Short-Duration Systemic Lidocaine for the Management of Refractory Chronic Pain in Pediatrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Demographic Information
2.3. Lidocaine Infusion
2.4. Tolerability of the Infusion
2.5. Pain Scores
2.6. Psychological Questionnaires
- Functional Disability Inventory (FDI): The FDI is a validated 15-item self-report questionnaire examining one’s ability to complete physical tasks. The FDI has been validated for use in pediatric chronic widespread pain [19].
- Pain Catastrophizing Scale for Children (PCS-C) [20] is a validated 13-item self-report measure of negative thoughts and behaviors related to pain.
- Fear of Pain Questionnaire, Child Report (FOPQ-C) [21] is a 24-item measure that assesses child perceptions of pain-related fears and avoidance behaviors.
- Pediatric Quality of Life Inventory (PedsQL) [22] is a self-reported instrument that measures physical, social, emotional, and school function on a 5-point Likert rating scale. It is well-validated for use with youth with chronic pain.
2.7. Data Analysis
3. Results
3.1. Population Description
3.2. Lidocaine Infusions
3.3. Events and Side Effects
3.4. Immediate Response to LI
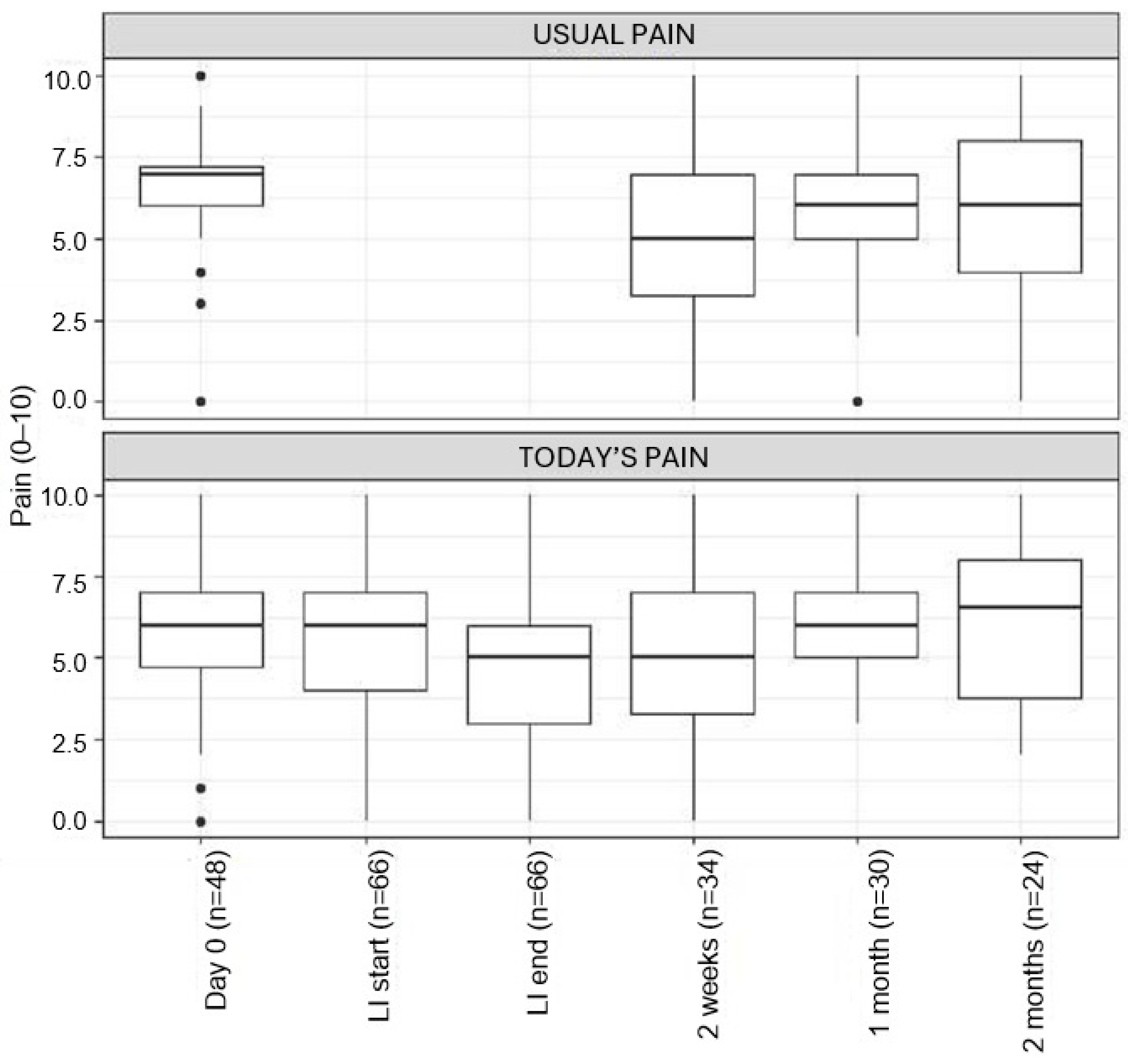
3.5. Prospective Follow-Up
Pain Scores
3.6. Psychological Functioning
3.7. Physical Functioning
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LI | Lidocaine infusion |
| BCH | Boston Children’s Hospital |
| MPC | Multidisciplinary Pain Clinic |
| CPDR | Chronic Pain Data Repository |
| LAST | Local anesthetic severe toxicity |
| NRS | Numeric Rating Scale |
| FLACC | Face, Legs, Activity, Cry Consolability scale |
| INRS | Individualized Numeric Rating Scale |
| FDI | Functional Disability Inventory |
| PCS-C | Pain Catastrophizing Scale for Children |
| FOPQ-C | Fear of Pain Questionnaire, Child Report |
| PedsQL | Pediatric Quality of Life Inventory |
| ED | Emergency Department |
References
- Simons, L.E.; Sieberg, C.B.; Conroy, C.; Randall, E.T.; Shulman, J.; Borsook, D.; Berde, C.; Sethna, N.F.; Logan, D.E. Children with Chronic Pain: Response Trajectories Following Intensive Pain Rehabilitation Treatment. J. Pain 2018, 19, 207–218. [Google Scholar] [CrossRef]
- Golzari, S.E.; Soleimanpour, H.; Mahmoodpoor, A.; Safari, S.; Ala, A. Lidocaine and Pain Management in the Emergency Department: A Review Article. Anesthesiol. Pain Med. 2014, 4, e15444. [Google Scholar] [CrossRef]
