Maternal Overweight and Obesity Alter Neurodevelopmental Trajectories During the First Year of Life: Findings from the OBESO Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Maternal and Infant Variables
2.3. Neurodevelopment Assessment
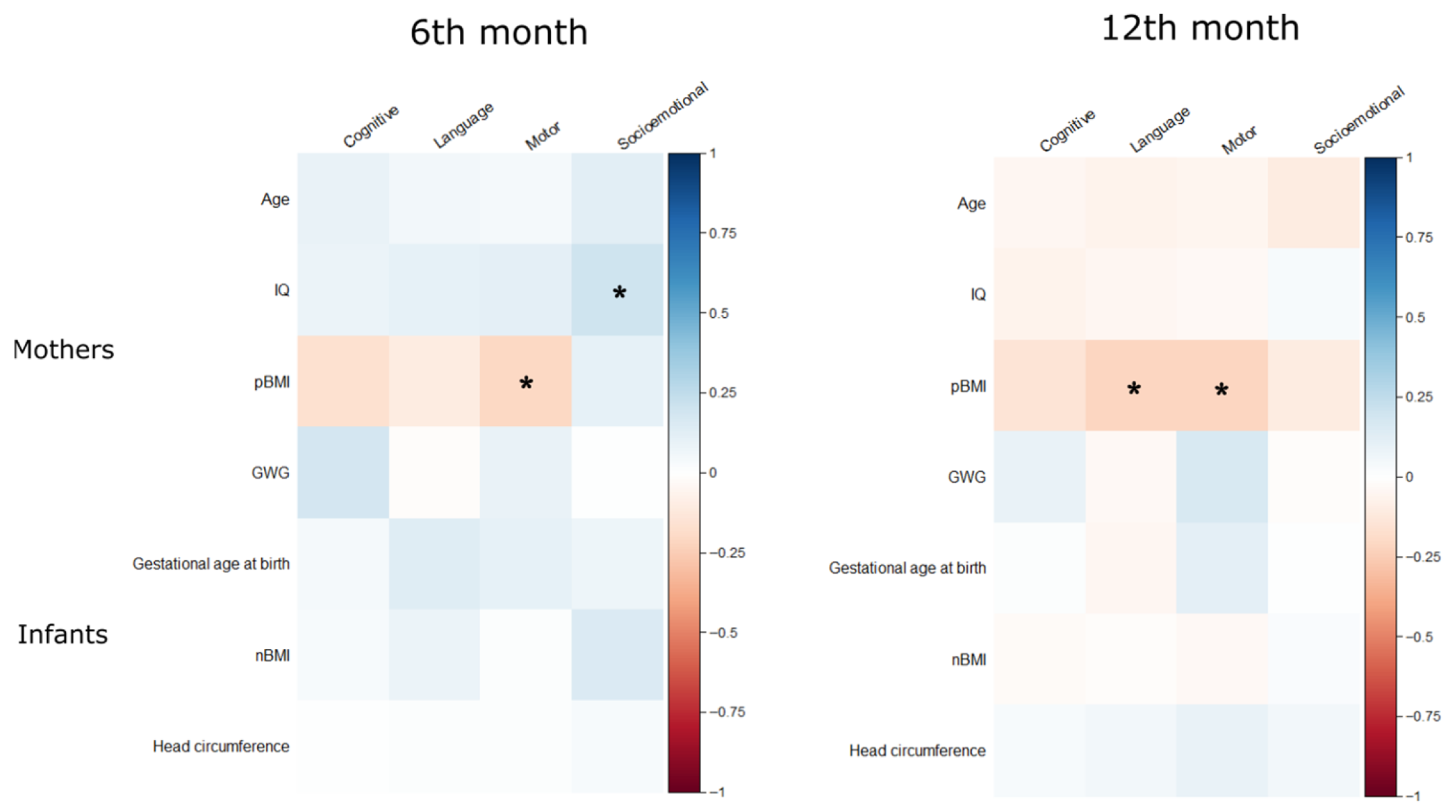
2.4. Statistical Analysis
3. Results
3.1. Maternal and Neonatal Characteristics
3.2. Neurodevelopmental Outcomes
3.3. Developmental Trajectories
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OBESO | Cohort Epigenetic and Biochemical Origin of Overweight and Obesity. |
| pBMI | Pregestational body mass index |
| IQ | Intelligence Quotient. |
| PREOBE | Study of maternal nutrition and genetic on the fetal adiposity programming |
| PREDO | Prediction and prevention of preeclampsia and intrauterine growth restriction study |
| INPer | National Institute of Perinatology Mexico |
| WHO | World Health Organization |
| GWG | Gestational weight gain |
| BSID-III | Bayley Scales of Infant Development Third Edition |
| nBMI | Newborn body mass index |
| Shanghai MCPC | The Shanghai Maternal-Child Pairs Cohort |
| MRI | Magnetic Resonance Imaging |
| DOHaD | Developmental Origins of Health and Disease |
References
- Obesity and Overweight [Internet]. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 October 2023).
