BioCeramic extrusion (BioCeramic was extruded/BioCeramic was not extruded) (p = 0.954), and a significant effect of pulpal diagnosis (symptomatic/asymptomatic) (p = 0.002). Finally, the Kruskal–Wallis test revealed no significant effects on child behavior (p = 0.843) or the cause of pulp necrosis (p = 0.130) on one-day postoperative pain.
Table 5 presents the linear regression results to identify the factor most influencing one-day postoperative pain among the following variables: apical barrier method, pain before the apical barrier procedure, periapical diagnosis, and apical barrier duration.
For the three-day postoperative pain, the Spearman test indicated a positive correlation but not significant regarding age (r = 0.526, p = 0.147), positive significant correlation regarding the one day postoperative pain level (r = 0.487, p < 0.001), negative but not significant correlation regarding working length (r = −0.012, p = 0.903), negative but not significant correlation regarding apical size (r = −0.086, p = 0.393), and positive significant correlation regarding apical barrier visit duration (r = 0.241, p = 0.016). Moreover, the Mann–Whitney test indicated no effects regarding gender (boys/girls) (p = 0.699), no effect regarding BioCeramic extrusion (BioCeramic was extruded/BioCeramic was not extruded) (p = 0.721), and a significant effect of pulpal diagnosis (symptomatic/asymptomatic) (p = 0.011). Finally, the Kruskal–Wallis test revealed no significant effects on child behavior (p = 0.564) or the cause of pulp necrosis (p = 0.470) on three-day postoperative pain.
Table 6 presents the linear regression results to identify the factor most influencing three-day postoperative pain among the following variables: apical barrier method, presence of one-day postoperative pain, periapical diagnosis, and apical barrier duration.
For the one-week postoperative pain, the Spearman test indicated a positive correlation but not significant regarding age (r = 0.008, p = 0.937), positive but not significant correlation regarding the three-day postoperative pain level (r = 0.0122, p = 0.876), positive but not significant correlation regarding working length (r = 0.134, p = 0.183), negative but not significant correlation regarding apical size (r = −0.068, p = 0.504), positive but not significant correlation regarding apical barrier visit duration (r = 0.027, p = 0.786). Moreover, the Mann–Whitney test indicated no effects regarding gender (boys/girls) (p = 0.549), no effect regarding BioCeramic extrusion (BioCeramic was extruded/BioCeramic was not extruded) (p = 0.807), and no effect of pulpal diagnosis (symptomatic/asymptomatic) (p = 0.71). Finally, the Kruskal–Wallis test revealed no significant effects on child behavior (p = 0.528) or the cause of pulp necrosis (p = 0.838) on one-week postoperative pain.
One week postoperatively, the multiple linear regression analysis revealed that the only factor influencing pain intensity was the use of the apical barrier method (β = 0.054, CI: 0.002–0.093, p = 0.021).
3.3. Analysis of BioCeramic Extrusion and Contributing Factors Across Study Groups
Table 7 presents the comparison results between the study groups regarding the BioCeramic extrusion according to the evaluated teeth.
The chi-square test showed a significant difference between the groups (p = 0.002). Pairwise comparisons using Fisher’s Exact Test were conducted to assess differences in the presence of BioCeramic extrusion among the three study groups. A statistically significant difference was found between the BPAP and SBS groups (p = 0.001), indicating a higher incidence of BioCeramic extrusion in the SBS group. However, no significant difference was detected between the BPAP and BPSM groups (p = 0.287). The comparison between the SBS and BPSM groups approached statistical significance but did not reach it (p = 0.080). BioCeramic extrusion among the groups can be summarized as SBS − BPSM > BPAP.
For the BioCeramic extrusion, the Mann–Whitney test indicated an effect regarding apical size (p = 0.003), no effect regarding the working length (p = 0.977), and no effect regarding the apical barrier visit duration (p = 0.409). Finally, the Chi-Square test indicated no effect on child behavior (p = 0.487) regarding the BioCeramic extrusion.
Table 8 presents the binary logistic regression coefficients analyzing the factors influencing BioCeramic extrusion, specifically the apical barrier method and apical size.