- Hall, E.A.; Sauer, H.E.; Davis, M.S.; Anghelescu, D.L. Lidocaine Infusions for Pain Management in Pediatrics. Pediatr. Drugs 2021, 23, 349–359. [Google Scholar] [CrossRef]
- Hachenberg, T. Perioperative management with short-acting intravenous anesthetics. Anaesthesiol. Reanim. 2000, 25, 144–150. [Google Scholar] [PubMed]
- de Jong, R.H.; Nace, R.A. Nerve Impulse Conduction During Intravenous Lidocaine Injection. Anesthesiology 1968, 29, 22–27. [Google Scholar] [CrossRef]
- Yousefshahi, F.; Predescu, O.; Francisco Asenjo, J. The Efficacy of Systemic Lidocaine in the Management of Chronic Pain: A Literature Review. Anesthesiol. Pain Med. 2017, 7, e44732. [Google Scholar] [CrossRef]
- Lee, J.T.; Sanderson, C.R.; Xuan, W.; Agar, M. Lidocaine for Cancer Pain in Adults: A Systematic Review and Meta-Analysis. J. Palliat. Med. 2019, 22, 326–334. [Google Scholar] [CrossRef]
- Beaussier, M.; Delbos, A.; Maurice-Szamburski, A.; Ecoffey, C.; Mercadal, L. Perioperative Use of Intravenous Lidocaine. Drugs 2018, 78, 1229–1246. [Google Scholar] [CrossRef] [PubMed]
- Daykin, H. The Efficacy and Safety of Intravenous Lidocaine for Analgesia in the Older Adult: A Literature Review. Br. J. Pain 2017, 11, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Ayulo, M.A.; Phillips, K.E.; Tripathi, S. Safety and Efficacy of IV Lidocaine in the Treatment of Children and Adolescents With Status Migraine. Pediatr. Crit. Care Med. 2018, 19, 755–759. [Google Scholar] [CrossRef]
- Nathan, A.; Rose, J.B.; Guite, J.W.; Hehir, D.; Milovcich, K. Primary Erythromelalgia in a Child Responding to Intravenous Lidocaine and Oral Mexiletine Treatment. PEDIATRICS 2005, 115, e504–e507. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.F.; Kraychete, D.C. The analgesic effect of intravenous lidocaine in the treatment of chronic pain: A literature review. Rev. Bras. Reumatol. 2014, 54, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Marmura, M.J. Intravenous Lidocaine and Mexiletine in the Management of Trigeminal Autonomic Cephalalgias. Curr. Pain Headache Rep. 2010, 14, 145–150. [Google Scholar] [CrossRef]
- Mooney, J.J.; Pagel, P.S.; Kundu, A. Safety, Tolerability, and Short-Term Efficacy of Intravenous Lidocaine Infusions for the Treatment of Chronic Pain in Adolescents and Young Adults: A Preliminary Report. Pain Med. 2014, 15, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Lemming, K.; Fang, G.; Buck, M.L. Safety and Tolerability of Lidocaine Infusions as a Component of Multimodal Postoperative Analgesia in Children. J. Pediatr. Pharmacol. Ther. 2019, 24, 34–38. [Google Scholar] [CrossRef]