- Campos-Nonato, I.; Galván-Valencia, Ó.; Hernández-Barrera, L.; Oviedo-Solís, C.; Barquera, S. Prevalence of obesity and associated risk factors in Mexican adults: Results of the Ensanut 2022. Salud Publica Mex. 2023, 65, s238–s247. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L.; Bilbo, S.D. Developmental programming of brain and behavior by perinatal diet: Focus on inflammatory mechanisms. Dialogues Clin. Neurosci. 2014, 16, 307–320. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Hao, X.; Lu, J.; Yan, S.; Tao, F.; Huang, K. Maternal Pre-Pregnancy Body Mass Index, Gestational Weight Gain and Children’s Cognitive Development: A Birth Cohort Study. Nutrients 2022, 14, 4613. [Google Scholar] [CrossRef]
- Huang, L.; Yu, X.; Keim, S.; Li, L.; Zhang, L.; Zhang, J. Maternal prepregnancy obesity and child neurodevelopment in the Collaborative Perinatal Project. Int. J. Epidemiol. 2014, 43, 783–792. [Google Scholar] [CrossRef]
- Tong, L.; Kalish, B.T. The impact of maternal obesity on childhood neurodevelopment. J. Perinatol. 2021, 41, 928–939. [Google Scholar] [CrossRef]
- Edlow, A.G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagn. 2017, 37, 95–110. [Google Scholar] [CrossRef]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. 2018, 19, 464–484. [Google Scholar] [CrossRef]
- Adane, A.A.; Mishra, G.D.; Tooth, L.R. Maternal pre-pregnancy obesity and childhood physical and cognitive development of children: A systematic review. Int. J. Obes. 2016, 40, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Gage, S.H.; Lawlor, D.A.; Tilling, K.; Fraser, A. Associations of maternal weight gain in pregnancy with offspring cognition in childhood and adolescence: Findings from the Avon Longitudinal Study of Parents and Children. Am. J. Epidemiol. 2013, 177, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Rajasilta, O.; Häkkinen, S.; Björnsdotter, M.; Scheinin, N.M.; Lehtola, S.J.; Saunavaara, J.; Parkkola, R.; Lähdesmäki, T.; Karlsson, L.; Karlsson, H.; et al. Maternal pre-pregnancy BMI associates with neonate local and distal functional connectivity of the left superior frontal gyrus. Sci. Rep. 2021, 11, 19182. [Google Scholar] [CrossRef]
- Nazzari, S.; Frigerio, A. The programming role of maternal antenatal inflammation on infants’ early neurodevelopment: A review of human studies: Special Section on “Translational and Neuroscience Studies in Affective Disorders” Section Editor, Maria Nobile MD, PhD. J. Affect. Disord. 2020, 263, 739–746. [Google Scholar] [CrossRef]
- van der Burg, J.W.; Sen, S.; Chomitz, V.R.; Seidell, J.C.; Leviton, A.; Dammann, O. The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatr. Res. 2016, 79, 3–12. [Google Scholar] [CrossRef]
- Agarwal, S.; Scher, M.S. Fetal-neonatal neurology program development: Continuum of care during the first 1000 days. J. Perinatol. 2022, 42, 165–168. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Early Diagnostics and Early Intervention in Neurodevelopmental Disorders-Age-Dependent Challenges and Opportunities. J. Clin. Med. Res. 2021, 10, 861. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: A systematic review. Transl. Psychiatry. 2021, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Darling, J.C.; Bamidis, P.D.; Burberry, J.; Rudolf, M.C.J. The First Thousand Days: Early, integrated and evidence-based approaches to improving child health: Coming to a population near you? Arch. Dis. Child. 2020, 105, 837–841. [Google Scholar] [CrossRef]
- Scher, M.S. “The First Thousand Days” Define a Fetal/Neonatal Neurology Program. Front. Pediatr. 2021, 9, 683138. [Google Scholar] [CrossRef]
- Zapata-Tarrés, M.M. The first 1000 days of life: The great opportunity. Bol. Med. Hosp. Infant. Mex. 2025, 82 (Suppl. 1), 1–2. [Google Scholar] [CrossRef] [PubMed]
- Draper, C.E.; Yousafzai, A.K.; McCoy, D.C.; Cuartas, J.; Obradović, J.; Bhopal, S.; Fisher, J.; Jeong, J.; Klingberg, S.; Milner, K.; et al. The next 1000 days: Building on early investments for the health and development of young children. Lancet 2024, 404, 2094–2116. [Google Scholar] [CrossRef]
- Dan, B. The first 1000 days: A critical window for neurodevelopmental trajectories and interventions—And for parents. Dev. Med. Child. Neurol. 2025, 67, 1108–1109. [Google Scholar] [CrossRef] [PubMed]
- Torres-Espinola, F.J.; Berglund, S.K.; García-Valdés, L.M.; Segura, M.T.; Jerez, A.; Campos, D.; Moreno-Torres, R.; Rueda, R.; Catena, A.; Pérez-García, M.; et al. Maternal Obesity, Overweight and Gestational Diabetes Affect the Offspring Neurodevelopment at 6 and 18 Months of Age—A Follow Up from the PREOBE Cohort. PLoS ONE 2015, 10, e0133010. [Google Scholar] [CrossRef]
- Girchenko, P.; Lahti, M.; Tuovinen, S.; Savolainen, K.; Lahti, J.; Binder, E.B.; Reynolds, R.M.; Entringer, S.; Buss, C.; Wadhwa, P.D.; et al. Cohort Profile: Prediction and prevention of preeclampsia and intrauterine growth restriction (PREDO) study. Int. J. Epidemiol. 2017, 46, 1380–1381g. [Google Scholar]
- Kato, T.; Nishimura, T.; Takahashi, N.; Harada, T.; Okumura, A.; Iwabuchi, T.; Nomura, Y.; Senju, A.; Tsuchiya, K.J.; Takei, N. Identification of neurodevelopmental transition patterns from infancy to early childhood and risk factors predicting descending transition. Sci. Rep. 2022, 12, 4822. [Google Scholar] [CrossRef]
- Lohman, T.J.; Roache, A.F.; Martorell, R. (Eds.) Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988; 192p. [Google Scholar]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; 160p. [Google Scholar]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence—Second Edition (WASI II); Pearson: San Antonio, TX, USA, 2011. [Google Scholar]
- Institute of Medicine, National Research Council. Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009; Available online: https://nap.nationalacademies.org/read/12584/chapter/2 (accessed on 8 October 2025).
