Interleukin-12 as a Predictor for Unresponsiveness to the Hepatitis B Vaccine: A Novel Cut-Off Point in Children
Abstract
1. Introduction
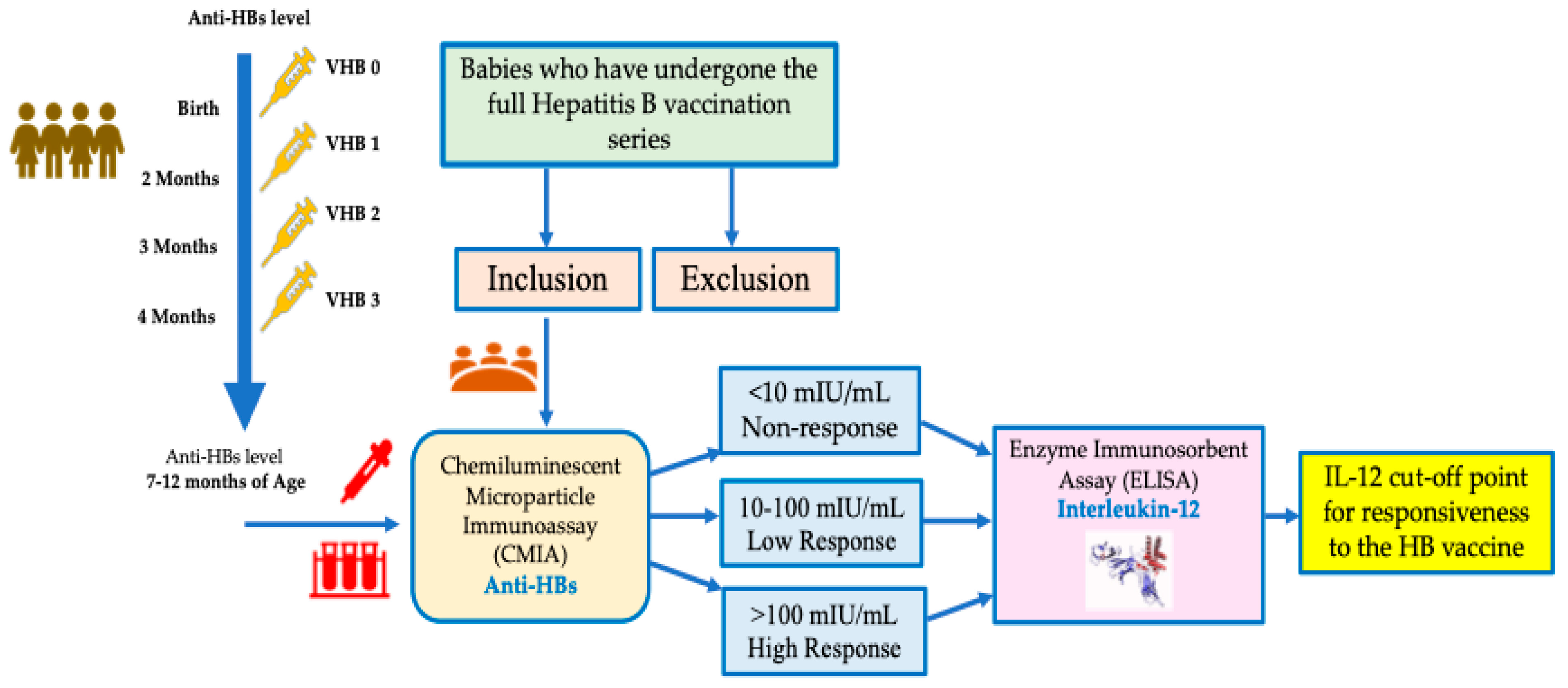
2. Materials and Methods
2.1. Participants and Procedure
2.2. Serum Sampling
2.3. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Results and Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUROC | Area Under the Receiver Operating Characteristics Curve |
| CD | Cluster of differentiation |
| CMIA | Chemiluminescent Microparticle Immunoassay |
| HB | Hepatitis B |
| HBV | Hepatitis B virus |
| HR | High Response |
| IL-12 | Interleukin-12 |
| LR | Low Response |
| NPV | Negative predictive value |
| NR | Non-Response |
| OR | Odd Ratio |
| PPV | positive predictive value |
| ROC | Receiver Operating Characteristics |
| WHO | World Health Organization |
References
- World Health Organization. Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 4 March 2023).
- Tahir, A.; Shinkafi, S.H.; Alshrari, A.S.; Yunusa, A.; Umar, M.T.; Hudu, S.A.; Jimoh, A.O. A Comprehensive Review of Hepatitis B Vaccine Nonresponse and Associated Risk Factors. Vaccines 2024, 12, 710. [Google Scholar] [CrossRef]
- Razavi-Shearer, D. Global HBV Disease Burden and Current Care Gaps. Clin. Liver Dis. 2024, 23, 710. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Hepatitis B Is Preventable with Safe and Effective Vaccines. Available online: https://www.who.int/southeastasia/activities/hepatitis-b-is-preventable-with-safe-and-effective-vaccines (accessed on 20 May 2023).
