Evaluation of the Effects of Lipoxin A4 and Resolvin D1 on the Severity of Transient Tachypnea of the Newborn: A Prospective Study
Abstract
Highlights
- LXA4 and RvD1 levels were found to be lower in infants with severe TTN.
- RvD1 and PLR are significant in predicting infants with severe TTN.
- Infants with high LXA4 and RvD1 levels have milder TTN.
- RvD1 and PLR are specific and sensitive biomarkers in predicting infants with severe TTN.
Abstract
1. Introduction
2. Materials and Methods
- Presence of respiratory distress symptoms such as grunting, tachypnea, nasal flaring, and chest retractions that start during the first six hours of life and last for a minimum of twelve hours.
- A chest radiograph showing at least one of the following features: symmetrical perihilar congestion, fluid in the interlobar fissures (fissure sign), prominent pulmonary vasculature, and hyperinflation of the lungs.
- Elimination of additional possible reasons for respiratory distress, including metabolic disorders, congenital pneumonia, surfactant deficiency, congenital heart disease, and meconium aspiration syndrome.
- Upper chest movement:
- ○
- Synchronous: 0 points
- ○
- Delayed inspiration: 1 point
- ○
- See-saw movement: 2 points
- Lower chest retraction:
- ○
- None: 0 points
- ○
- Barely visible: 1 point
- ○
- Easily visible: 2 points
- Xiphoid retraction:
- ○
- None: 0 points
- ○
- Barely visible: 1 point
- ○
- Easily visible: 2 points
- Nasal flaring:
- ○
- None: 0 points
- ○
- Barely visible: 1 point
- ○
- Easily visible: 2 points
- Expiratory grunting:
- ○
- None: 0 points
- ○
- Audible with stethoscope: 1 point
- ○
- Audible without stethoscope: 2 points
2.1. Exclusion Criteria
- Infants with respiratory distress symptoms lasting less than 12 h;
- Infants born at 34 weeks gestation or less;
- Infants with congenital heart disease during prenatal follow-up;
- Infants without an informed consent form.
2.2. Statistical Analysis
3. Findings
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Consortium on Safe Labor; Hibbard, J.U.; Wilkins, I.; Sun, L.; Gregory, K.; Haberman, S.; Hoffman, M.; Kominiarek, M.A.; Reddy, U.; Bailit, J.; et al. Respiratory morbidity in late preterm births. JAMA 2010, 304, 419–425. [Google Scholar] [CrossRef]
- Hansen, A.K.; Wisborg, K.; Uldbjerg, N.; Henriksen, T.B. Risk of respiratory morbidity in term infants delivered by elective caesarean section: Cohort study. BMJ 2008, 336, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Ozkiraz, S.; Gokmen, Z.; Boke, S.B.; Kilicdag, H.; Ozel, D.; Sert, A. Lactate and lactate dehydrogenase in predicting the severity of transient tachypnea of the newborn. J. Matern. Fetal Neonatal Med. 2013, 26, 1245–1248. [Google Scholar] [CrossRef]