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy [Internet]; WHO: Geneva, Switzerland, 2013; Available online: https://www.who.int/publications-detail-redirect/9789241506823 (accessed on 27 May 2025).
- World Health Organization. WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia [Internet]; WHO: Geneva, Switzerland, 2011; Available online: https://www.who.int/publications/i/item/9789241548335 (accessed on 27 May 2025).
- World Health Organization. Preterm Birth [Internet]; WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 27 May 2025).
- Bayley, N. Bayley Scales of Infant and Toddler Development; PsychCorp; Harcourt Assessment: San Antonio, TX, USA, 2005; 163p. [Google Scholar]
- Dong, X.; Zhou, A. Associations of maternal pre-pregnancy body mass index and gestational weight gain with risk of offspring neurodevelopment at 2 years: A Chinese birth cohort study. Front. Pediatr. 2023, 11, 1165743. [Google Scholar] [CrossRef] [PubMed]
- Duffany, K.O.; McVeigh, K.H.; Kershaw, T.S.; Lipkind, H.S.; Ickovics, J.R. Maternal Obesity: Risks for Developmental Delays in Early Childhood. Matern. Child. Health J. 2016, 20, 219–230. [Google Scholar] [CrossRef]
- Heikura, U.; Taanila, A.; Hartikainen, A.L.; Olsen, P.; Linna, S.L.; von Wendt, L.; Järvelin, M.R. Variations in prenatal sociodemographic factors associated with intellectual disability: A study of the 20-year interval between two birth cohorts in northern Finland. Am. J. Epidemiol. 2008, 167, 169–177. [Google Scholar] [CrossRef]
- Babaei, M.; Machle, C.J.; Mokhtari, P.; Ottino González, J.; Schmidt, K.A.; Alderete, T.L.; Adise, S.; Peterson, B.S.; Goran, M.I. Pre-pregnancy maternal obesity and infant neurodevelopmental outcomes in Latino infants. Obesity 2024, 32, 979–988. [Google Scholar] [CrossRef]
- Girchenko, P.; Tuovinen, S.; Lahti-Pulkkinen, M.; Lahti, J.; Savolainen, K.; Heinonen, K.; Pyhälä, R.; Reynolds, R.M.; Hämäläinen, E.; Villa, P.M.; et al. Maternal early pregnancy obesity and related pregnancy and pre-pregnancy disorders: Associations with child developmental milestones in the prospective PREDO Study. Int. J. Obes. 2018, 42, 995–1007. [Google Scholar] [CrossRef]
- Basatemur, E.; Gardiner, J.; Williams, C.; Melhuish, E.; Barnes, J.; Sutcliffe, A. Maternal prepregnancy BMI and child cognition: A longitudinal cohort study. Pediatrics 2013, 131, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Arabiat, D.; Al Jabery, M.; Jenkins, M.; Kemp, V.; Whitehead, L.; Adams, G. Language abilities in children born to mothers diagnosed with diabetes: A systematic review and meta-analysis. Early Hum. Dev. 2021, 159, 105420. [Google Scholar] [CrossRef]
- Nichols, A.R.; Rundle, A.G.; Factor-Litvak, P.; Insel, B.J.; Hoepner, L.; Rauh, V.; Perera, F.; Widen, E.M. Prepregnancy obesity is associated with lower psychomotor development scores in boys at age 3 in a low-income, minority birth cohort. J. Dev. Orig. Health Dis. 2020, 11, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Berglund, S.K.; Torres-Espínola, F.J.; García-Valdés, L.; Segura, M.T.; Martínez-Zaldívar, C.; Padilla, C.; Rueda, R.; García, M.P.; McArdle, H.J.; Campoy, C. The impacts of maternal iron deficiency and being overweight during pregnancy on neurodevelopment of the offspring. Br. J. Nutr. 2017, 118, 533–540. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, X.; Wei, Q.; Zhang, Y.; Wang, L.; Shi, H. Maternal Pre-Pregnancy BMI and Gestational Weight Gain Modified the Association between Prenatal Depressive Symptoms and Toddler’s Emotional and Behavioral Problems: A Prospective Cohort Study. Nutrients 2022, 15, 181. [Google Scholar] [CrossRef] [PubMed]