- Batko, I.; Kościelniak-Merak, B.; Tomasik, P.J.; Kobylarz, K.; Wordliczek, J. Lidocaine as an Element of Multimodal Analgesic Therapy in Major Spine Surgical Procedures in Children: A Prospective, Randomized, Double-Blind Study. Pharmacol. Rep. 2020, 72, 744–755. [Google Scholar] [CrossRef]
- e Silva, L.O.J.; Scherber, K.; Cabrera, D.; Motov, S.; Erwin, P.J.; West, C.P.; Murad, M.H.; Bellolio, M.F. Safety and Efficacy of Intravenous Lidocaine for Pain Management in the Emergency Department: A Systematic Review. Ann. Emerg. Med. 2018, 72, 135–144.e3. [Google Scholar] [CrossRef]
- Donado, C.; Lobo, K.; Berde, C.; Bourgeois, F. Developing a Pediatric Pain Data Repository. JAMIA Open 2019, 3, 31–36. [Google Scholar] [CrossRef]
- Kashikar-Zuck, S.; Flowers, S.R.; Claar, R.L.; Guite, J.W.; Logan, D.E.; Lynch-Jordan, A.M.; Palermo, T.M.; Wilson, A.C. Clinical Utility and Validity of the Functional Disability Inventory (FDI) among a Multicenter Sample of Youth with Chronic Pain. Pain 2011, 152, 1600–1607. [Google Scholar] [CrossRef]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The Pain Catastrophizing Scale: Development and Validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Simons, L.E.; Sieberg, C.B.; Carpino, E.; Logan, D.; Berde, C. The Fear of Pain Questionnaire (FOPQ): Assessment of Pain-Related Fear among Children and Adolescents with Chronic Pain. J. Pain 2011, 12, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Varni, J.W.; Seid, M.; Rode, C.A. The PedsQL: Measurement Model for the Pediatric Quality of Life Inventory. Med. Care 1999, 37, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Riley, W.; Stone, A.; Rothrock, N.; Reeve, B.; Yount, S.; Amtmann, D.; Bode, R.; Buysse, D.; Choi, S.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) Developed and Tested Its First Wave of Adult Self-Reported Health Outcome Item Banks: 2005–2008. J. Clin. Epidemiol. 2010, 63, 1179–1194. [Google Scholar] [CrossRef]
- Jacobson, C.J.; Farrell, J.E.; Kashikar-Zuck, S.; Seid, M.; Verkamp, E.; Dewitt, E.M. Disclosure and Self-Report of Emotional, Social, and Physical Health in Children and Adolescents with Chronic Pain--a Qualitative Study of PROMIS Pediatric Measures. J. Pediatr. Psychol. 2013, 38, 82–93. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2018. Available online: https://www.R-project.org/ (accessed on 23 September 2025).