- Lakshminrusimha, S.; Keszler, M. Persistent pulmonary hypertension of the newborn. Neoreviews 2015, 16, e680–e692. [Google Scholar] [CrossRef]
- Bak, S.Y.; Shin, Y.H.; Jeon, J.H.; Park, K.H.; Kang, J.H.; Cha, D.H.; Han, M.Y.; Jo, H.S.; Lee, K.H.; Lee, C.A. Prognostic factors for treatment outcomes in transient tachypnea of the newborn. Pediatr. Int. 2012, 54, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J. Does promoting resolution instead of inhibiting inflammation represent the new paradigm in treating infections? Mol. Aspects Med. 2017, 58, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Recchiuti, A.; Serhan, C.N. Pro-resolving lipid mediators (SPMs) and their actions in regulating miRNA in novel resolution circuits in inflammation. Front. Immunol. 2012, 3, 298. [Google Scholar]
- Kasap, B.; Duman, N.; Özer, E.; Tatlı, M.; Kumral, A.; Ozkan, H. Transient tachypnea of the newborn: Predictive factor for prolonged tachypnea. Pediatr. Int. 2008, 50, 81–84. [Google Scholar] [CrossRef]
- Hedstrom, A.B.; Gove, N.E.; Mayock, D.E.; Batra, M. Performance of the Silverman Andersen Respiratory Severity Score in predicting PCO2 and respiratory support in newborns: A prospective cohort study. J. Perinatol. 2018, 38, 505–511. [Google Scholar] [CrossRef]
- Eriksson, L.; Haglund, B.; Odlind, V.; Altman, M.; Ewald, U.; Kieler, H. Perinatal conditions related to growth restriction and inflammation are associated with an increased risk of bronchopulmonary dysplasia. Acta Paediatr. 2015, 104, 259–263. [Google Scholar] [CrossRef]
- Strunk, T.; Inder, T.; Wang, X.; Burgner, D.; Mallard, C.; Levy, O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect. Dis. 2014, 14, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.F.; Secher, N.J. Low consumption of seafood in early pregnancy as a risk factor for preterm delivery: Prospective cohort study. BMJ 2002, 324, 447. [Google Scholar] [CrossRef]
- Olsen, S.F.; Østerdal, M.L.; Salvig, J.D.; Weber, T.; Tabor, A.; Secher, N.J. Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: A randomized clinical trial. Eur. J. Clin. Nutr. 2007, 61, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.H.; Shih, C.H.; Yu, Y.B.; Hsu, H.C. Plasma levels in sepsis patients of annexin A1, lipoxin A4, macrophage inflammatory protein-3α, and neutrophil gelatinase-associated lipocalin. J. Chin. Med. Assoc. 2013, 76, 486–490. [Google Scholar] [CrossRef]
- Jain, L.; Eaton, D.C. Physiology of fetal lung fluid clearance and the effect of labor. Semin. Perinatol. 2006, 30, 34–43. [Google Scholar] [CrossRef]