- Girchenko, P.; Lahti-Pulkkinen, M.; Lahti, J.; Pesonen, A.-K.; Hämäläinen, E.; Villa, P.M.; Kajantie, E.; Laivuori, H.; Reynolds, R.M.; Räikkönen, K. Neonatal regulatory behavior problems are predicted by maternal early pregnancy overweight and obesity: Findings from the prospective PREDO Study. Pediatr. Res. 2018, 84, 875–881. [Google Scholar] [CrossRef]
- Mina, T.H.; Lahti, M.; Drake, A.J.; Denison, F.C.; Räikkönen, K.; E Norman, J.; Reynolds, R.M. Prenatal exposure to maternal very severe obesity is associated with impaired neurodevelopment and executive functioning in children. Pediatr. Res. 2017, 82, 47–54. [Google Scholar] [CrossRef]
- Robinson, S.L.; Ghassabian, A.; Sundaram, R.; Trinh, M.-H.; Lin, T.-C.; Bell, E.M.; Yeung, E. Parental Weight Status and Offspring Behavioral Problems and Psychiatric Symptoms. J. Pediatr. 2020, 220, 227–236.e1. [Google Scholar] [CrossRef] [PubMed]
- Neri, C.; Edlow, A.G. Effects of Maternal Obesity on Fetal Programming: Molecular Approaches. Cold Spring Harb. Perspect. Med. 2015, 6, a026591. [Google Scholar] [CrossRef]
- Na, X.; Phelan, N.E.; Tadros, M.R.; Wu, Z.; Andres, A.; Badger, T.M.; Glasier, C.M.; Ramakrishnaiah, R.R.; Rowell, A.C.; Wang, L.; et al. Maternal Obesity during Pregnancy is Associated with Lower Cortical Thickness in the Neonate Brain. AJNR Am. J. Neuroradiol. 2021, 42, 2238–2244. [Google Scholar] [CrossRef]
- Ionescu, M.I.; Zahiu, C.D.M.; Vlad, A.; Galos, F.; Gradisteanu Pircalabioru, G.; Zagrean, A.M.; O’Mahony, S.M. Nurturing development: How a mother’s nutrition shapes offspring’s brain through the gut. Nutr. Neurosci. 2025, 28, 50–72. [Google Scholar] [CrossRef]
- Widen, E.M.; Kahn, L.G.; Cirillo, P.; Cohn, B.; Kezios, K.L.; Factor-Litvak, P. Prepregnancy overweight and obesity are associated with impaired child neurodevelopment. Matern. Child. Nutr. 2018, 14, e12481. [Google Scholar] [CrossRef]
- The CHILD Study Investigators; Krzeczkowski, J.E.; Lau, A.; Fitzpatrick, J.; Tamana, S.; Smithson, L.; de Souza, R.; Kozyrskyj, A.L.; Lefebvre, D.; Becker, A.B.; et al. Maternal Metabolic Complications in Pregnancy and Offspring Behavior Problems at 2 Years of Age. Matern. Child. Health J. 2019, 23, 746–755. [Google Scholar] [CrossRef]
- Costa Wiltgen, A.; Valentini, N.C.; Beltram Marcelino, T.; Santos Pinto Guimarães, L.; Homrich Da Silva, C.; Rombaldi Bernardi, J.; Goldani, M.Z. Different intrauterine environments and children motor development in the first 6 months of life: A prospective longitudinal cohort. Sci. Rep. 2023, 13, 10325. [Google Scholar] [CrossRef]
- Islam, M.M.; Khan, M.N. Early childhood development and its association with maternal parity. Child. Care Health Dev. 2023, 49, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Glynn, L.M. Increasing parity is associated with cumulative effects on memory. J. Women’s Health 2012, 21, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Chen, C.T.; Yang, H.J.; Chou, P. Family structure, birth order, and aggressive behaviors among school-aged boys with attention deficit hyperactivity disorder (ADHD). Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 661–670. [Google Scholar] [CrossRef]