- Vervoort, T.; Goubert, L.; Eccleston, C.; Bijttebier, P.; Crombez, G. Catastrophic Thinking about Pain Is Independently Associated with Pain Severity, Disability, and Somatic Complaints in School Children and Children with Chronic Pain. J. Pediatr. Psychol. 2006, 31, 674–683. [Google Scholar] [CrossRef]
- Samwel, H.J.A.; Evers, A.W.M.; Crul, B.J.P.; Kraaimaat, F.W. The Role of Helplessness, Fear of Pain, and Passive Pain-Coping in Chronic Pain Patients. Clin. J. Pain 2006, 22, 245–251. [Google Scholar] [CrossRef]
- Marks, D.M.; Newhouse, A. Durability of Benefit From Repeated Intravenous Lidocaine Infusions in Fibromyalgia Patients: A Case Series and Literature Review. Prim. Care Companion CNS Disord. 2015, 17, 26280. [Google Scholar] [CrossRef][Green Version]
- Wilderman, I.; Pugacheva, O.; Perelman, V.S.; Wansbrough, M.C.T.; Voznyak, Y.; Zolnierczyk, L. Repeated Intravenous Lidocaine Infusions for Patients with Fibromyalgia: Higher Doses of Lidocaine Have a Stronger and Longer-Lasting Effect on Pain Reduction. Pain Med. 2020, 21, 1230–1239. [Google Scholar] [CrossRef]
- Iacob, E.; Hagn, E.E.; Sindt, J.; Brogan, S.; Tadler, S.C.; Kennington, K.S.; Hare, B.D.; Bokat, C.E.; Donaldson, G.W.; Okifuji, A.; et al. Tertiary Care Clinical Experience with Intravenous Lidocaine Infusions for the Treatment of Chronic Pain. Pain Med. 2018, 19, 1245–1253. [Google Scholar] [CrossRef]
- Schafranski, M.D.; Malucelli, T.; Machado, F.; Takeshi, H.; Kaiber, F.; Schmidt, C.; Harth, F. Intravenous Lidocaine for Fibromyalgia Syndrome: An Open Trial. Clin. Rheumatol. 2009, 28, 853–855. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Potential Exclusion Criteria |
|---|---|
The patient has debilitating pain from
|
|
| Lidocaine Dosing | Infuse over | |
|---|---|---|
| Initial dose | <50 kg: 2 mg/kg IV >/=50 kg: 100 mg IV | 30 min |
| Subsequent dose | <50 kg: 2 mg/kg IV >/=50 kg: 100 mg IV | Subsequent 60 min |
| Total dose | <50 kg: 4 mg/kg IV >/=50 kg: 200 mg IV | Total 90 min |
| No LI | Yes LI | ||
|---|---|---|---|
| (n = 2489) | (n = 126) | p-Value | |
| Age (mean; SD) | 14.92 (3.36) | 15.50 (2.34) | 0.056 |
| Gender | |||
| Female | 1954 (78.5) | 112 (88.9) | 0.043 * |
| Male | 499 (20.0) | 13 (10.3) | |
| Prefer to Self-Describe | 14 (0.6) | 0 (0.0) | |
| Transgender | 22 (0.9) | 1 (0.8) | |
| Developmental history | |||
| Problems during pregnancy | 660 (27.6) | 27 (22.0) | 0.204 |
| Walking by 8 months | 2265 (94.2) | 121 (97.6) | 0.162 |
| Talking by 18 months | 2184 (91.3) | 119 (96.7) | 0.052 |
| Prematurity | 320 (13.5) | 13 (11.6) | 0.743 |
| Early sensitivity | 544 (22.7) | 21 (17.2) | 0.195 |
| Use of assistant devices | |||
| Boot | 133 (5.3) | 5 (4.0) | 0.639 |