- Flodby, P.; Kim, Y.H.; Beard, L.L.; Gao, D.; Ji, Y.; Kage, H.; Liebler, J.M.; Minoo, P.; Kim, K.; Borok, Z.; et al. Knockout mice reveal a major role for alveolar epithelial type I cells in alveolar fluid clearance. Am. J. Respir. Cell Mol. Biol. 2016, 55, 395–406. [Google Scholar] [CrossRef]
- Johnson, M.D.; Widdicombe, J.H.; Allen, L.; Barbry, P.; Dobbs, L.G. Alveolar epithelial type I cells contain transport proteins and transport sodium. Proc. Natl. Acad. Sci. USA 2002, 99, 1966–1971. [Google Scholar] [CrossRef]
- Matalon, S.; O’Brodovich, H. Sodium channels in alveolar epithelial cells: Molecular characterization, biophysical properties, and physiological significance. Annu. Rev. Physiol. 1999, 61, 627–661. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol. Aspects Med. 2017, 64, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Robriquet, L.; Folkesson, H.G.; Matthay, M.A. Protective effect of endogenous beta-adrenergic tone on lung fluid balance in acute bacterial pneumonia in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L769–L776. [Google Scholar]
- Perkins, G.D.; McAuley, D.F.; Thickett, D.R.; Gao, F. The beta-agonist lung injury trial (BALTI): A randomized placebo-controlled clinical trial. Am. J. Respir. Crit. Care Med. 2006, 173, 281–287. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.; Lian, Q.Q.; Yang, L.; Qi, W.; Wu, D.; Zheng, X.; Liu, Y.; Li, W.; Jin, S.; et al. Contribution of CFTR to alveolar fluid clearance by lipoxin A4 via PI3K/Akt pathway in LPS-induced acute lung injury. Mediat. Inflamm. 2013, 2013, 862628. [Google Scholar] [CrossRef]
- Wang, Q.; Lian, Q.Q.; Li, R.; Ying, B.-Y.; He, Q.; Chen, F.; Zheng, X.; Yang, Y.; Wu, D.-R.; Zheng, S.-X.; et al. Lipoxin A4 activates alveolar epithelial sodium channel, Na/K-ATPase, and increases alveolar fluid clearance. Am. J. Respir. Cell. Mol. Biol. 2013, 48, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zheng, X.; Cheng, Y.; Zhang, Y.-L.; Wen, H.-X.; Tao, Z.; Li, H.; Hao, Y.; Gao, Y.; Yang, L.-M.; et al. Resolvin D1 stimulates alveolar fluid clearance through alveolar epithelial sodium channel and Na/K-ATPase via ALX/cAMP/PI3K pathway in LPS-induced acute lung injury. J. Immunol. 2014, 192, 3765–3777. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, N.; Abdulnour, R.E.; Walker, K.H.; Engstrom, B.D.; Levy, B.D. Specialized pro-resolving mediators in innate and adaptive immune responses in airway diseases. Physiol. Rev. 2018, 98, 1335–1370. [Google Scholar] [CrossRef]