- Reimelt, C.; Wolff, N.; Hölling, H.; Mogwitz, S.; Ehrlich, S.; Martini, J.; Roessner, V. Siblings and Birth Order-Are They Important for the Occurrence of ADHD? J. Atten. Disord. 2021, 25, 81–90. [Google Scholar] [CrossRef]
- Sajewicz-Radtke, U.; Łada-Maśko, A.; Olech, M.; Jurek, P.; Bieleninik, Ł.; Radtke, B.M. Association between parental education level and intelligence quotient of children referred to the mental healthcare system: A cross-sectional study in Poland. Sci. Rep. 2025, 15, 4142. [Google Scholar] [CrossRef] [PubMed]
- Lean, R.E.; Paul, R.A.; Smyser, C.D.; Rogers, C.E. Maternal intelligence quotient (IQ) predicts IQ and language in very preterm children at age 5 years. J. Child. Psychol. Psychiatry 2018, 59, 150–159. [Google Scholar] [CrossRef]
- Harding, J.F. Increases in maternal education and low-income children’s cognitive and behavioral outcomes. Dev. Psychol. 2015, 51, 583–599. [Google Scholar] [CrossRef]
- Zhao, Y.K.; Li, M.; Shi, T.T.; Feng, M.M.; Hu, L.L. Association of premature birth and maternal education level on attention deficit hyperactivity disorder in children: A meta-analysis. World J. Psychiatry 2024, 14, 1956–1970. [Google Scholar] [CrossRef]
- Jackson, M.; Kiernan, K.; McLanahan, S. Maternal Education, Changing Family Circumstances, and Children’s Skill Development in the United States and UK. Ann. Am. Acad. Pol. Soc. Sci. 2017, 674, 59–84. [Google Scholar] [CrossRef] [PubMed]
- Bando, R.; Lopez-Boo, F.; Fernald, L.; Gertler, P.; Reynolds, S. Gender Differences in Early Child Development: Evidence from Large-Scale Studies of Very Young Children in Nine Countries. J. Econ. Race Policy 2024, 7, 82–92. [Google Scholar] [CrossRef]
- Brandlistuen, R.E.; Flatø, M.; Stoltenberg, C.; Helland, S.S.; Wang, M.V. Gender gaps in preschool age: A study of behavior, neurodevelopment and pre-academic skills. Scand. J. Public Health 2021, 49, 503–510. [Google Scholar] [CrossRef]
- Rice, L.C.; Rochowiak, R.N.; Plotkin, M.R.; Rosch, K.S.; Mostofsky, S.H.; Crocetti, D. Sex Differences and Behavioral Associations with Typically Developing Pediatric Regional Cerebellar Gray Matter Volume. Cerebellum 2024, 23, 589–600. [Google Scholar] [CrossRef]
- Cook, K.M.; De Asis-Cruz, J.; Lopez, C.; Quistorff, J.; Kapse, K.; Andersen, N.; Vezina, G.; Limperopoulos, C. Robust sex differences in functional brain connectivity are present in utero. Cereb. Cortex 2022, 33, 2441–2454. [Google Scholar] [CrossRef] [PubMed]
- Short, A.K.; Baram, T.Z. Early-life adversity and neurological disease: Age-old questions and novel answers. Nat. Rev. Neurol. 2019, 15, 657–669. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Bhavnani, S.; Betancourt, T.S.; Tomlinson, M.; Patel, V. Adverse childhood experiences and lifelong health. Nat. Med. 2023, 29, 1639–1648. [Google Scholar] [CrossRef]

| Normal Weight (n = 41) | Overweight/Obesity (n = 56) | p Value | |
|---|---|---|---|
| Mothers | |||
| Age (years) | 29.85 ± 4.59 | 30.42 ± 5.51 | 0.75 |
| Education level | |||
| Middle and high school | 28 (28.9%) | 37 (38.1%) | 0.26 |
| Higher education | 13 (13.4%) | 19 (19.6%) | 0.29 |
| IQ | 91.0 ± 12.0 | 89.9 ± 10.4 | 0.65 |
| Parity | |||
| Nulliparous | 27 (27.8%) | 32 (32.9%) | 0.51 |