- World Health Organization. Hepatitis B Vaccines: WHO Position Paper, July 2017—Recommendations. Vaccine 2019, 37, 223–225. [Google Scholar] [CrossRef]
- Walayat, S. Recent Advances in Vaccination of Non-Responders to Standard Dose Hepatitis B Virus Vaccine. World J. Hepatol. 2015, 7, 2503. [Google Scholar] [CrossRef]
- Mormile, R. Hepatitis B Vaccine Non Response: A Predictor of Latent Autoimmunity? Med. Hypotheses 2017, 104, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, L.; Albarran, B.; Salmen, S.; Borges, L.; Fields, H.; Montes, H.; Soyano, A.; Diaz, Y.; Berrueta, L. The Nonresponse to Hepatitis B Vaccination Is Associated with Impaired Lymphocyte Activation. Virology 2004, 326, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Xu, W.; Shao, X.; Yue, Y.; Hu, L.; Xu, H.; Yuan, Z.; Zheng, X.; Xiong, S. Coimmunization with RANTES Plasmid Polarized Th1 Immune Response against Hepatitis B Virus Envelope via Recruitment of Dendritic Cells. Antivir. Res. 2007, 76, 140–149. [Google Scholar] [CrossRef]
- Bello, N.; Hudu, S.A.; Alshrari, A.S.; Imam, M.U.; Jimoh, A.O. Overview of Hepatitis B Vaccine Non-Response and Associated B Cell Amnesia: A Scoping Review. Pathogens 2024, 13, 554. [Google Scholar] [CrossRef]
- Watford, W.T.; Moriguchi, M.; Morinobu, A.; O’Shea, J.J. The Biology of IL-12: Coordinating Innate and Adaptive Immune Responses. Cytokine Growth Factor Rev. 2003, 14, 361–368. [Google Scholar] [CrossRef]
- Roh, E.Y.; Song, E.Y.; Yoon, J.H.; Oh, S.; Chang, J.Y.; Park, H.; Seo, S.H.; Shin, S. Effects of Interleukin-4 and Interleukin-12b Gene Polymorphisms on Hepatitis B Virus Vaccination. Ann. Hepatol. 2017, 16, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.A.; El-Ballat, M.A.F.; El-Sherif, A.M.; Al-Shurbagy, M.A.; Kayioum, M.M.A. Interleukin-12 Level and Its Relation Ti Hepatits B Virus Vaccine Response in Hemodialysis Patients. Al-Azhar Med. J. 2017, 46, 113–120. [Google Scholar] [CrossRef]
- Siemens. Advia Anti-HBs2 (aHBs2) Assay for the Detection of Antibodies to Hepatitis B Surface Antigen. Advia 2017, 1–47. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf10/p100039c.pdf (accessed on 8 March 2023).
- Elabscience Human IL-12 (Interleukin 12) Elisa Kit. Available online: https://www.elabscience.com/p/human-il-12-interleukin-12-elisa-kit (accessed on 8 March 2023).
- Karabay, O.; Hamarat, K.F.; Guney Eskiler, G.; Aydin, A. The Association of Systemic Inflammatory Response with Hepatitis B Vaccine Unresponsiveness. Viruses 2025, 17, 338. [Google Scholar] [CrossRef] [PubMed]