- Levy, B.D.; Kohli, P.; Gotlinger, K.; Haworth, O.; Hong, S.; Kazani, S.; Israel, E.; Haley, K.J.; Serhan, C.N. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J. Immunol. 2007, 178, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Planagumà, A.; Kazani, S.; Marigowda, G.; Haworth, O.; Mariani, T.J.; Israel, E.; Bleecker, E.R.; Curran-Everett, D.; Erzurum, S.C.; Calhoun, W.J.; et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am. J. Respir. Crit. Care. Med. 2008, 178, 574–582. [Google Scholar] [CrossRef]
- Rogers, L.K.; Valentine, C.J.; Pennell, M.; Velten, M.; Britt, R.D.; Dingess, K.; Zhao, X.; Welty, S.E.; Tipple, T.E. Maternal docosahexaenoic acid supplementation decreases lung inflammation in hyperoxia-exposed newborn mice. J. Nutr. 2011, 141, 214–222. [Google Scholar] [CrossRef]
- Ma, L.; Li, N.; Liu, X.; Shaw, L.; Calzi, S.L.; Grant, M.B.; Neu, J.N. Arginyl-glutamine dipeptide or docosahexaenoic acid attenuate hyperoxia-induced lung injury in neonatal mice. Nutrition 2012, 28, 1186–1191. [Google Scholar] [CrossRef]
- Higgins, G.; Fustero Torre, C.; Tyrrell, J.; McNally, P.; Harvey, B.J.; Urbach, V. Lipoxin A4 prevents tight junction disruption and delays colonization of cystic fibrosis bronchial epithelial cells by Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L1053–L1061. [Google Scholar] [CrossRef]
- Fukunaga, K.; Kohli, P.; Bonnans, C.; Fredenburgh, L.E.; Levy, B.D. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J. Immunol. 2005, 174, 5033–5039. [Google Scholar] [CrossRef]
- Sahanic, S.; Löffler-Ragg, J.; Tymoszuk, P.; Hilbe, R.; Demetz, E.; Masanetz, R.K.; Theurl, M.; Holfeld, J.; Gollmann-Tepeköylü, C.; Tzankov, A.; et al. The role of innate immunity and bioactive lipid mediators in COVID-19 and influenza. Front. Physiol. 2021, 12, 688946. [Google Scholar] [CrossRef]
- Souza, P.R.; Marques, R.M.; Gomez, E.A.; Colas, R.A.; De Matteis, R.; Zak, A.; Patel, M.; Collier, D.J.; Dalli, J. Enriched marine oil supplements increase peripheral blood specialized pro-resolving mediators and reprogram host immune responses: A randomized placebo-controlled study. Circ. Res. 2020, 126, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E.; Colombo, J.; Gajewski, B.J.; Gustafson, K.M.; Mundy, D.; Yeast, J.; Georgieff, M.K.; Markley, L.A.; Kerling, E.H.; Shaddy, D.J. DHA supplementation and pregnancy outcomes. Am. J. Clin. Nutr. 2013, 97, 808–815. [Google Scholar] [CrossRef]
- Emmett, P.M.; Jones, L.R.; Northstone, K. Dietary patterns in the Avon Longitudinal Study of Parents and Children. Nutr. Rev. 2015, 73 (Suppl. S3), 207–230. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Lyden, E.; Anderson-Berry, A.; Hanson, C. Omega-3 fatty acid intake of pregnant women and women of childbearing age in the United States: Potential for deficiency? Nutrients 2017, 9, 197. [Google Scholar] [CrossRef]