| Multiparous | 14 (14.43%) | 24 (24.7%) | 0.10 |
| Gestational weight gain (kg) | 8.36 ± 3.88 | 6.16 ± 5.32 | 0.02 |
| Gestational weight gain (Diagnostic) | |||
| Insufficient | 15 (15.4%) | 15 (15.4%) | 0.99 |
| Adequate | 14 (14.4%) | 12 (12.4%) | 0.69 |
| Excessive | 12 (12.4%) | 27 (27.8%) | 0.02 |
| Gestational diabetes/preeclampsia | |||
| Yes | 6 (6.2%) | 11 (11.3%) | 0.23 |
| No | 35 (13.0%) | 45 (46.4%) | 0.26 |
| Mode of delivery | |||
| Vaginal | 22 (22.7%) | 27 (27.8%) | 0.48 |
| Cesarean section | 19 (19.6%) | 29 (29.9%) | 0.15 |
| Infants | |||
| Gestational age at birth (weeks) | 38.72 ± 1.32 | 38.55 ± 1.92 | 0.59 |
| Sex | |||
| Male | 21 (21.6%) | 32 (32.9%) | 0.13 |
| Female | 20 (20.6%) | 24 (24.7%) | 0.55 |
| Preterm birth | |||
| Yes | 4 (4.1%) | 6 (6.2%) | 0.52 |
| No | 37 (38.1%) | 60 (51.5%) | |
| nBMI | 12.410 ± 1.48 | 12.97 ± 1.56 | 0.89 |
| Head circumference birth (cm) | 33.4 ± 1.39 | 33.5 ± 1.72 | 0.55 |
| Birth weight (g) | 2900 ± 396 | 2900 ± 500 | 0.97 |
| Birth length (cm) | 47.30 ± 2.42 | 47.13 ± 2.79 | 0.72 |
| Apgar 1 min | 7.28 ± 2.06 | 7.81 ± 1.2 | 0.14 |
| Apgar 5 min | 8.95 ± 0.39 | 8.94 ± 0.30 | 0.94 |
| 6th Month | 12th Month | Total (97) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cognitive | Language | Motor | Socioemotional | Cognitive | Language | Motor | Socioemotional | ||
| Mothers (n = 97) | |||||||||
| Education level | |||||||||
| Middle and high school | 96.54 ± 9.96 | 90.00 ± 9.57 | 88.46 ± 11.24 | 97.77 ± 14.20 | 102.46 ± 10.87 | 88.14 ± 9.55 | 89.72 ± 9.22 | 94.94 ± 12.65 | 65 (67.0%) |
| Higher education | 96.87 ± 11.48 | 87.97 ± 10.75 | 87.53 ± 12.38 | 100.00 ± 15.03 | 100.31 ± 11.57 | 82.56 ± 16.29 | 88.88 ± 12.90 | 98.44 ± 11.94 | 32 (33.0%) |
| p value | 0.895 | 0.506 | 0.677 | 0.431 | 0.234 | 0.097 | 0.292 | 0.25 | 0.356 |
| Parity | |||||||||
| Nulliparous | 96.69 ± 10.93 | 89.49 ± 9.21 | 87.39 ± 12.34 | 101.53 ± 13.01 | 101.10 ± 11.75 | 85.92 ± 14.64 | 88.54 ± 12.28 | 98.05 ± 11.89 | 38 (39.2%) |
| Multiparous | 96.58 ± 10.3 | 89.08 ± 11.15 | 89.34 ± 10.33 | 93.82 ± 15.44 | 102.76 ± 10.05 | 86.89 ± 7.82 | 90.84 ± 6.87 | 93.05 ± 12.88 | 59 (60.8%) |
| p value | 0.937 | 0.584 | 0.467 | 0.013 | 0312 | 0.888 | 0.18 | 0.041 | 0.459 |
| Pregestational BMI | |||||||||
| Normal weight | 98.66 ± 10.25 | 91.41 ± 9.89 | 90.17 ± 10.24 | 98.05 ± 16.08 | 103.66 ± 10.73 | 90.56 ± 7.43 | 91.02 ± 10.71 | 99.15 ± 9.41 | 41 (42.3%) |
| Overweight/obesity | 95.18 ± 10.40 | 87.80 ± 9.82 | 86.68 ± 12.34 | 98.84 ± 13.25 | 100.36 ± 11.24 | 83.18 ± 14.29 | 88.29 ± 10.31 | 93.86 ± 13.96 | 56 (57.7%) |
| p value | 0.135 | 0.149 | 0.117 | 0.791 | 0.31 | 0.002 | 0.396 | 0.031 | 0.878 |
| Gestational weight gain | |||||||||
| Weight loss | 93.33 ± 13.29 | 89.67 ± 9.35 | 84.17 ± 19.02 | 97.50 ± 13.69 | 101.67 ± 18.35 | 87.67 ± 14.72 | 85.50 ± 13.07 | 95.83 ± 25.58 | 6 (6.2%) |
| Insufficient | 94.60 ± 9.89 | 90.48 ± 10.08 | 90.60 ± 10.86 | 95.40 ± 14.36 | 102.00 ± 10.31 | 88.72 ± 10.11 | 90.64 ± 12.23 | 96.00 ± 9.57 | 25 (25.8%) |
| Adequate | 97.50 ± 11.07 | 88.42 ± 11.07 | 86.65 ± 11.27 | 102.88 ± 14.01 | 102.12 ± 11.33 | 87.27 ± 7.58 | 87.54 ± 8.56 | 97.50 ± 12.19 | 27 (27.8%) |
| Excessive | 97.82 ± 10.12 | 88.95 ± 10.12 | 88.05 ± 11.14 | 97.56 ± 14.86 | 101.54 ± 10.65 | 83.69 ± 15.60 | 90.51 ± 10.35 | 95.28 ± 12.20 | 39 (40.2%) |