- Salama, I.I.; Sami, S.M.; Elserougy, S.M.; Emam, H.M.; Salama, S.I.; Elhariri, H.M.; Hemeda, S.A.; Hassanain, A.I.; Abdel Mohsen, A.M.; Fouad, W.A.; et al. Humoral Immune Memory to Hepatitis B Vaccine after Primary Vaccination of Children and Adolescents in Assiut, Egypt. Oman Med. J. 2020, 35, e175. [Google Scholar] [CrossRef]
- Lawton, G. You’re Only as Young as Your Immune System. New Sci. 2020, 245, 44–48. [Google Scholar] [CrossRef]
- Kimera, A.; Rujumba, J.; Ndikuno, C.K.; Ndeezi, G.; Mukiza, N.; Basenero, A.; Piloya, T.; Bakeera-Kitaka, S.; Nabulya, R.; Nalumansi, A.M.; et al. Immune Response and Associated Factors to Hepatitis B Vaccination among Children under Five Attending Care at Mulago Hospital. BMC Pediatr. 2025, 25, 175. [Google Scholar] [CrossRef]
- Zhong, S.; Zhang, T.; Tang, L.; Li, Y. Cytokines and Chemokines in HBV Infection. Front. Mol. Biosci. 2021, 8, 805625. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.R.d.A.; Beghini, D.G.; Lemos, A.S.; Martinelli, K.G.; de Mello, V.d.M.; de Almeida, N.A.A.; Lewis-Ximenez, L.L.; de Paula, V.S. Cytokines Profile in Patients with Acute and Chronic Hepatitis B Infection. Microbiol. Immunol. 2022, 66, 31–39. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Gou, C.; Jin, H.; Zhang, C.; Liu, Y.; Wang, Y.; Wang, X. Serum Interleukins as Potential Prognostic Biomarkers in HBV-Related Acute-on-Chronic Liver Failure. Mediat. Inflamm. 2022, 2022, 7794890. [Google Scholar] [CrossRef]
- Rahimkhani, A.; Haghighat, S.; Noorbazargan, H.; Mahdavi, M. Improvement of Hepatitis B Vaccine to Induce IFN-γ Cytokine Response: A New Formulation. Microb. Pathog. 2021, 160, 105184. [Google Scholar] [CrossRef]
- Dimitriadis, K.; Katelani, S.; Pappa, M.; Fragkoulis, G.E.; Androutsakos, T. The Role of Interleukins in HBV Infection: A Narrative Review. J. Pers. Med. 2023, 13, 1675. [Google Scholar] [CrossRef] [PubMed]
- Grzegorzewska, A.E.; Wobszal, P.M.; Mostowska, A.; Jagodziński, P.P. Antibodies to Hepatitis B Virus Surface Antigen and Interleukin 12 and Interleukin 18 Gene Polymorphisms in Hemodialysis Patients. BMC Nephrol. 2012, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Grzegorzewska, A.E.; Wobszal, P.M.; Sowińska, A.; Mostowska, A.; Jagodziński, P.P. Association of the Interleukin-12 Polymorphic Variants with the Development of Antibodies to Surface Antigen of Hepatitis B Virus in Hemodialysis Patients in Response to Vaccination or Infection. Mol. Biol. Rep. 2013, 40, 6899–6911. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | n = 155(%) |