- Palmer, D.J.; Sullivan, T.; Gold, M.S.; Prescott, S.L.; Heddle, R.; Gibson, R.A.; Makrides, M. Effect of n-3 long-chain polyunsaturated fatty acid supplementation in pregnancy on infants’ allergies in the first year of life: Randomized controlled trial. BMJ 2012, 344, e184. [Google Scholar] [CrossRef]
- Furuhjelm, C.; Warstedt, K.; Larsson, J.; Fredriksson, M.; Fagerås Böttcher, M.; Fälth-Magnusson, K.; Duchén, K. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr. 2009, 98, 1461–1467. [Google Scholar] [CrossRef]
- Furuhjelm, C.; Warstedt, K.; Fageras, M.; Fälth-Magnusson, K.; Larsson, J.; Fredriksson, M.; Duchén, K. Allergic disease in infants up to 2 years of age in relation to plasma omega-3 fatty acids and maternal fish oil supplementation. Pediatr. Allergy Immunol. 2011, 22, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.F.; Osterdal, M.L.; Salvig, J.D.; Mortensen, L.M.; Rytter, D.; Secher, N.J.; Henriksen, T.B. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized trial. Am. J. Clin. Nutr. 2008, 88, 167–175. [Google Scholar] [CrossRef]
- Imhoff-Kunsch, B.; Stein, A.D.; Martorell, R.; Parra-Cabrera, S.; Romieu, I.; Ramakrishnan, U. Prenatal docosahexaenoic acid supplementation and infant morbidity: Randomized controlled trial. Pediatrics 2011, 128, e505–e512. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Troxler, H.; Klinke, G.; Rogler, D.; Braegger, C.; Hersberger, M. High levels of anti-inflammatory and pro-resolving lipid mediators in human milk during the first month of lactation. Lipids Health Dis. 2013, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Malamas, A.; Chranioti, A.; Tsakalidis, C.; Dimitrakos, S.A.; Mataftsi, A. The omega-3 and retinopathy of prematurity relationship. Int. J. Ophthalmol. 2017, 10, 300–305. [Google Scholar] [CrossRef]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Increased dietary intake of omega-3 polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Altas, O.F.; Kizilkaya, M. The effects of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and prognostic markers in determining mortality in patients with pneumonia in intensive care. Medeni. Med. J. 2021, 36, 130–137. [Google Scholar] [PubMed]
- Zheng, H.H.; Xiang, Y.; Wang, Y.; Zhao, Q.-S.; Dai, R. Clinical value of blood-related indexes in the diagnosis of bacterial infectious pneumonia in children. Transl. Pediatr. 2022, 11, 114–119. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, Z.; Huang, W.; Huang, K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am. J. Emerg. Med. 2020, 38, 641–647. [Google Scholar] [PubMed]
- Bolat, F.; Haspolat, N.Y.; Bolat, G.; Şahin, M. Simple hematological markers in predicting the severity of transient tachypnea of newborn: New wine of old bottles. J. Trop. Pediatr. 2021, 67, fmab100. [Google Scholar] [CrossRef] [PubMed]