3.4. Analysis of Apical Barrier Visit Duration and Contributing Factors Across Study Groups
Table 9 presents the comparison results between the study groups regarding the apical barrier visit duration minutes among groups.
The One-way ANOVA test revealed statistically significant differences in apical barrier procedure duration and postoperative pain among the three study groups (BPAP, SBS, and BPSM) (p < 0.001).
Table 10 presents the results of pairwise comparisons between the study groups regarding the duration of the apical barrier procedure.
Apical barrier duration procedure among the groups can be summarized as SBS < BPSM < BPAP.
For the apical barrier procedure duration, the Spearman test revealed a positive but nonsignificant correlation with working length (r = 0.133, p = 0.187) and a positive, significant correlation with apical size (r = 0.289, p = 0.003). Finally, the Kruskal–Wallis test revealed significant effects regarding the apical barrier method (p < 0.001) and child behavior (p = 0.007) on the duration of the apical barrier procedure. Specifically, children with negative behavior required a more extended treatment duration than those with upbeat and definitely positive behavior, according to the Frankl scale.
Table 11 presents the linear regression results to identify the factor most influencing the apical barrier visit duration among the following variables: apical size, apical barrier method, and child behavior.
4. Discussion
In light of the increasing prevalence of necrotic immature permanent teeth requiring endodontic management in children aged 6–12 years [
36], the significance of this study lies in several key aspects. First, it represents the first randomized controlled clinical trial to evaluate the impact of different bioceramic application methods on critical clinical outcomes, including postoperative pain, treatment duration, and the incidence of obturation material extrusion, with the aim of identifying the most effective approach for pediatric patients. Second, it explores the influence of various contributing factors that cannot be fully standardized in a randomized controlled setting—such as apical size, working length, etiology of pulpal necrosis, preoperative symptoms, and children’s behavior—in addition to the selected apical barrier method, all of which collectively shape the overall treatment outcomes.
The use of bioceramics in the presented study has been investigated through three distinct methods. For instance, Sockalingam et al. [
14] and Ghaly et al. [
29] recommended the application of BioCeramic Putty as an apical plug. Rencher et al. [
37] demonstrated that BioCeramic putty and BioCeramic sealer were employed for retrograde filling after apicoectomy. Additionally, BioCeramic sealer use in conjunction with the single-cone method was based on manufacturer guidelines, with reports of high success rates in managing large periapical lesions in closed-apex anterior teeth [
38]. However, this method requires modifications for open-apex cases, as the gutta-percha cone cannot achieve tug-back at the open apex. Instead, tug-back is accomplished at the apical third of the root, where the BioCeramic sealer’s high flowability seals the gap between the gutta-percha cone and apical walls.
The present findings indicate a direct association between the apical barrier method using bioceramics and postoperative pain, as the different methods employed demonstrated varying effects on pain intensity. Accordingly, the null hypothesis (A) was rejected. These results highlight the importance of considering the treatment method in patients who are particularly sensitive to endodontic procedures, to minimize discomfort in children during sessions involving apical barrier formation. The regression results indicate that the apical barrier method continued to exert a significant influence on the outcomes even one week after treatment.
The most clinically significant finding was the substantial difference in immediate postoperative pain between treatment groups. On the first day post-treatment, pain levels varied dramatically: BPAP showed the highest pain, followed by BPSM, while SBS demonstrated the lowest pain levels.
This pain hierarchy (SBS < BPSM < BPAP) suggests that the apical barrier method employed in the apical barrier approach may play a role in influencing postoperative pain when managing necrotic immature permanent teeth. The SBS method is less traumatic to the periapical tissues, potentially contributing to reduced pain levels. The significantly lower pain reported in the SBS group may be attributed to the less invasive nature of the single cone obturation technique, which avoids the use of hand pluggers for apical adaptation. In contrast, both the BPSM and BPAP groups involved the use of hand pluggers to adapt the bioceramic material in the apical third, potentially causing additional trauma to the periodontal ligament and contributing to increased postoperative discomfort. This finding is somewhat in agreement with the study by Ruiz-Cano et al. [
39], which showed a tendency for lower postoperative pain—though not statistically significant—when comparing warm vertical compaction to the single cone technique, both using a bioceramic sealer, in the treatment of mature permanent teeth in adult patients after 24 h of the treatment.