|---|---|
| Sex | |
| Boy | 77 (49.7) |
| Girl | 78 (50.3) |
| Age (months) | |
| 7–<9 | 39 (25.2) |
| 9–12 | 116 (74.8) |
| The age at which the primary Hepatitis B vaccination series was completed (months) | |
| ≤6 | 92 (59.4) |
| >6 | 63 (40.6) |
| Anti-HB titer (mIU/mL) | |
| <10 (NR) | 7 (4.5) |
| 10–100 (LR) | 25 (16.1) |
| >100 (HR) | 123 (79.4) |
| Variable | Statistical Measures | Data Normality Test (p * Value) | |||
|---|---|---|---|---|---|
| Mean | SD | Median | Range | ||
| Age (months) | 9.7 | 1.64 | 10.0 | 7–12 | <0.001 |
| Anti-HBs (mIU/mL) | 407.85 | 314.88 | 370.60 | 0.1–1000 | 0.001 |
| IL-12 (pg/mL) | 86.88 | 183.84 | 14.33 | 0.02–814.78 | <0.001 |
| Variable | Anti-HB Titer (mIU/mL) | p * Value | r | p ** Value | ||
|---|---|---|---|---|---|---|
| <10 (NR) n = 7 | 10–100 (LR) n = 25 | >100 (HR) n = 123 | ||||
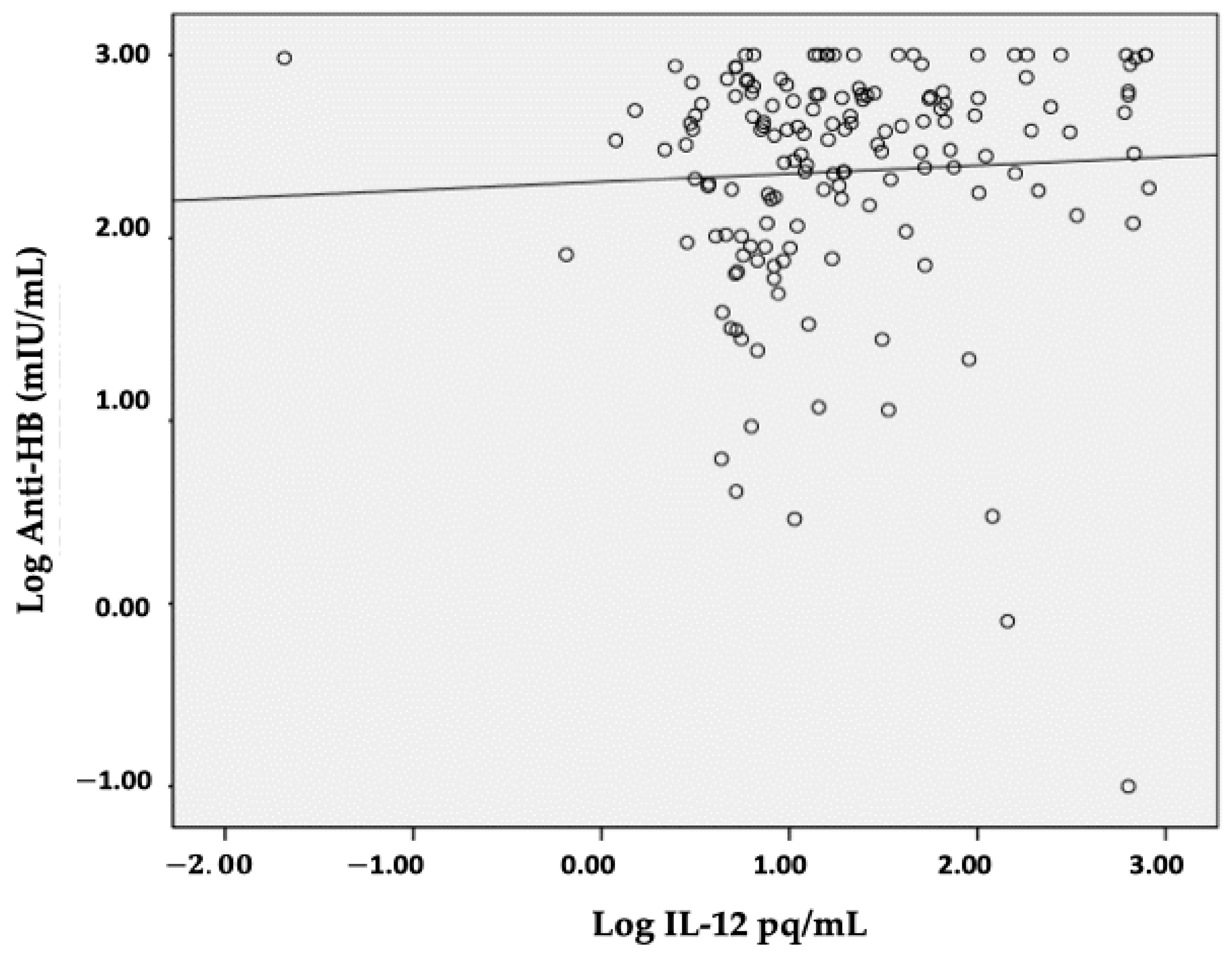
| IL-12 (pg/mL) | 10.65 (4.34–634.26) | 7.42 (0.65–90.09) | 17.26 (0.02–814.77) | 0.013 | 0.193 | 0.016 |
| Anti-HB Response | Cut-Off IL-12 Level | AUROC (CI 95%) | p Value |
|---|---|---|---|
| >10 mIU/mL (LR) | ≤110.39 pg/mL | 0.516 (0.435–0.597) | 0.906 |
| >100 mIU/mL (HR) | >10.65 pg/mL | 0.649 (0.565–0.724) | 0.004 |
| Cut-Off Value | Anti-HBs (mIU/mL) | p * Value | OR (CI 95%) | |
|---|---|---|---|---|
| >100 | ≤100 | |||
| IL-12 (pg/mL): | 0.001 | |||
| >10.65 | 79 | 10 | 3.95 (1.72–9.09) | |
| ≤10.65 | 44 | 22 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermaya, Y.S.; Sribudiani, Y.; Ghrahani, R.; Hock, Q.S.; Prasetyo, D. Interleukin-12 as a Predictor for Unresponsiveness to the Hepatitis B Vaccine: A Novel Cut-Off Point in Children. Children 2025, 12, 1400. https://doi.org/10.3390/children12101400
Ermaya YS, Sribudiani Y, Ghrahani R, Hock QS, Prasetyo D. Interleukin-12 as a Predictor for Unresponsiveness to the Hepatitis B Vaccine: A Novel Cut-Off Point in Children. Children. 2025; 12(10):1400. https://doi.org/10.3390/children12101400
Chicago/Turabian StyleErmaya, Yudith Setiati, Yunia Sribudiani, Reni Ghrahani, Quak Seng Hock, and Dwi Prasetyo. 2025. "Interleukin-12 as a Predictor for Unresponsiveness to the Hepatitis B Vaccine: A Novel Cut-Off Point in Children" Children 12, no. 10: 1400. https://doi.org/10.3390/children12101400
APA StyleErmaya, Y. S., Sribudiani, Y., Ghrahani, R., Hock, Q. S., & Prasetyo, D. (2025). Interleukin-12 as a Predictor for Unresponsiveness to the Hepatitis B Vaccine: A Novel Cut-Off Point in Children. Children, 12(10), 1400. https://doi.org/10.3390/children12101400