| Crutches | 263 (10.6) | 11 (8.7) | 0.612 |
| Walker | 57 (2.3) | 1 (0.8) | 0.422 |
| Wheelchair | 197 (7.9) | 8 (6.3) | 0.64 |
| School | |||
| Enrolled in school | 2255 (92.2) | 121 (97.6) | 0.042 * |
| Home schooled | 262 (11.6) | 9 (7.5) | 0.217 |
| Missed school day due to pain (mean; SD) | 20.4 (28.0) | 22.7 (27.9) | 0.423 |
| Plan 504 | 556 (26.8) | 47 (49.5) | <0.001 ** |
| Home tutoring | 185 (8.9) | 13 (13.8) | 0.154 |
| Plan IEP | 378 (18.2) | 12 (13.0) | 0.26 |
| Gym at school | |||
| Modified gym | 434 (18.9) | 24 (21.1) | 0.636 |
| No | 1448 (63.2) | 67 (58.8) | |
| Yes | 410 (17.9) | 23 (20.2) | |
| Extracurricular activities | 1379 (57.3) | 82 (66.1) | 0.066 |
| Limited extra activities due to pain | 2186 (91.3) | 116 (93.5) | 0.482 |
| Sleep | |||
| Wake up at night times | |||
| 0 | 753 (31.9) | 26 (22.2) | 0.014 * |
| 1–2 | 1069 (45.3) | 51 (43.6) | |
| 3–4 | 417 (17.7) | 29 (24.8) | |
| 5+ | 119 (5.0) | 11 (9.4) | |
| Caffeinated drinks day | |||
| 0 | 1707 (71.5) | 80 (66.1) | 0.603 |
| 1–2 | 652 (27.3) | 39 (32.2) | |
| 3–4 | 27 (1.1) | 2 (1.7) | |
| 5+ | 2 (0.1) | 0 (0.0) | |
| Tired in the morning (yes) | 1321 (55.0) | 79 (64.8) | 0.044 * |
| Health utilization | |||
| Physician visits (mean; SD) | 5.32 (4.74) | 5.55 (3.88) | 0.594 |
| Emergency room visits (mean; SD) | 0.87 (2.03) | 0.65 (1.26) | 0.224 |
| Overnight hospitalizations (mean; SD) | 0.39 (1.71) | 0.15 (0.63) | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riley, B.; Shusterman, C.; O’Neil, T.; Donado, C.; Lobo, K.; Koka, A.; Nelson, S.; Ribeiro, M.; Dinakar, P.; Solodiuk, J.; et al. Short-Duration Systemic Lidocaine for the Management of Refractory Chronic Pain in Pediatrics. Children 2025, 12, 1349. https://doi.org/10.3390/children12101349
Riley B, Shusterman C, O’Neil T, Donado C, Lobo K, Koka A, Nelson S, Ribeiro M, Dinakar P, Solodiuk J, et al. Short-Duration Systemic Lidocaine for the Management of Refractory Chronic Pain in Pediatrics. Children. 2025; 12(10):1349. https://doi.org/10.3390/children12101349
Chicago/Turabian StyleRiley, Bobbie, Christine Shusterman, Teresa O’Neil, Carolina Donado, Kimberly Lobo, Anjali Koka, Sarah Nelson, Monique Ribeiro, Pradeep Dinakar, Jean Solodiuk, and et al. 2025. "Short-Duration Systemic Lidocaine for the Management of Refractory Chronic Pain in Pediatrics" Children 12, no. 10: 1349. https://doi.org/10.3390/children12101349
APA StyleRiley, B., Shusterman, C., O’Neil, T., Donado, C., Lobo, K., Koka, A., Nelson, S., Ribeiro, M., Dinakar, P., Solodiuk, J., Schechter, N., & Greco, C. (2025). Short-Duration Systemic Lidocaine for the Management of Refractory Chronic Pain in Pediatrics. Children, 12(10), 1349. https://doi.org/10.3390/children12101349