By day three, pain levels had substantially decreased across all groups, with no significant differences, and by two weeks, all patients were pain-free. This temporal pattern is consistent with recent studies on bioceramic materials, which demonstrate rapid healing and reduced tissue irritation [
7,
9].
The reduced postoperative pain observed within groups in this study may be attributed to their distinct immunomodulatory and bioactive properties. Calcium-silicate-based formulations promote a healing response dominated by M2 macrophages rather than the pro-inflammatory M1 phenotype. This polarization leads to downregulation of key pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, which are directly associated with periapical inflammation and postoperative discomfort [
40,
41]. Both in vitro and in vivo studies have consistently demonstrated that BioCeramics induce a minimal cytotoxic and inflammatory response [
42,
43], likely contributing to the lower incidence of pain following treatment.
Several factors influenced postoperative pain intensity in the current sample. A significant positive correlation was found between preoperative pain and postoperative pain, as well as with the periapical diagnosis, indicating that patients with pre-existing discomfort were more likely to experience post-treatment pain. Notably, regression analysis revealed that these two factors had a greater impact on postoperative pain than other variables (apical barrier visit duration and apical barrier method) on both the first day and three days after treatment. Based on these findings, it is recommended to adopt the less painful method (SBS) when managing cases with acute periapical inflammation and high levels of preoperative pain during apical barrier procedures. Similar evidence in mature teeth supports that a longer duration of preoperative pain significantly predicts increased postoperative discomfort [
44].
Longer procedure duration correlated with increased postoperative pain, suggesting that extended treatment times may contribute to tissue trauma and subsequent discomfort. While this specific correlation has not been extensively detailed in previous studies, earlier systematic reviews have primarily focused on comparing postoperative pain between single-visit and multiple-visit endodontic treatments, rather than examining the impact of procedure duration per se on pain outcomes [
45]. Therefore, it is recommended that studies focusing on apical barrier methods consider both the visit duration and the presence of preoperative pain in acute cases, as these variables may significantly influence postoperative pain outcomes.
It is worth noting that, among the treated samples in this study across all groups, larger apical diameters were not associated with increased postoperative pain. This finding may be logical, as the immature apices were not subjected to any mechanical enlargement. A recent well-designed study has suggested that the degree of apical preparation significantly influences the severity of postoperative pain [
31]. Regarding irrigant extrusion, although a wider apical diameter is generally associated with an increased risk of irrigant extrusion beyond the apex [
46], the irrigation protocol used in this study—which involved one side vented irrigation needle to deliver the solution and another tip connected to a saliva ejector for simultaneous aspiration—seemed effective in preventing irrigant extrusion, and therefore did not contribute to increased postoperative discomfort.
The present findings indicate a direct association between the apical barrier method using bioceramics and BioCeramic extrusion, as the BioCeramic extrusion rates among groups revealed important method considerations. Accordingly, the null hypothesis (B) was rejected. The BPAP group showed the lowest extrusion rate, while the SBS and BPSM groups demonstrated higher rates. This finding suggests that the putty consistency in BPAP may provide better control during placement, reducing the likelihood of material overflow beyond the apex. On the other hand, the higher extrusion rates in the SBS and BPSM groups may be related to the flowable nature of sealers and the technique-sensitive nature of these procedures.
Sealers show variable extrusion rates depending on their consistency and application method; Specifically, the bioceramic sealer demonstrated a high extrusion rate, which can be attributed to its excessive flowability and high fluidity [
47,
48].
It is noteworthy that, within the scope of this study, the BioCeramic extrusion in all study groups did not result in increased postoperative pain. This finding is consistent with previous studies that investigated the impact of sealer extrusion in both mature [
47,
49] and cases with open apices [
20,
50]. In line with the existing literature [
49], and considering the context of treating nonvital immature permanent teeth, we do not recommend any apical extrusion of the obturation material, as the long-term fate of extruded BioCeramic materials remains unclear—particularly regarding its effect on periapical healing, bone regeneration, and the density of newly formed bone.