| p value | 0.769 | 0.673 | 0.682 | 0.405 | 0.830 | 0.615 | 0.482 | 0.977 | 0.102 |
| GDM/ preeclampsia | |||||||||
| No | 95.81 ± 10.75 | 89.03 ± 10.30 | 87.68 ± 11.73 | 98.12 ± 14.35 | 101.88 ± 11.46 | 85.74 ± 13.18 | 89.56 ± 11.31 | 95.95 ± 13.46 | 80 (82.5%) |
| Yes | 100.59 ± 7.88 | 90.76 ± 8.29 | 90.41 ± 10.89 | 100.29 ± 15.15 | 101.18 ± 9.44 | 88.94 ± 7.28 | 88.88 ± 5.59 | 96.76 ± 6.11 | 17 (17.5%) |
| p value | 0.072 | 0.358 | 0.466 | 0.388 | 0.969 | 0.393 | 0.759 | 0.897 | 0.78 |
| Route of delivery | |||||||||
| Vaginal | 96.84 ± 10.83 | 89.41 ± 10.10 | 88.20 ± 11.82 | 99.49 ± 11.82 | 101.53 ± 14.08 | 84.08 ± 10.95 | 89.45 ± 13.93 | 95.92 ± 9.07 | 49 (50.5%) |
| Cesarean section | 96.46 ± 10.10 | 89.25 ± 9.93 | 88.10 ± 9.93 | 97.50 ± 11.44 | 101.98 ± 14.88 | 88.56 ± 11.33 | 89.44 ± 10.23 | 96.27 ± 11.90 | 48 (49.5%) |
| p value | 0.991 | 0.902 | 0.942 | 0.514 | 0.658 | 0.858 | 0.954 | 0.954 | 0.89 |
| Infants (n = 97) | |||||||||
| Sex | |||||||||
| Male | 95.00 ± 10.38 | 89.42 ± 8.88 | 87.32 ± 11.40 | 99.25 ± 15.11 | 99.53 ± 11.06 | 86.43 ± 9.92 | 90.08 ± 9.12 | 95.49 ± 13.32 | 53 (54.6%) |
| Female | 98.64 ± 10.25 | 89.23 ± 11.23 | 89.16 ± 11.83 | 97.61 ± 13.70 | 104.43 ± 10.63 | 86.14 ± 14.95 | 88.68 ± 12.05 | 96.82 ± 11.47 | 44 (45.4%) |
| p value | 0.049 | 0.861 | 0.371 | 0.633 | 0.37 | 0.651 | 0.263 | 0.72 | 0.3 |
| Preterm birth | |||||||||
| No | 96.67 ± 10.61 | 89.61 ± 9.89 | 88.14 ± 12.04 | 98.33 ± 14.38 | 101.78 ± 11.28 | 86.17 ± 12.75 | 89.33 ± 10.94 | 96.09 ± 12.56 | 87 (89.7%) |
| Yes | 96.50 ± 9.14 | 86.90 ± 10.84 | 88.30 ± 6.70 | 100.00 ± 15.63 | 101.50 ± 9.73 | 87.40 ± 8.96 | 90.40 ± 5.80 | 96.10 ± 12.24 | 10 (10.3%) |
| p value | 0.962 | 0.363 | 0.858 | 0.711 | 0.914 | 0.830 | 0.525 | 0.962 | 0.12 |
| 6 Months | ||||||
|---|---|---|---|---|---|---|
| Female | Male | |||||
| Normal Weight | Overweight/Obesity | p Value | Normal Weight | Overweight/Obesity | p Value | |
| Cognitive | 101.25 ± 10.24 | 96.46 ± 9.94 | 0.125 | 96.19 ± 9.86 | 94.22 ± 10.78 | 0.496 |
| Language | 92.20 ± 11.99 | 86.75 ± 10.15 | 0.116 | 90.67 ± 7.60 | 88.59 ± 9.66 | 0.388 |
| Motor | 91.50 ± 9.55 | 87.21 ± 13.33 | 0.222 | 88.90 ± 10.94 | 86.28 ± 11.75 | 0.412 |
| Socioemotional | 97.00 ± 14.99 | 98.12 ± 12.84 | 0.793 | 99.05 ± 17.37 | 99.38 ± 13.72 | 0.942 |
| 12 Months | ||||||
| Cognitive | 104.00 ± 11.54 | 104.79 ± 10.05 | 0.812 | 103.33 ± 10.17 | 97.03 ± 11.06 | 0.039 |
| Language | 90.80 ± 8.32 | 82.25 ± 18.05 | 0.046 | 90.33 ± 6.67 | 83.88 ± 10.92 | 0.010 |
| Motor | 90.85 ± 12.67 | 86.88 ± 11.46 | 0.286 | 91.19 ± 8.77 | 89.34 ± 9.40 | 0.470 |
| Socioemotional | 98.00 ± 6.57 | 95.83 ± 14.42 | 0.515 | 100.24 ± 11.56 | 92.38 ± 13.64 | 0.029 |
| Predictor | β | 95% CI | p Value | R2m | R2c | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Cognitive | 0.11 | 0.39 | ||||
| Overweight/obesity | −3.21348 | −6.79072 | 0.363772 | 0.077681 | ||
| Higher education | −0.54558 | −4.38046 | 3.289294 | 0.778076 | ||
| Multiparous | 0.718202 | −3.02169 | 4.458094 | 0.703685 | ||
| Maternal IQ | −0.02192 | −0.18905 | 0.145208 | 0.795001 | ||
| Gestational age at birth | 0.078311 | −1.07223 | 1.228852 | 0.892726 | ||
| nBMI | 0.06884 | −1.28041 | 1.418093 | 0.91948 | ||
| Female | 4.046038 | 0.466961 | 7.625115 | 0.027165 | ||
| Language | 0.1 | 0.26 | ||||