| Mild Group (n = 31) | Severe Group (n = 31) | p | |
|---|---|---|---|
| Gestational Week | 37.16 ± 1.46 | 36.84 ± 1.37 | 0.374 |
| Delivery mode, n (%) C/S NSVD | 15 (48.1) 16 (51.9) | 24 (77.4) 7 (22.6) | 0.025 |
| Sex, n (%) Male Female | 19 (61.3) 12(38.7) | 16 (51.6) 15 (48.4) | 0.365 |
| Birth Weight (gram) | 3152.58 ± 229.47 | 3094.19 ± 201.92 | 0.292 |
| LXA4 (ng/mL) | 1.11 ± 0.60 | 0.73 ± 0.38 | 0.005 |
| RVD1 (ng/mL) | 1.40 ± 0.81 | 0.86 ± 0.38 | 0.002 |
| RDW (%) | 17.05 ± 1.31 | 17.62 ± 1.69 | 0.138 |
| NLR | 1.29 ± 0.86 | 1.91 ± 1.44 | 0.044 |
| PLR | 50.76 ±21.77 | 65.72 ± 29.7 | 0.027 |
| Variables | β | SE | p | OR | 95% CI for OR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Constant | 3.493 | 1.090 | 0.001 | 32.898 | ||
| RVD1 | −1.731 | 0.621 | 0.005 | 0.177 | 0.052 | 0.598 |
| PLR | −0.028 | 0.012 | 0.022 | 0.972 | 0.949 | 0.996 |
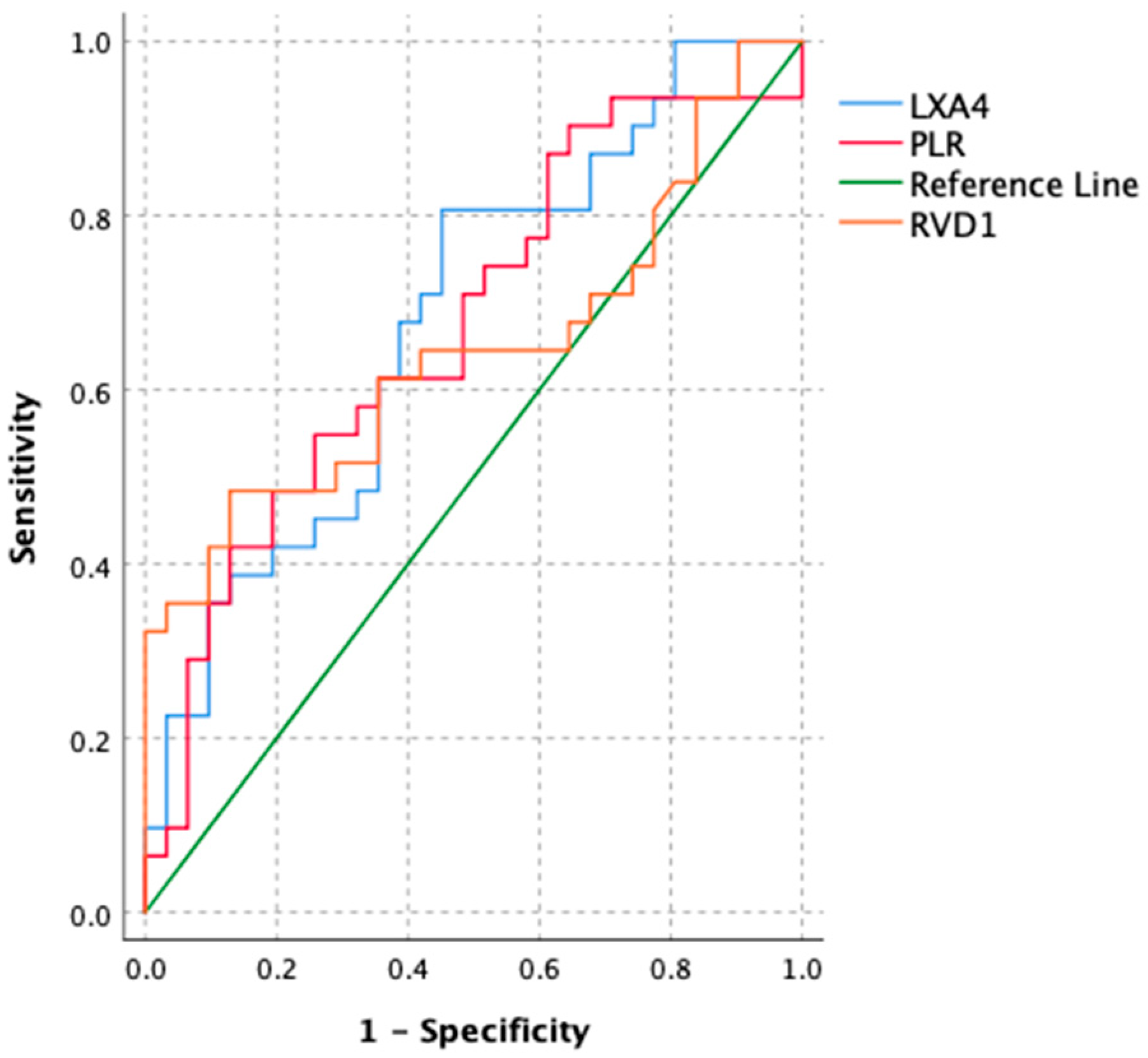
| Variables | Cutoff | Sensitivity | Specificity | Youden Index | AUC (95% CI) | p |
|---|---|---|---|---|---|---|
| LXA4 | 0.678 | 0.806 | 0.548 | 0.354 | 0.682 (0.549–0.814) | 0.007 |
| RVD1 | 1.22 | 0.484 | 0.871 | 0.355 | 0.646 (0.505–0.814) | 0.043 |
| PLR | 61.89 | 0.548 | 0.742 | 0.290 | 0.671(0.536–0.807) | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çığrı, E.; Çatan İnan, F.; Gülten, S.; Bildirici, M.A.; Gökkaya, A.E.; Asıleren, M.; Yıldız, F.; Kabukcu, H.O. Evaluation of the Effects of Lipoxin A4 and Resolvin D1 on the Severity of Transient Tachypnea of the Newborn: A Prospective Study. Children 2025, 12, 1421. https://doi.org/10.3390/children12101421
Çığrı E, Çatan İnan F, Gülten S, Bildirici MA, Gökkaya AE, Asıleren M, Yıldız F, Kabukcu HO. Evaluation of the Effects of Lipoxin A4 and Resolvin D1 on the Severity of Transient Tachypnea of the Newborn: A Prospective Study. Children. 2025; 12(10):1421. https://doi.org/10.3390/children12101421
Chicago/Turabian StyleÇığrı, Emrah, Funda Çatan İnan, Sedat Gülten, Mehmet Akif Bildirici, Ayşe Ece Gökkaya, Metin Asıleren, Fethiye Yıldız, and Hilmi Onur Kabukcu. 2025. "Evaluation of the Effects of Lipoxin A4 and Resolvin D1 on the Severity of Transient Tachypnea of the Newborn: A Prospective Study" Children 12, no. 10: 1421. https://doi.org/10.3390/children12101421
APA StyleÇığrı, E., Çatan İnan, F., Gülten, S., Bildirici, M. A., Gökkaya, A. E., Asıleren, M., Yıldız, F., & Kabukcu, H. O. (2025). Evaluation of the Effects of Lipoxin A4 and Resolvin D1 on the Severity of Transient Tachypnea of the Newborn: A Prospective Study. Children, 12(10), 1421. https://doi.org/10.3390/children12101421