Apical size significantly influenced BioCeramic extrusion risk, with larger apical openings predisposing to material overflow. This correlation highlights the importance of carefully selecting cases and modifying methods based on anatomical considerations [
51].
It is noteworthy that, based on the logistic regression results regarding the factors influencing BioCeramic extrusion, the apical barrier method itself proved to be more important than the size of the apical foramen. This highlights the crucial role of proper practice and training in each method, as well as the need to adopt the less extrusion-prone method (BPAP) to minimize the risk of sealer extrusion, particularly in wider apical foramina.
The present findings indicate a direct association between the apical barrier method using bioceramics and apical barrier visit duration, as the duration among groups revealed important method considerations. Accordingly, the null hypothesis (C) was rejected.
Samples in the SBS group required the shortest time, followed by BPSM and BPAP, taking the longest. The efficiency advantage of the SBS method may be related to the simplified single-cone obturation procedure compared to the more complex putty manipulation required in other methods. Moreover, it is noteworthy that the SBS method did not require an additional session for filling the remaining portion of the canal. Once the gutta-percha was severed, the final restoration could be accomplished immediately in routine cases that did not necessitate a post-and-core build-up.
Recent clinical reviews confirm that single cone obturation with bioceramic sealers represents a simplified approach that has gained widespread acceptance [
52]. Contemporary studies demonstrate that the single cone technique can be used with a bioceramic sealer, which makes obturation faster [
53].
Longer procedure times correlated with larger apical sizes, likely due to increased difficulty in achieving adequate seal with wider apical openings. This study also demonstrated that children with definitely negative and negative behavior required longer treatment times regardless of the apical barrier method used. This finding aligns with previous systematic reviews that confirmed a relationship between treatment session duration and child behavior [
54]. The use of the SBS method may be advisable in cases involving uncooperative pediatric patients who require apical barrier procedures.
It is likely that the use of the modified cannula [
34] for the BioCeramic putty in the BPAP and BPSM groups, combined with the application of ready-to-use premixed BioCeramic in the SBS and BPSM groups, contributed to reducing the influence of WL on the overall treatment duration.
It was observed that the size of the apical foramen and child behavior had a greater impact on the duration of apical barrier visit procedures than the apical barrier method itself. Therefore, these factors should be carefully considered, favoring faster methods in uncooperative children, along with an accurate assessment of the immature permanent tooth anatomy, when selecting the most appropriate bioceramic apical barrier method.
These findings suggest that the SBS method may be advantageous for children who require faster, less painful treatment sessions, although clinicians must weigh the increased risk of extrusion. Conversely, BPAP may be preferred in cases with wide apices or when extrusion must be avoided, despite its drawbacks in time and discomfort. Clinical decision-making should therefore integrate patient cooperation, apex morphology, and operator skill rather than relying on a single technique.
While this study focused on immediate outcomes, it is essential to contextualize these findings within long-term success data from recent literature, which demonstrates high success rates with bioceramic materials in pediatric endodontics across different methods [
7,
29]. As a next step, a dedicated report will address the long-term effects of the three tested methods on the healing of periapical lesions in immature permanent teeth, as well as the impact of sealer extrusion on the healing process.
One of the limitations of this study is the inability to apply all methods to the same child, as it was not feasible to recruit children with three upper incisors in comparable conditions for these treatments. Additionally, operator blinding was not feasible, and extrusion was recorded only as present/absent, not quantitatively. Cost analysis was also not addressed. Future studies should adopt standardized extrusion measurement and incorporate economic considerations to inform evidence-based guidelines more effectively.
While the present trial highlights short-term differences in pain, extrusion, and treatment duration across bioceramic apical barrier methods, these findings should also be interpreted in the broader context of regenerative endodontics. Advances in pulp revascularization and tissue engineering increasingly emphasize biologic healing and root maturation as ultimate goals. The predictable control of extrusion and minimization of postoperative discomfort observed in this study provide a clinical foundation upon which regenerative protocols can build. Long-term evaluations comparing apical barrier techniques with regenerative approaches will be essential to determine not only periapical healing but also the potential for continued root development, dentin thickness, and preservation of esthetics in growing children.