| Overweight/obesity | −5.44304 | −8.91935 | −1.96672 | 0.002505 | ||
| Higher education | −3.83191 | −7.55858 | −0.10523 | 0.044001 | ||
| Multiparous | 0.256382 | −3.37799 | 3.890751 | 0.888843 | ||
| Maternal IQ | 0.003298 | −0.15911 | 0.165711 | 0.967905 | ||
| Gestational age at birth | 0.251222 | −0.86686 | 1.369301 | 0.656352 | ||
| nBMI | 0.196762 | −1.11442 | 1.507947 | 0.766264 | ||
| Female | −0.8206 | −4.29869 | 2.657494 | 0.640362 | ||
| Motor | 0.05 | 0.56 | ||||
| Overweight/obesity | −3.16637 | −7.17375 | 0.841017 | 0.119969 | ||
| Higher education | −0.6864 | −4.98239 | 3.609589 | 0.751628 | ||
| Multiparous | 2.779343 | −1.41024 | 6.968927 | 0.190836 | ||
| Maternal IQ | 0.01883 | −0.16839 | 0.206053 | 0.842065 | ||
| Gestational age at birth | 0.957744 | −0.33114 | 2.24663 | 0.143344 | ||
| nBMI | −0.53423 | −2.04572 | 0.977261 | 0.484332 | ||
| Female | −0.53801 | −4.54744 | 3.471425 | 0.790375 | ||
| Socioemotional | 0.12 | 0.4 | ||||
| Overweight/obesity | −1.53999 | −5.91128 | 2.831306 | 0.485751 | ||
| Higher education | 2.42417 | −2.26194 | 7.110275 | 0.30679 | ||
| Multiparous | −7.06145 | −11.6315 | −2.49141 | 0.002836 | ||
| Maternal IQ | 0.24423 | 0.040004 | 0.448455 | 0.019637 | ||
| Gestational age at birth | −0.36472 | −1.77065 | 1.041205 | 0.607511 | ||
| nBMI | 1.704818 | 0.056069 | 3.353567 | 0.042855 | ||
| Female | 0.387941 | −3.98559 | 4.76147 | 0.860499 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canul-Euan, A.A.; Mendoza-Ortega, J.A.; Solis-Paredes, J.M.; Borboa-Olivares, H.; Martínez-Medina, S.; Hernández-Chávez, C.; Gil-Martínez, G.; Osorio-Valencia, E.; Torres-Calapiz, M.; Suárez-Rico, B.V.; et al. Maternal Overweight and Obesity Alter Neurodevelopmental Trajectories During the First Year of Life: Findings from the OBESO Cohort. Children 2025, 12, 1385. https://doi.org/10.3390/children12101385
Canul-Euan AA, Mendoza-Ortega JA, Solis-Paredes JM, Borboa-Olivares H, Martínez-Medina S, Hernández-Chávez C, Gil-Martínez G, Osorio-Valencia E, Torres-Calapiz M, Suárez-Rico BV, et al. Maternal Overweight and Obesity Alter Neurodevelopmental Trajectories During the First Year of Life: Findings from the OBESO Cohort. Children. 2025; 12(10):1385. https://doi.org/10.3390/children12101385
Chicago/Turabian StyleCanul-Euan, Arturo Alejandro, Jonatan Alejandro Mendoza-Ortega, Juan Mario Solis-Paredes, Héctor Borboa-Olivares, Sandra Martínez-Medina, Carmen Hernández-Chávez, Gabriela Gil-Martínez, Erika Osorio-Valencia, Mariana Torres-Calapiz, Blanca Vianey Suárez-Rico, and et al. 2025. "Maternal Overweight and Obesity Alter Neurodevelopmental Trajectories During the First Year of Life: Findings from the OBESO Cohort" Children 12, no. 10: 1385. https://doi.org/10.3390/children12101385
APA StyleCanul-Euan, A. A., Mendoza-Ortega, J. A., Solis-Paredes, J. M., Borboa-Olivares, H., Martínez-Medina, S., Hernández-Chávez, C., Gil-Martínez, G., Osorio-Valencia, E., Torres-Calapiz, M., Suárez-Rico, B. V., González-Ludlow, I., Rodríguez-Hernández, C., Rodríguez-Cano, A., Reyes-Muñoz, E., Camacho-Arroyo, I., Hernandez, S. L., Perichart-Perera, O., & Estrada-Gutierrez, G. (2025). Maternal Overweight and Obesity Alter Neurodevelopmental Trajectories During the First Year of Life: Findings from the OBESO Cohort. Children, 12(10), 1385. https://doi.org/10.3390/children12101385









